Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Clinicians should assess all patients for factors increasing the risk of venous thromboembolic events (VTEs).
Having total hip arthroplasty (THA) puts a patient in a high-risk group.
THA patients are often older, with additional comorbidities that increase the risk of VTE.
Multiple methods of VTE prophylaxis are available.
Chemoprophylaxis
Aspirin
Vitamin K antagonist (VKA; warfarin)
Low-molecular-weight heparin (LMWH)
Factor Xa inhibitors
Direct thrombin inhibitors
Bleeding is a concern for all types of chemoprophylaxis.
Mechanical prophylaxis
Boots
Calf length
Thigh length
Portable or stationary pump
Vena cava filter
Continue to assess clinically for VTE during hospitalization and at follow-up.
Regulations set by the Surgical Care Improvement Project (SCIP) and Joint Commission (formerly the Joint Commission on Accreditation of Healthcare Organizations [JCAHO]) mandate that some type of prophylaxis be prescribed for THA patients.
Prophylaxis for venous thromboembolic events (VTEs) for total hip arthroplasty (THA) patients is an accepted necessity. However, the optimal method and duration of prophylaxis are the topics of ongoing controversy. The incidence of VTE, including deep venous thrombosis (DVT) and pulmonary embolism (PE), were unacceptably high before any type of prophylaxis ( Table 26.1 ). Although prophylaxis has been recommended since 1986, more is known about the epidemiology, risk factors, and methods for determining the presence of VTE, leading to new methods of prophylaxis. Low-molecular-weight heparins (LMWHs) have been available since 1993 and new oral anticoagulants have now been approved. Compression devices have also been available for many years, but recently developed portable compression devices may alter nonpharmacologic prophylaxis. Various guidelines are available, although confusion often surrounds which guidelines should be followed for optimal patient outcomes. A government agency, the Surgical Care Improvement Project (SCIP), and the Joint Commission (formerly the Joint Commission on Accreditation of Healthcare Organizations [JCAHO]), the accreditation body for hospitals, have mandated that THA patients be assessed and provided with prophylaxis after surgery.
| DVT Prevalence | Proximal DVT Prevalence | PE Prevalence | Fatal PE Prevalence | |
|---|---|---|---|---|
| THA | 42%–57% | 18%–36% | 0.9%–28% | 0.1%–2.0% |
| HFS | 46%–60% | 23%–30% | 3%–11% | 0.3%–7.5% |
Thromboprophylaxis in THA begins with assessment of each patient's risk for VTE. Given that surgery, especially orthopedic surgery of the lower extremity, automatically places patients in the high-risk category, initial assessment for other factors that put the patient at even higher risk of VTE is done during preparation for surgery. This assessment may affect the type of prophylaxis prescribed and the length of time that prophylaxis should be continued by the patient. Many available assessment tools provide checklists for risks and often suggest prophylaxis based on these risks ( Box 26.1 ). Patients should be clinically assessed during the recovery period for any signs or symptoms of VTE. Assessments should be documented in patients’ records to communicate with other health care professionals regarding assessment findings. Patients should be educated about clinical VTE signs and symptoms so that they can appropriately report these findings to their physician. Clinical signs alone are notoriously flawed with respect to an accurate diagnosis and should be adjudicated by objective tests, which presently are carried out by duplex ultrasound for DVT and spiral computed tomography (CT) for PE. Screening ultrasound in asymptomatic patients is specifically contraindicated.
Institutionalization (hospital/nursing home)
Trauma (pelvic, lower extremity)
Major orthopedic surgery
Antiphospholipid syndrome
Malignancy with chemotherapy or radiotherapy
Stroke
Malignancy with no therapies
Inherited thrombophilia/hypofibrinolysis
Limited mobility, including lower extremity paresis
History of VTE
Tamoxifen or raloxifene therapy
ASA score—3
Oral contraceptives or hormone replacement therapy (HRT)
Advanced age
Obesity
Diabetes mellitus
Coronary artery disease
Congestive heart disease
Varicose veins
Smoking, current/former
Female gender
Black race
ABO blood group
ASA, American Society of Anesthesiologists; VTE, venous thromboembolism.
