Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A rapid decline in glomerular filtration evidenced by the accumulation of nitrogenous waste products (blood urea nitrogen and creatinine) reflects acute kidney injury (AKI), a common major medical problem that occurs in 7%–18% of hospitalized patients including 50%–60% of those admitted to intensive care units. AKI manifesting in the early postoperative phase is particularly common after some surgeries, complicating up to 20%–70% of cardiac, vascular, major trauma, and hepatobiliary procedures. The importance of postoperative AKI lies in its consistent association with worsened outcome, including higher in-hospital mortality, delayed recovery in survivors, and overall increased cost. Even evidence of minor renal impairment carries with it a related risk of major postoperative complications and mortality.
Clinical practice modifications can eliminate or limit renal insult in cohorts and reduce the burden of postoperative AKI. In contrast, once AKI has occurred, beyond dialysis the portfolio of evidence-based interventions that improve outcome is sparse to nil. This unfortunate observation has gained attention from research agencies such as the United States National Institutes of Health and the Cochrane Database of Systematic Reviews. In this context, the aim of this chapter is to review evidence (or lack thereof) for putative renal preservation strategies, including those directed at the postoperative critically-ill patient.
The kidneys constitute less than 0.5% of body weight but receive 25% of cardiac output, making them the most highly perfused major organs. Among their many active roles are regulation of body fluid composition and volume; the kidneys filter equivalent to a 12-oz (355 mL) can of soda every 3 minutes, returning all but 1% (approximately 4 mL of urine) to the circulation. The ability of the kidneys to control the contents of urine precisely is the basis of whole body homeostasis. As highlighted by the renal physiologist, Dr. Homer Smith: “It is no exaggeration to say that the composition of the blood is determined not by what the mouth ingests but by what the kidneys keep; they are the master chemists of our internal environment, which, so to speak, they synthesize in reverse.” Hence, acute derangements that have systemic effects are thought to underpin some of the risk for adverse outcome related to AKI, a notably different state from compensated chronic kidney disease (CKD) where homeostasis is maintained in the context of limited renal reserve.
Although some elements of perioperative AKI pathophysiology are mechanistically well understood and available to guide the clinician, much is still not known. Nonetheless, identified factors can be divided generally into culprits suspected of adding to a cumulative burden of renal insult and those identifying kidney vulnerability. Pathophysiologic contributors to renal insult are discussed in elsewhere in this textbook, and mechanistic understanding of their role in AKI (and potential avoidance) remains the most important overarching renal preservation principle. Sometimes a sole AKI insult is evident (e.g., renal artery atheroembolism), but AKI is most commonly a multifactorial problem. For specific procedures and even individual patients, renal insults may be obvious, and sometimes their effects are avoidable or can be attenuated through thoughtful clinical care. Vulnerabilities such as comorbid disease (e.g., diabetes) are also extensively discussed in elsewhere in this textbook. However, beyond CKD these premorbid conditions only modestly predict variability in postoperative creatinine rise.
To understand the “renal paradox” (that such a highly perfused organ is so susceptible to injury) requires an appreciation of normal kidney vasculature. The most highly studied pathophysiologic model of AKI is cardiac surgery, which has provided many insights about the kidney response to stress that are generalizable.
Normal blood flow is unevenly distributed within the kidney, with the renal medulla disproportionately under-perfused (5% total). Notably, such heterogeneous blood flow facilitates “mission critical” urine concentrating ability of the kidney, but also places the medulla at particular risk of ischemia. To add to this “low-flow” threat, medullary perfusion is notably inefficient; with entering and exiting vessels arranged in parallel, allowing “escape” of oxygen prior to its arrival at tissues. Known as oxygen countercurrent exchange, this oddity is a byproduct of sluggish perfusion that also allows creation of the medullary urea gradient, an essential ingredient for the urine concentrating process. Finally, adequacy of perfusion is further challenged by high oxygen demands of the outer medulla as a result of active solute transport (thick ascending limb [mTAL] of the loop of Henle). These challenges result in a very low medullary pO 2 even in healthy individuals (10–20 mmHg; Fig. 17.1 ), with medullary oxygen needs met through extraction at the highest levels in the body (79%). Originally reported in the 1960s, this important phenomenon is referred to as medullary hypoxia . Local paracrine systems (e.g., nitric oxide and the renin-angiotensin system) mediate the precarious balance between medullary oxygen demand and delivery to maintain local homeostasis. For clinicians, an additional important consideration is that increased renal blood flow does not provide a luxurious supply of oxygen to the medulla, since equivalent increases in glomerular filtration and tubular active transport reabsorption occur, negating any potential gains.
