Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A range of vasospastic and inflammatory disorders can present with ischaemia and thus come to the attention of the vascular clinician. These include primary and secondary Raynaud’s phenomenon (RP), and conditions that cause vasculitis (the vasculitides). Diagnostic accuracy is important as management is directed by the underlying pathogenic process, be it vasospasm or inflammation. Diagnosis can be challenging, not least because presenting features of the vasculitides and some of the connective tissue diseases associated with secondary Raynaud’s are non-specific, and features of atherosclerosis, vasculitis and vasospasm may overlap. The aim of this chapter is to provide the vascular clinician with a grounding of knowledge in these conditions so that the initial diagnosis can be made. It describes the clinical features, diagnostic tests, with particular emphasis on serology and imaging, and delineates treatment, highlighting guidelines where they exist.
Vasospasm is the key feature of RP, illustrated in Fig. 12.1 . Maurice Raynaud’s original description was of episodic digital ischaemia induced by cold and emotion. There is no single accepted clinical definition of RP. The classic ‘French tricolour’ is pallor (vasospasm), then cyanosis (deoxygenation of static venous blood) and finally rubor (reactive hyperaemia). This full triphasic colour change is not essential for the diagnosis of RP, and a history of monophasic cold-induced blanching may be sufficient. The condition may be asymmetrical and may not involve the whole digit. Other stimuli can provoke attacks, such as stress, chemicals (including drugs and those in tobacco smoke ), and trauma. In addition to the digits, vasospasm may involve the nose, tongue, ear lobes and nipples.
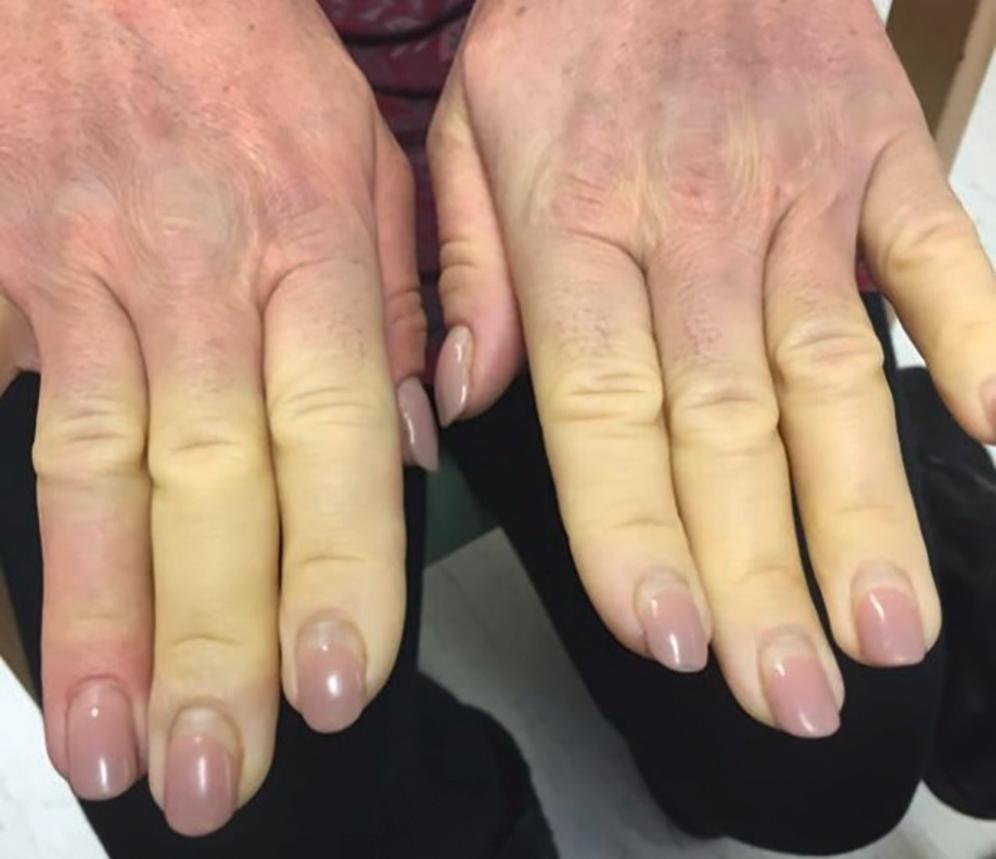
There is inconsistent terminology around RP. The nomenclature recommended in recent European Society for Vascular Medicine (ESVM) guidelines suggests the terms primary Raynaud’s phenomenon ( PRP ) where there is no underlying associated condition, and secondary Raynaud’s phenomenon ( SRP ) where there is. The terms Raynaud’s disease and Raynaud’s syndrome are best avoided.