Evidence suggests that proximal DVT is more important than distal DVT in terms of its sequelae and risk of development of subsequent PE. Proximal DVT occurs in veins above the knee, from the popliteal upward. Because these vessels are larger, the thrombosis is usually more significant and, if it migrates proximally, could cause a larger PE. Studies have shown that between 20% and 30% of thromboses that originate in the distal veins propagate to proximal veins and can cause PE. Calf vein thrombi are not totally benign: a high proportion leave residual venous abnormalities, including persistent occlusion and/or venous valvular incompetence, and postthrombotic syndrome (PTS) develops in 5% of patients after total knee arthroplasty (TKA) and THA. Therefore, prophylaxis to prevent proximal and distal DVT, as well as PE, appears to be important.
Genetic factors, including mutations of factor V Leiden, prothrombin gene G20210A , have been reported to increase the risk of VTE in the population. One study of TKA or THA patients indicated that the prothrombin gene mutation G20210A was significantly represented in those in this group with symptomatic VTE ( P = .0002). A tendency toward increased risk of VTE was found with factor V Leiden mutation ( P = .09). Men with the factor V Leiden mutation were found to have a six-fold increased rate of VTE recurrences versus major bleeds and a three-fold increase of DVT recurrence with no difference in PE recurrence compared with women after discontinuing anticoagulants. However, because 90% of the population who had these genetic risk factors did not have a VTE, general preoperative genotype screening is of questionable value.
Clotting factors, which have been associated with VTE, include increased levels of factor VII and fibronectin. However, these factors have not been examined in relation to orthopedic surgery patients and VTE. Also implicated is a low level of high-density lipoprotein (HDL), although this has not been studied in the orthopedic surgical patient. Another study reports a positive relationship between plasma cholesterol ester transfer proteins and increased coagulability in young males, although, again, whether this would transfer to surgical patients is not known.
Two genetic variants of the enzyme that metabolizes warfarin, cytochrome P-450 2C9 (CYP2C9) and vitamin K epoxide reductase (VKORC1), have been associated with differences in patient response to warfarin doses. One study of this genetic-based dosing in TKA and THA has been reported. This study of 92 patients proposed an algorithm for warfarin dosing after orthopedic surgery that took into consideration genetic type, clinical variables, current medication, and preoperative and postoperative laboratory values. With validations, a safer, more effective process for initiating warfarin therapy could be provided.
Doppler duplex ultrasound is a major tool used in practice and research to detect DVT in patients following hip and knee arthroplasty surgery. Because Doppler ultrasound is noninvasive, has almost no contraindications, and can be used as a repeated measure, it has become the most widely used test for clinical detection of symptomatic and asymptomatic DVT. Screening has not been shown to be effective in changing an existing protocol because of short hospital stays after THA. Although compression venous Doppler ultrasound has been shown to have high specificity in the thigh in almost all institutions, this specificity is not maintained in the calf by all institutions.
After lower extremity orthopedic surgery, 50% of deaths are caused by vascular events and death may occur within a matter of minutes, making prophylaxis critical. Symptoms most predictive of pulmonary embolism are tachycardia, low oxygen saturation, and shortness of breath. Spiral CT scan of the chest is the preferred method of confirming the diagnosis of PE.
With the increased awareness on the part of the orthopedic surgeon of the risk of VTE after total hip replacement (THR) surgery and the need for prophylaxis comes a competing awareness of the risk for bleeding with any of the potent anticoagulants. Bleeding in these cases can compromise the results of the surgical procedure. Data from multiple large nonrandomized trials and a systematic review seem to confirm the effectiveness of aspirin (acetylsalicylic acid [ASA]) as a part of a multimodal VTE prophylaxis regimen. Based on these trials, both the American Academy of Orthopaedic Surgeons (AAOS) guidelines and CHEST Guidelines from the American College of Chest Physicians (ACCP) include ASA using their classification as “moderate evidence” in the AAOS guidelines and grade 1B in the Chest Guidelines. No consensus has been established concerning the dosage, with recommendations of 81 mg twice daily, 150 mg once daily or 325 mg once or twice daily. Almost all studies using aspirin indicate a decrease in all-cause mortality, indicating a potential effect on the arterial side, as many all-cause mortality cases were secondary to cardiac deaths. Protocols often use aspirin up to 6 weeks postoperatively, but few are recommending use beyond that time frame. As in all pharmacologic usage, tracking compliance is difficult in the outpatient setting.