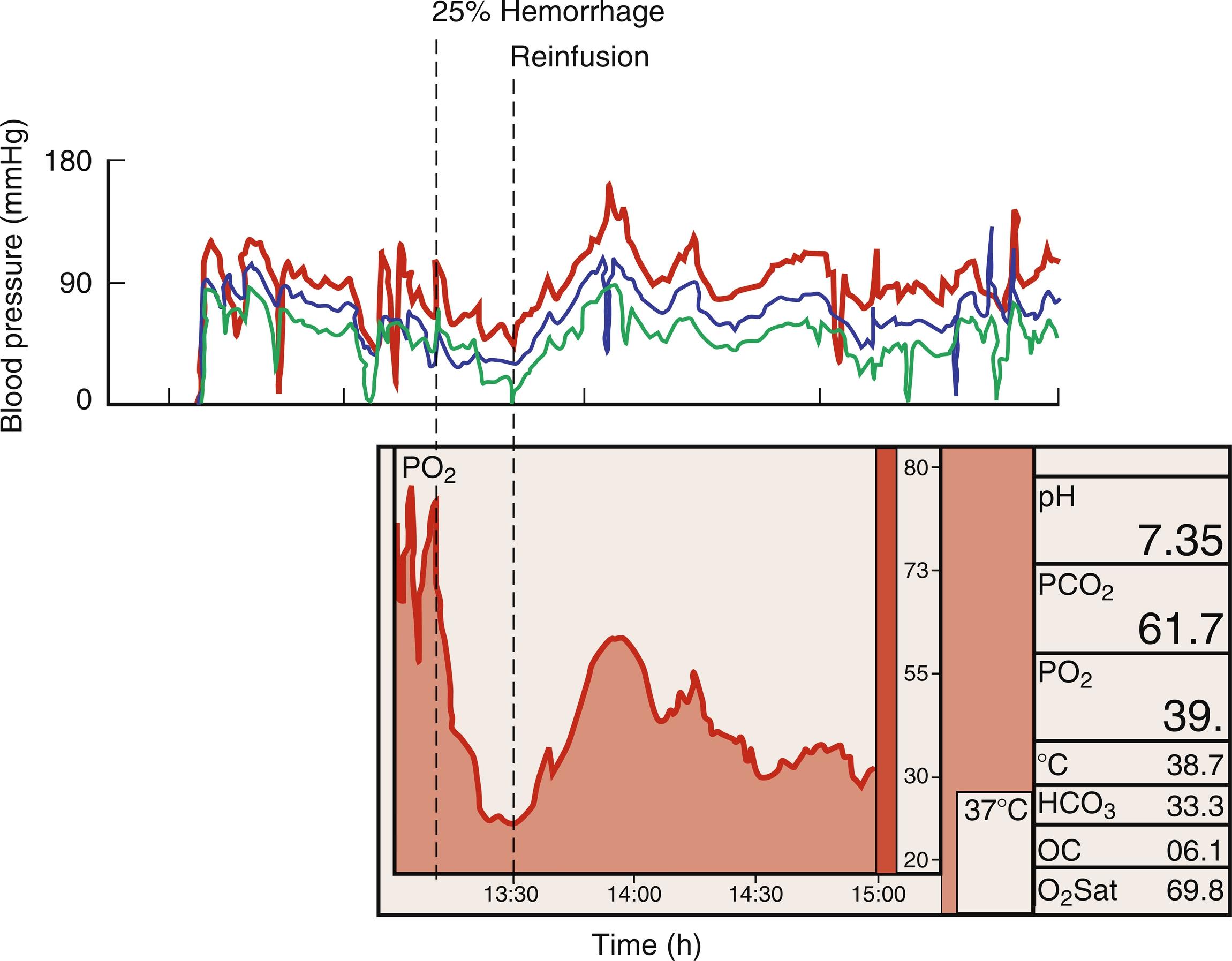
Direct measurement demonstrates that medullary hypoxia is exacerbated by common perioperative occurrences such as dehydration, hemorrhage, low cardiac output, and cardiopulmonary bypass. In addition to the direct effects of such conditions, related homeostatic fluid retention responses may also add to renal risk. Interestingly, urine pO 2 measurement may be useful as a sensitive monitor of medullary perfusion ; one clinical study found that a drop in urine oxygen levels after cardiac surgery predicted postoperative creatinine rise.