![]() Use the term ‘Raynaud’s phenomenon’.
Use the term ‘Raynaud’s phenomenon’.
![]() Colour change may only be monophasic – have a low index of suspicion for diagnosis.
Colour change may only be monophasic – have a low index of suspicion for diagnosis.
![]() Primary Raynaud’s phenomenon (PRP) is not associated with any underlying condition.
Primary Raynaud’s phenomenon (PRP) is not associated with any underlying condition.
![]() Secondary Raynaud’s phenomenon (SRP) is associated with an underlying condition, most often rheumatological.
Secondary Raynaud’s phenomenon (SRP) is associated with an underlying condition, most often rheumatological.
A meta-analysis of observational studies suggests a prevalence of RP of approximately 5% in the UK but may affect 20–30% of women in younger age groups. Primary RP comprises 80–90% cases, is nine times more common in women and has a genetic contribution in 50% of cases. , ,
This can be difficult but is critical to management. Conditions associated with SRP are shown in Box 12.1 and features, which can be helpful to distinguish them in Table 12.1 .
![]() Distinguishing PRP and SRP can be clinically difficult.
Distinguishing PRP and SRP can be clinically difficult.
![]() Have a low threshold for further investigation and referral to rheumatology.
Have a low threshold for further investigation and referral to rheumatology.
Systemic sclerosis
Idiopathic inflammatory myositis, amyopathic disease, anti-synthetase syndrome
Systemic lupus erythematosus
Sjögren’s syndrome
Mixed connective tissue diseases/overlap syndromes
Undifferentiated connective tissue diseases
Rheumatoid arthritis
Atherosclerosis
Buerger’s disease (thromboangiitis obliterans)
Microemboli
Thoracic outlet syndrome (especially cervical ribs)
Cryoglobulinaemia, cryofibrinogenaemia
Paraprotein
Malignancy, paraneoplastic phenomenon
Anti-migraine therapies: ergot alkaloids, e.g., methysergide
Non-selective beta-blockers, including eye drops
Bromocriptine
Clonidine
Cytotoxics, e.g., bleomycin, cisplatin
Ciclosporin
Drugs of misuse: amphetamines, cannabis, cocaine
Ephedrine, in ear, nose and throat preparations
Interferon α and β
Oestrogen replacement therapy without progesterone
Vinyl chloride monomers
Vibration white finger disease
Frozen food industry
Reflex sympathetic dystrophy
Hypothyroidism
Phaeochromocytoma
| Primary Raynaud’s | Secondary Raynaud’s | |
|---|---|---|
| Age at onset | Generally young | Can be older |
| Involvement of thumbs | Usually spared | Involved |
| Temperature trigger | Severe coldLong exposure | Trivial temperature dropShort exposure |
| Recovery time on rewarming | Short; often <10 minutes | Long; may be >30 minutes |
| Ischaemic damage | AbsentNo pain between episodes | Digital pain, pitting, ulcers, gangrene |
| ANA | NegativeWeak positive 1 in 80 | Positive; >1 in 80Supplementary antibodies positive; ENA, SSc specific, myositis specific |
| Nailfold capillaroscopy | Normal | Abnormal |
| Peripheral pulses | Normal | Impaired/absent if obstructive cause |
| ESR, plasma viscosity | Normal | Raised if hyperviscosity causal |
PRP is generally a benign condition with few if any significant sequelae. Nevertheless, individuals with PRP still report impaired quality of life.