Historically, the most common pharmacologic agent of choice for the orthopedic surgeon remained adjusted-dose warfarin sodium, a vitamin K inhibitor with monitoring by an international normalized ratio (INR) both in hospital and into the postoperative rehabilitation period. The drug can be started preoperatively or the first postoperative day; full anticoagulation would not be expected for approximately 3 days. The INR for medical patients is a prolongation on the INR to 2 to 3, but many orthopedic protocols use an INR of 1.5 to 1.9 with other low-dose regimes of an INR of 1.5 to 2.5 to avoid the risk of excessive bleeding ( Table 26.2 ). The half-life of warfarin is 36 to 42 hours, and its effects can be reversed with vitamin K. Because warfarin requires monitoring, compliance of the use of warfarin can easily be tracked. The effectiveness of adjusted-dose warfarin is shown in Table 26.3 . Many factors, including medications, smoking, alcohol, foods, and changes in activity, may interact with warfarin. Because many patients are discharged with warfarin prophylaxis, patients must be made aware of these interactions and must monitor themselves for any symptoms of over-anticoagulation. Optimal use of warfarin requires consideration of the time frame of its effects, use of the INR for close monitoring of effects and interacting factors, patient education, and a systematic approach. As with many of the pharmacologic agents, warfarin is often being used in conjunction with external intermittent compression devices, at least during the hospitalization period.
| Drug | Manufacturer/Type of Drug | Dosing | Start Time |
|---|---|---|---|
| Enoxaparin (Lovenox) | Sanofi-Aventis (Bridgewater, NJ)/LMWH | 30 mg SC; 40 mg SC may be used for extended prophylaxis | Start 12–24 h after surgery, then twice daily for 7–10 d; may extend prophylaxis for 35 d after initial 7–10 d. |
| Dalteparin (Fragmin) | Pfizer (Brooklyn, NY)/LMWH (FDA approved only for total hip arthroplasty) |
2500 U (half dose) SC 5000 U (full dose) SC |
May start within 2 h before surgery with half dose or start 4–8 h after surgery with half dose, then full dose once daily; may extend to 14 d. |
| Fondaparinux (Arixtra) | GlaxoSmithKline (Research Park, NC)/synthetic pentasaccharide | 2.5 mg SC | Start approximately 6–8 h after surgery or the next day, then once daily for 7–10 d; may extend for 35 d. |
| Warfarin (Coumadin) | Bristol-Myers Squibb (Princeton, NJ)/VKA | 2–10 mg oral to maintain INR of 1.5–2.5 | Start 1–12 h before or after surgery; may extend for 35 d. |
| Dabigatran | Boehringer Ingelheim, Ingelheim am Rhein, Germany | Administered orally, half dose the first day and a full dose (220 mg) daily | Day of surgery; extend for 28–35 d. |
| Apixaban | Bristol-Myers Squibb (Princeton, NJ) | Orally, twice daily doses (2.5 mg each) | Started day of surgery and continued for 35 d. |
| Rivaroxaban | Bayer HealthCare (Seattle, WA) | Once daily in a fixed dose (10 mg) | Started day of surgery and continued for 35 d. |
| Agent | Number of Studies/Patients | PE, % (95% CI) | Proximal DVT, % (95% CI) | Total DVT, % (95% CI) |
|---|---|---|---|---|
| Placebo | 13/947 | 1.5 (0.8–2.6) | 25.8 (21–31) | 48.5 (43–54) |
| Pneumatic compression | 5/431 | 0.3 (0.01–1.4) | 13.3 (10–18) | 20.7 (15–29) |
| Warfarin | 12/1493 | 0.2 (0.02–0.6) | 6.3 (5–8) | 23.2 (19–28) |
| Low-dose unfractionated heparin | 11/1859 | 1.4 (0.9–2.0) | 19.0 (13–27) | 31.1 (23–41) |
| Aspirin | 8/687 | 1.3 (0.6–2.5) | 11.4 (7–18) | 30.6 (21–42) |
| LMWH | 21/5512 | 0.4 (0.2–0.6) | 7.7 (6–10) | 17.7 (15–21) |
| Fondaparinux | 2/2255 | 0.3 (0.2–0.4) | 1.2 (1–2) | 4.7 (4–6) |
| Rivaroxaban | 2/2149 | 0.6 (0.17–2.13) | 0.36 (0.2–0.62) | |
| Dabigatran | 3/2575 | 6.4% | 6.3% | |
| Apixaban | 4/2708 | 1.72 (0.52–5.69) | 0.55 (0.32–0.95) |
The category of drugs most commonly used worldwide for VTE prophylaxis is LMWHs. These drugs are administered subcutaneously and do not require laboratory monitoring or dose adjustment. Extensive data have shown that this category of drugs is safe and effective, although concern still exists about related bleeding. The point at which each of these agents provides inhibition in the coagulation cascade is shown in Fig. 26.1 .