Neonates are born with their adult number of nephrons, which mature and grow throughout infancy to reach full function at around the age of 2 years. Glomerular filtration is reduced because of relatively low systemic blood pressure and high renal vascular resistance and in early infancy tubular transport mechanisms are incompletely developed, reducing the reserve for toxin elimination and increasing the risk of (sodium and water) fluid retention. Finally, as a result of the immaturity of the tubule in premature infants and newborns, measures normally used to differentiate pre- from intrarenal injury may be misleading (i.e., urine osmolality, urine sodium, and fractional excretion of sodium).
During pregnancy, hormonal effects contribute to a systemically vasodilated state. Renal changes also include dilation of the urinary collecting system and increases in renal plasma flow and glomerular filtration (hyperfiltration), which can be complicated by urine protein wasting. In the setting of CKD, the demands of pregnancy can overwhelm renal reserve and increase the risk of accelerated deterioration of renal function and associated adverse maternal and fetal outcomes. Treatment of hypertension, proteinuria, and nephrotic syndrome is particularly important in the management of parturients.
Beyond the age of 50 years, kidney parenchyma becomes smaller mainly because of accelerated cortical loss and the crowding effects of benign cysts. On a microscopic level, there are fewer functional nephrons and nephrosclerosis is more prevalent. Some functional compensation is achieved through hypertrophy of remaining nephrons, but averaged over cohorts, glomerular filtration falls accordingly, declining by roughly 0.75 mL/min per year after the age of 30 years. Therefore, even in the absence of renal disease geriatric patients commonly meet CKD criteria, and because of limited reserve are at a higher risk of sustaining AKI to levels requiring dialysis.
Cardiac surgery is the most common source of postoperative AKI in the United States , occurring following up to 30% of such procedures. Observations in postcardiac surgery AKI cohorts commonly generalize to other surgical settings. Therefore, postcardiac surgery AKI (often referred to as CS-AKI) is a prevalent perioperative AKI research model and often the source of evidence to support or to refute putative renal preservation strategies.
Notably, although the physiologic renal trespass of major surgery is well represented by cardiac surgery, elements such as aortic cross clamping and cardiopulmonary bypass differentiate cardiovascular from most other major surgeries. Although the immediate ischemic consequences of cross clamping on the kidney require little explanation, the complex effects on renal perfusion of cardiopulmonary bypass (and other circulatory assist devices) are reviewed elsewhere.
Lack of consensus on a “gold standard” definition for AKI previously hampered progress in its research. One review from the mid-1990s identified 28 perioperative studies, with no two using the same criteria. More recently, consensus AKI criteria have allowed for more uniform reporting and comparison of outcomes. Furthermore, the search for reliable early biomarkers that enable prompt AKI detection earlier than the measurement of serum creatinine accumulation alone is a continuously evolving field.
Consensus criteria have been developed to facilitate AKI diagnosis, four of which are worthy of mention ( Table 17.1 ). Limited to surgical populations, the Society of Thoracic Surgeons postoperative AKI criteria include either new need for dialysis or a serum creatinine rise of at least a 50% from baseline to exceed 2 mg/dL (177 μmol/L). However, three more recently developed (related) definitions are the most commonly used to contrast nonsurgical and surgical studies. These include: (1) RIFLE (Risk, Injury, Failure, Loss, End-stage kidney disease), (2) AKIN (Acute Kidney Injury Network), and (3) KDIGO (Kidney Disease: Improving Global Outcomes) criteria. The most current of these (KDIGO) defines AKI as either (1) a serum creatinine rise ≥ 0.3 mg/dL (≥ 26.5 μmol/L) within a 48-hour period; or (2) a ≥ 50% serum creatinine rise within the prior 7 days; or (3) urine volume < 0.5 mL/kg/h for 6 hours. Each definition uses serum creatinine and urine output criteria to define AKI. Although these consensus criteria have brought stability to the definition of AKI, they are not without their problems, as discussed later.