SRP is more severe, has the potential to cause ischaemic damage to the digit ( Fig. 12.2 ) and often requires pharmacological intervention. Recognition of SRP is important as it enables identification of an associated reversible cause (see Box 12.1 ), or connective tissue disorder (CTD) and the opportunity to optimise management of this at an early stage.
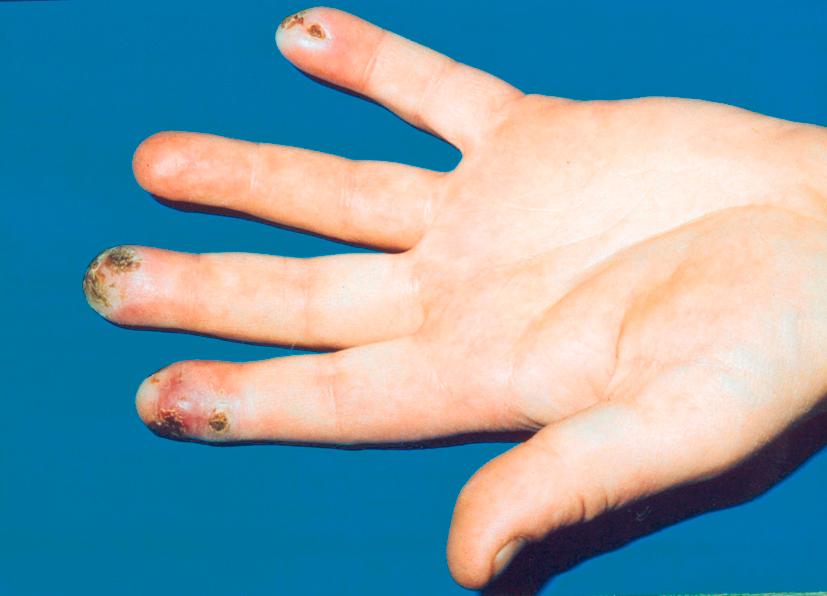
The age of onset is one useful feature, SRP generally occurring at a later age, for example, at a median of 36 years compared to 14 years in PRP. Around 80% of patients over 60 years presenting with new digital ischaemia will have an associated condition. Conversely RP occurring in very young children, whilst rare, is frequently caused by an underlying CTD. Thumb involvement suggests SRP.
The CTDs are the most common group of disorders underlying SRP, with the prevalence of RP in each listed in Table 12.2 . Of these, systemic sclerosis (SSc) is the most frequent association and linked with digit loss in up to 16% of patients. A ‘top to toe’ screen to identify possible features of a CTD is recommended ( Box 12.2 ). Nonetheless, SRP may be the only feature of an underlying CTD and precede other symptoms by years. One study reported that 12.6% of patients referred for assessment of RP progressed to SSc after a median follow-up of 4 years. Another reported a 9% transition in patients presenting with RP alone and 30% from ‘possible’ secondary RP after a 12.4-year mean follow-up.
![]() Systemic sclerosis (SSc) and systemic lupus erythematosus (SLE) are the commonest and most serious connective tissue disorders (CTDs) associated with SRP.
Systemic sclerosis (SSc) and systemic lupus erythematosus (SLE) are the commonest and most serious connective tissue disorders (CTDs) associated with SRP.
![]() RP may be the only presenting symptom.