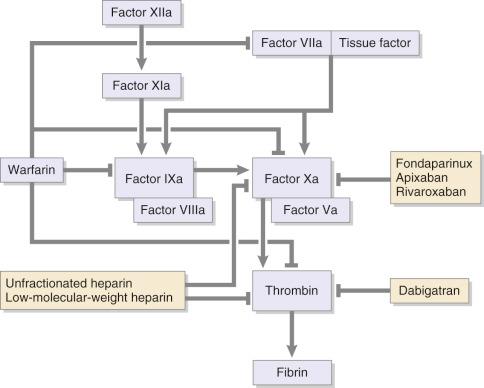
LMWHs were approved for prophylactic use for THA in 1993 and have been adopted in practice by many clinicians. The LMWHs most commonly used in North America are enoxaparin and dalteparin (see Table 26.2 ). LMWHs have been studied extensively and are highly effective and generally safe. Pooled results from clinical trials are shown in Table 26.3 .
LMWHs are pharmacologically different from unfractionated heparin. LMWHs bind less to proteins and endothelial cells, resulting in a more predictable dose response, a dose-independent mechanism of clearance, and a longer plasma half-life.
With highly predictable pharmacokinetic properties and high bioavailability, LMWHs have the ability to target factor Xa while affecting factor IIa to a lesser extent (see Fig. 26.1 ) and are associated with a lower incidence of thrombocytopenia than unfractionated heparin. The half-life of LMWH is 4.5 hours, and its effects can be reversed by protamine sulfate. Its propitious pharmacokinetics allows LMWH administration subcutaneously once or twice daily without requisite monitoring of drug levels or activity.
The possibility of increased bleeding remains a concern with LMWH prophylaxis ( Table 26.4 ).
| Agent | Number of Patients | Major | Minor |
|---|---|---|---|
| Placebo | 713 | 0.6% | 3.0% |
| Pneumatic compression | 388 | 0 | 4.1% |
| Warfarin | 1381 | 1.7% | 5.7% |
| Low-dose unfractionated heparin | 1992 | 3.5% | 13.5% |
| Aspirin | 687 | 0.7% | 1.2% |
| LMWH | 5412 | 2.2% | 10.5% |
| Fondaparinux | 2268 | 0.3% | 2.7% |
| Rivaroxaban | 6183 | 0.4% | 3.1% |
| Dabigatran | 4374 | 5.6% | 6.0% |
| Apixaban | 2673 | 0.8% | 6.9% |
Fondaparinux, a synthetic pentasaccharide given subcutaneously that anticoagulates through inhibition of factor Xa (see Fig. 26.1 ), has been shown to provide effective prophylaxis in a once-daily dose in THA and hip fracture surgery (see Table 26.2 ). This pentasaccharide has a half-life of 18 hours with no known antidote. The reported total incidence of DVT in THA is shown in Table 26.3 . The bleeding rate is reported in Table 26.4 .
Rivaroxaban is an orally administered selective direct inhibitor of factor Xa with a half-life of approximately 9 hours and a rapid onset of action, reaching maximum serum concentration in less than 2 hours (see Table 26.2 ). Rivaroxaban has been reported in two clinical trials after THA, both comparing rivaroxaban with enoxaparin. One trial with treatment for 35 days reported a VTE rate of 1.1% (18/1595) with rivaroxaban and 3.7% (58/1558) with enoxaparin. The other trial, with 31 to 39 days of treatment with rivaroxaban and 10 to 14 days of enoxaparin treatment, reported a VTE rate of 2.0% (17/864) and 9.3% (81/869), respectively. Bleeding was reported to be similar with the two treatments in both studies.
Apixaban, also orally administered, is a selective factor Xa inhibitor with a half-life of approximately 12 hours and reaches maximum concentration within 3 to 4 hours. This drug is given in twice-daily doses for a period of 35 days ( Table 26.2 ). Apixaban clinical trials for prophylaxis in THA using the drug for 10 to 14 days compared with enoxaparin reported a VTE rate of 0.5% (10/2199) for apixaban and 1.1% (25/2195) for enoxaparin, with similar bleeding events.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here