| Serum Creatinine | Urine Output | |
|---|---|---|
| RIFLE criteria | ||
| Risk | Increase in SCr to ≥ 1.5 times baseline or decrease in GFR by > 25% within 7 days | < 0.5 mL/kg/h for > 6 h |
| Injury | Increase in SCr to > 2 times baseline or decrease in GFR by > 50% within 7 days | < 0.5 mL/kg/h for > 12 h |
| Failure | Increase in SCr to > 3 times baseline or decrease in GFR by > 75% within 7 days or increase in SCr to ≥ 4 mg/dL with an acute rise of 0.5 mg/dL | < 0.3 mL/kg/h for > 24 h or anuria for 12 h |
| Loss | Complete loss of kidney function requiring dialysis for > 4 weeks | |
| ESRD | Complete loss of kidney function requiring dialysis for > 3 months | |
| AKIN criteria | ||
| Stage 1 | Increase in SCr by ≥ 0.3 mg/dL or increase in SCr to ≥ 1.5 times baseline within 48 h | < 0.5 mL/kg/h for ≥ 6 h |
| Stage 2 | Increase in SCr to > 2 times baseline within 48 h | < 0.5 mL/kg/h for ≥ 12 h |
| Stage 3 | Increase in SCr to > 3 times baseline within 48 h or increase in SCr to ≥ 4 mg/dL with a rise of 0.5 mg/dL within 24 h or initiation of dialysis | < 0.3 mL/kg/h for ≥ 24 h or anuria for ≥ 12 h |
| KDIGO criteria | ||
| Stage 1 | Increase in SCr by ≥ 0.3 mg/dL within 48 h or increase in SCr to ≥ 1.5 times baseline within 7 days | < 0.5 mL/kg/h for ≥ 6 h |
| Stage 2 | Increase in SCr to > 2 times baseline within 7 days | < 0.5 mL/kg/h for ≥ 12 h |
| Stage 3 | Increase in SCr to > 3 times baseline within 7 days or increase in SCr to ≥ 4 mg/dL or initiation of dialysis | < 0.3 mL/kg/h for ≥ 24 h or anuria for ≥ 12 h |
Notably, all of the above-mentioned consensus definitions use changes in serum creatinine to reflect changes in glomerular filtration rate (GFR) and overall kidney function. One inconvenience of this approach is the nonlinear relationship between changes in GFR and serum creatinine. Additionally, serum creatinine does not generally reliably reflect CKD until GFR rates fall below 50 mL/min, hence serum creatinine may be “normal” in patients even with important underlying chronic renal impairment. Following surgery, creatinine accumulation requires up to 48 hours to confirm AKI and a rise that reflects significant kidney injury may occur while levels remain within the “normal” range (e.g., 50% increase). These factors represent sources of significant delay in the implementation of kidney protective strategies that confound study of their effectiveness. Furthermore, small changes in serum creatinine that never meet the threshold for AKI criteria are still associated with increased mortality and despite their subthreshold degree probably also reflect important AKI episodes.
Controversial (and often also difficult to measure perioperatively) is the significance of urine output as a diagnostic biomarker of postoperative AKI. This contrasts with ICU patients where urine output measurements add explanatory power to AKI predictive outcome models: inclusion of oliguria increases AKI incidence and isolated oliguria (i.e., exclusive of serum creatinine rise criteria) is associated with higher ICU mortality and 1-year mortality or need for renal replacement therapy (RRT). Furthermore, critically ill patients meeting both oliguria and creatinine criteria are at greatest risk. However, the value of immediate perioperative oliguria to define AKI in surgical populations is less clear. Alpert and colleagues documented oliguria in 137 aortic surgery patients given either crystalloid solution, mannitol, furosemide, or no intervention when urinary flow dropped below 0.125mL/kg/h. These authors from the 1980s found no correlation between intraoperative mean urinary output or lowest hourly urinary output and subsequent AKI development by change from pre to peak postoperative levels of serum creatinine. The findings of this study are consistent with data from several other more recent studies that suggest that oliguria in the immediate perioperative period is not as useful as a marker of AKI. For example, in a retrospective study of cardiac surgery patients, the addition of urine output to serum creatinine criteria (AKIN) considerably increased AKI incidence but added no prognostic value for either in-hospital mortality or new requirement for RRT. In contrast, another prospective study of cardiac surgical patients found meeting either creatinine-only or oliguria-only (KDIGO) criteria was related to equally elevated long-term mortality risk when compared with patients without AKI. Furthermore, patients meeting both creatinine and oliguria criteria in this study had additional long-term mortality risk above that of the patients meeting only one such criterion.