RP may be the only presenting symptom.
| Systemic sclerosis | 95% |
| Mixed connective tissue disease | 85% |
| Systemic lupus erythematosus | 29–40% |
| Idiopathic inflammatory myositis | 40% |
| Sjögren’s syndrome | 33% |
| Rheumatoid arthritis | 10% |
hair thinning, alopecia
dry mouth or eyes
oral ulcers
rash, photosensitivity
arthralgia
difficulty swallowing, choking
cough, breathlessness on exertion
muscle pain or weakness
fatigue
Close examination of the hands may reveal signs of ischaemic damage, which excludes PRP, and features of CTDs associated with SRP. Ischaemia is indicated by digital pulp loss, skin cracks or breaks, ulcers and at worse, gangrene. Features of CTDs include subcutaneous calcification (calcinosis cutis), ragged cuticles and visible nailfold capillaries. The latter are caused by abnormal giant capillary loops and haemorrhage from these, confirmed by capillaroscopy (see later section). The skin of the fingers should be examined for SSc where early features include puffiness followed by the classic waxy tightening of sclerodactyly, and ultimately progressive sclerodermatous change extending proximally up the arms and legs, involvement of the trunk, back and face with peri-oral puckering, tendon friction rubs and joint contractures. Roughening of the skin, on the radial aspect of the fingers and ulnar side of the thumb, called ‘mechanic’s hands’ is seen almost exclusively in inflammatory myositis.
Peripheral pulses should be checked for strength and symmetry, blood pressure compared in both arms, and subclavian arteries checked for bruits. Thoracic outlet syndromes can be checked using dynamic tests such as Adson’s.
Basic tests include full blood count, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), urea and electrolytes, liver and thyroid function tests, antinuclear antibody (ANA) and urinalysis. In systemic lupus erythematosus (SLE), myeloma and other hyperviscosity syndromes, the ESR may be raised but CRP normal. This should prompt serum protein electrophoresis and serum free light chain testing. Imaging for obstructive causes including a cervical rib may be appropriate.
Most patients with SSc or SLE will have a positive ANA. However, the test is positive in around 10% of healthy individuals, usually at low titre (1/80–1/160). If the ANA is positive, check extractable nuclear antigen (ENA) antibodies and anti-double stranded deoxyribonucleic acid antibodies and specific antibodies for SSc and myositis. Autoantibodies have clinical utility as they are associated with specific autoimmune diseases, patterns of disease and organ involvement, summarised in Table 12.3 . As such, their detection enables prediction of likely clinical phenotypes, greatly enhancing patient care, with targeted screening and preemptive treatment before critical organ damage ensues.
| Antibody | Disease | Specific association |
|---|---|---|
| Centromere | Limited SSc | |
| Topoisomerase I (Scl70) | Diffuse SSc | ILD, cardiac |
| RNA polymerase III | Diffuse SSc | Renal crisis |
| Double stranded DNA | SLE | Renal disease |
| RNP, Sm | SLE | PH |
| U1RNP | mixed CTD | |
| SSA (Ro), SSB (La) | Sjogren’s syndrome, SLE | |
| Jo-1, PL-7, PL-12 ∗ | Anti-synthetase syndrome | ILD |
| Pm/Scl-75/100 | Myositis, SSc | ILD |
| Ro-52 | Sjogren’s, myositis | ILD |
| MDA-5 | Dermatomyositis | ILD |
| SAE | Dermatomyositis | Oesophageal disease |
| TIF1γ, NXP-2 | Dermatomyositis | Malignancy |
| CCP | Rheumatoid arthritis | |
| ANCA, PR3, MPO | Small vessel vasculitis | ILD and renal disease |
∗ 8 antibodies are recognized in the anti-synthetase syndrome: Jo-1, PL-7, PL-12, EJ, Ha, KS, OJ, Zo.
SSc has the strongest association with RP. It is present in virtually all cases, sometimes preceding other clinical manifestations by several years. Limited SSc is associated with anti-centromere antibodies and specific clinical features and signs including telangiectasiae, calcinosis cutis, sclerodactyly and oesophagitis (previously known as ‘ CREST’ ). Diffuse cutaneous SSc is a more aggressive disorder associated with internal organ involvement including renal and interstitial lung disease (ILD) and pulmonary hypertension. Antibodies to topoisomerase I (Scl70) are associated with ILD and cardiac involvement and antibodies to ribonucleic acid (RNA) polymerase III with renal disease. , Early detection of ILD enables commencement of immunosuppressants such as cyclophosphamide and mycophenolate mofetil to prevent progression. Detection of anti-RNA polymerase III enables enhanced monitoring for renal crisis with blood pressure and estimated glomerular filtration rate (eGFR) assessment, avoidance of steroids, which increase the incidence, and early commencement of angiotensin-converting enzyme (ACE) inhibitors to significantly improve outcome from this rare but fatal complication.
Isolated patches of sclerodermatous skin, called morphoea , may also be associated with RP and the presence of autoantibodies or abnormal nailfold capillaries confers a high likelihood of a systemic disease.
In patients with SLE, the presence of RP associates with anti-Sm and anti-RNP autoantibodies , and a more benign disease course, including a lower incidence of renal disease, serositis, haemolysis and improved survival, , although a higher incidence of peripheral and central nervous system disease has been reported.
RP is a prevalent feature of the idiopathic inflammatory myopathies (IIM), reported in 78% of those with anti-PM/Scl-75/100 antibodies in which features of SSc and myositis are found. In the same series, the prevalence of RP was 39% in the anti-synthetase syndrome (ARS), 22% in dermatomyositis (DM), and 15% in necrotising myositis. Other skin signs are diagnostic of IIM, with mechanics hands a