Notably, perioperative oliguria is typical during uneventful surgical procedures and simply represents an appropriate homeostatic response to perioperative fasting and the normal response to anesthesia (i.e., acute renal “success”) as opposed to a pathologic response reflecting renal injury. In the absence of consensus, it is recommended that urine output criteria to diagnose AKI should be used cautiously in the immediate postsurgical setting, in contrast to nonsurgical and/or chronic critically ill patient populations. In addition, irrespective of surgical status, it appears that patients meeting both urine output and serum creatinine criteria are at highest risk of poor outcomes.
Long-term kidney outcomes are important and include persistent CKD, temporary or chronic need for dialysis, and death. Although consensus AKI criteria have improved the ability of clinicians to report and to compare acute renal events and their short-term consequences across populations, these do not reflect important long-term kidney outcomes. As a long-term measure of renal outcome, Major Adverse Kidney Events (MAKE) reflect chronic outcomes that may demonstrate improvements with effective renoprotective interventions (e.g., benefits seen in successful phase III renoprotection clinical trials). The MAKE metric is a composite of persistent renal dysfunction (25% or greater decline in eGFR), new hemodialysis, and death to identify poor outcomes following AKI. It is typically reported at 30 (MAKE30), 60 (MAKE60), or 90 (MAKE90) days after AKI diagnosis. . MAKE90 is particularly relevant because this MAKE version aligns with the 90-day criteria typically used in other CKD diagnostic criteria. The MAKE composite is important for clinicians to link AKI with chronic morbidities and for the assessment of meaningful outcomes in AKI intervention trials.
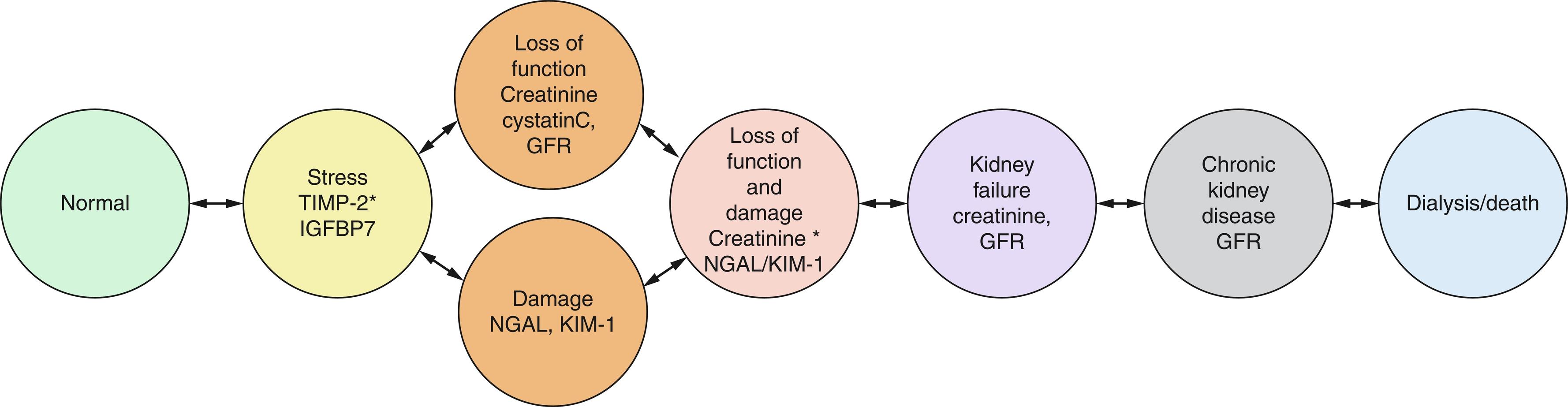
Several putative biomarkers are postulated to have better sensitivity and specificity than serum creatinine for detecting early kidney stress and AKI; however, these have yet to be widely adopted in the clinical setting. Although still requiring validation, these biomarkers have the advantage of both detecting AKI at earlier time points and discriminating types and degrees of injury as outlined later. AKI biomarkers may be useful to reflect progress along a continuum towards renal injury. In this continuum, an acute preinjury kidney stress phase typically precedes renal structural damage which proceeds overt functional loss. Thus, AKI biomarkers can be divided into those that detect: (1) kidney stress, (2) impaired function without structural damage, (3) structural damage with intact function, and (4) structural damage with loss of function. The framework for how these biomarkers could be useful to detect the phase of renal injury in hopes of guiding more specific interventions (e.g., preventing overt AKI, limiting kidney damage, etc.) is shown in Fig. 17.2 . Furthermore, studies have indicated that such early AKI biomarkers could be useful for long-term risk stratification following AKI. Nonetheless, to date serum creatinine remains a reliable and readily available gold standard test to diagnose AKI. In this context, some newer biomarkers (e.g., TIMP-2 * IGFBP7) may have potential to predict AKI and allow clinicians to intervene earlier. Further validation is needed for most of these potential clinical tools, with the hope that in the future potential biomarker profiles may enable more precise risk stratification and earlier intervention of patients with AKI.