pathognomonic feature occurring in 80% of patients with anti-PM/SCl-75/100 antibodies, 58% of those with ARS and 28% with DM. Thus the finding of co-existing skin signs including mechanics hands and the well described features of DM, such as Gottron’s papules and a heliotrope peri-orbital rash, reveal IIM as a secondary cause of RP, and enable screening for muscle and lung disease.
![]() Autoantibodies are increasingly used to predict specific clinical phenotypes in CTDs.
Autoantibodies are increasingly used to predict specific clinical phenotypes in CTDs.
![]() ANA is a useful screening test; when positive, further immunological tests and rheumatology referral should be considered.
ANA is a useful screening test; when positive, further immunological tests and rheumatology referral should be considered.
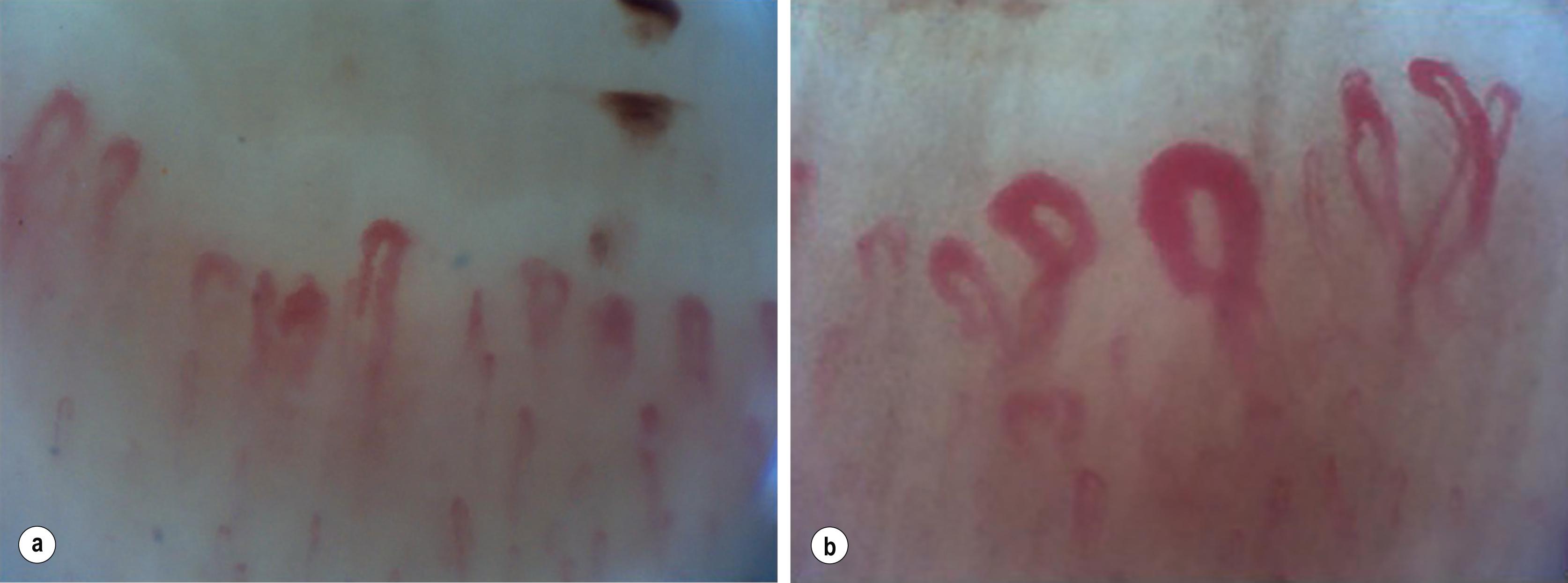
At the nailfold, the capillaries run in parallel to the skin surface enabling inspection of anatomical size and form. Nailfold capillaroscopy has become established as one of the most sensitive ways to screen for an associated CTD, especially SSc. This technique uses 200–600 x magnification to directly visualise the capillaries at the nail fold. Lower magnification may be used by a variety of devices such as an ophthalmoscope or dermatoscope, with less detail visible. Very large abnormal capillaries can just be seen with the naked eye. Normal vessels are uniform in diameter and lie equi-spaced and in parallel with a ‘hairpin’ structure. Characteristic abnormalities in CTDs ( Fig. 12.3 ), especially systemic sclerosis, include dilation, giant forms, distortion of shape, tortuosity, haemorrhage and regions of absence (drop-out). The combination of abnormal nailfold vessels and autoantibodies has been shown to predict progression to CTD; studies suggest that RP patients with both abnormalities are up to 60 times more likely to go on to develop SSc than those with neither. , It should be noted that nailfold changes also occur with trauma and in diabetes mellitus.
![]() The diagnostic value of nailfold capillaroscopy is now validated with standardisation and algorithms developed to detect SSc ,
The diagnostic value of nailfold capillaroscopy is now validated with standardisation and algorithms developed to detect SSc ,
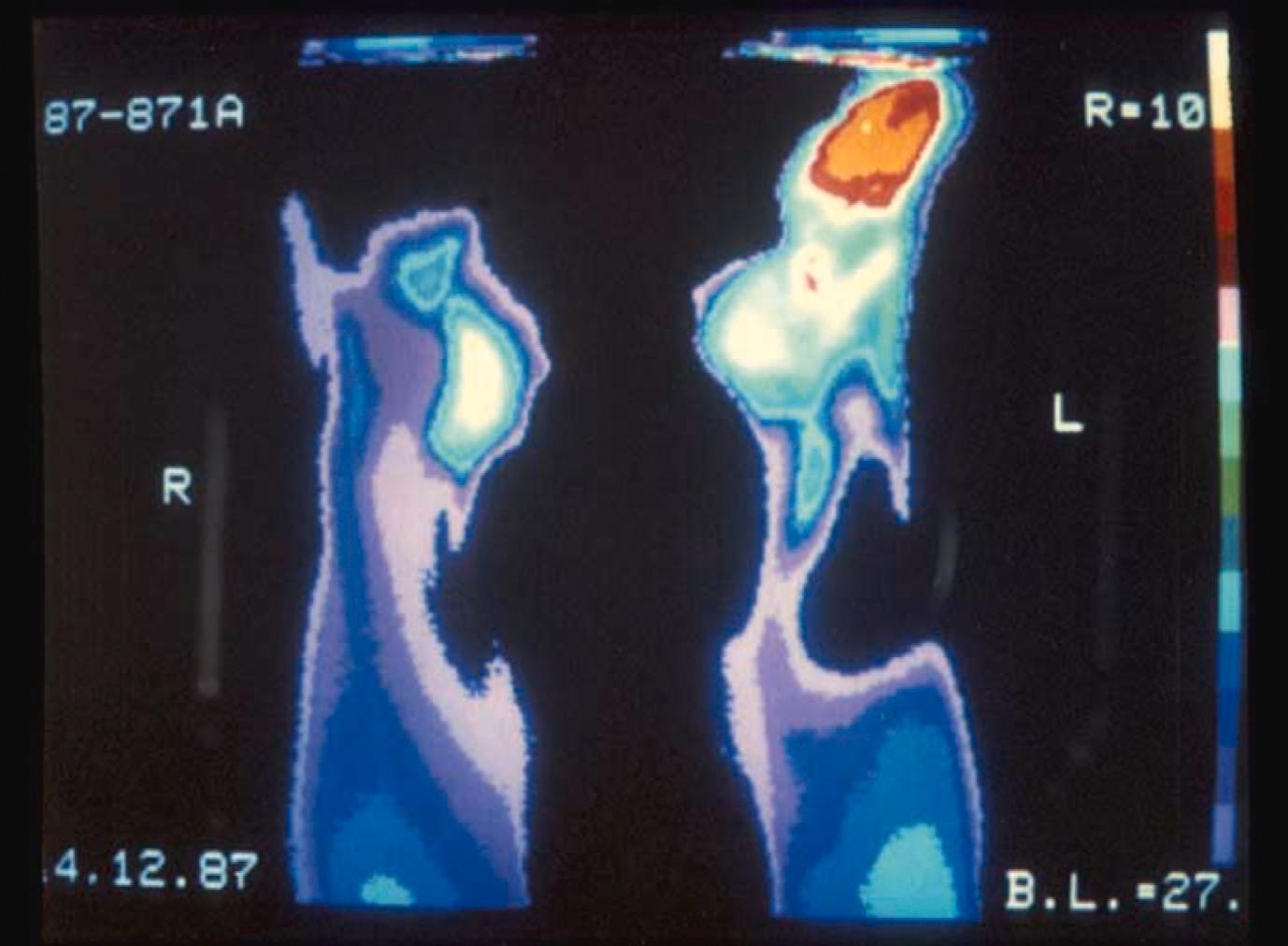
Thermography uses a thermal imaging camera to measure skin surface temperature, as an indirect measure of cutaneous blood flow ( Fig. 12.4 ). This can help distinguish SRP from PRP, given more severe disease in SRP, especially SSc. , This technique is yet to be routinely used in clinical practice, and requires specialised equipment and a temperature controlled room. It may also have potential as an indirect measure of response to therapy, though standardised protocols have not been established.
Hand–arm vibration syndrome (HAVS, previously known as vibration white finger ), is described in workers using vibrating tools such as chainsaws, pneumatic road drills and buffing machines. An estimated 4.2 million men and 667 000 women in Great Britain have occupational exposure to hand-transmitted vibration. Vasospasm in HAVS has been described in the toes as well as the fingers and is likely related to vibration-induced damage of the endothelium. The duration of exposure is important, as symptom severity and length of exposure are correlated. The latency period between exposure and disease is usually more than 5 years of full-time work. Resolution of symptoms may occur in up to one-third of cases if there is a change of job early in the disease course.
In the UK, HAVS has been a proscribed industrial disease since 1985. Patients may be eligible for industrial injuries disablement benefits if they fulfil certain criteria. It has been suggested that prolonged exposure to vibrating computer game controls could also cause HAVS.
Further examples of occupation-related RP include vinyl chloride disease, which is estimated to occur in 3% of exposed workers and can persist long after retirement, and nitrate exposure related to ammunitions work, where habituation to vasodilatory nitrates means that RP can develop away from the work environment.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here