Insulin-like growth factor-binding protein 7 (IGFBP7) and tissue inhibitor of metalloproteinases-2 (TIMP-2) are kidney stress biomarkers. Both substances arrest the G 1 phase of the cell cycle and levels of each can be measured in the urine. These kidney stress biomarkers were identified as the top performing among a collection of related biomarkers for detecting AKI in a variety of high-risk critically ill patients including those with sepsis, shock, major surgery, and trauma. Furthermore, a urinary TIMP-2 * IGFBP7 measurement greater than 0.3 is a strong predictor of AKI in critically ill patients. Several prospective analyses and a recent meta-analysis of cardiac surgery studies also support TIMP2 * IGFBP7 levels measured several hours after cardiac surgery as predictive of subsequent postoperative AKI. Finally, relative to long-term outcomes, TIMP-2 * IGFBP7 levels > 2.0 at ICU admission are associated with death or dialysis at 9 months (HR, 2.16; CI 1.32–3.53).
Beyond serum creatinine, steady-state cystatin C level is an alternative biomarker that reflects changes in kidney filtration function. Notably, the use of cystatin C does not appear to add significantly more information regarding AKI compared with serum creatinine. Cystatin C is a cysteine protease inhibitor protein produced by all nucleated cells, and is freely filtered by the glomerulus and completely reabsorbed in renal tubules. In a prospective observational study of septic ICU patients, urinary cystatin C levels were independently associated with sepsis, AKI, and mortality within 30 days. However, in a large meta-analysis, postoperative urinary Cystatin C poorly discriminated for cardiac surgery (CS) of AKI of any stage (composite AUC 0.63 [0.37–0.89]; diagnosis by AKIN), but plasma cystatin C was modestly more useful (composite AUC 0.69 [0.63–0.74]; diagnosis by RIFLE, AKIN, KDIGO).
The three most studied major biomarkers that indicate renal damage are: (1) neutrophil gelatinase-associated lipocalin (NGAL), (2) kidney injury molecule-1 (KIM-1), and (3) interleukin-18 (IL-18).
NGAL is a small protein with notable upregulation following acute kidney tubular injury, that can be measured either in the urine or plasma. However, in numerous studies both urine and plasma NGAL have modest predictive value for subsequent traditionally diagnosed AKI in critically ill patients. A meta-analysis by Ho et al. also demonstrated modest discrimination for AKI after cardiac surgery for urine NGAL (composite AUC 0.72 [0.66–0.79]) and plasma NGAL (composite AUC 0.71 [0.64–0.77]). Both urine and plasma NGAL have shown only a modest ability to predict AKI severity, renal recovery, and long-term outcomes.
Expression and release of KIM-1 is induced in the proximal tubule after renal injury. A meta-analysis revealed a sensitivity of 74% and specificity of 86% for urinary KIM-1 to diagnose AKI. Blood KIM-1 levels are elevated in patients with AKI and CKD of various etiologies. Urine levels of KIM-1 perform similarly to NGAL in discrimination for CS-AKI (composite AUC 0.72 [0.59–0.84]). Measurement of KIM-1 has not been widely adopted in practice.
IL-18 is a proinflammatory cytokine involved in the evolution of tubular ischemia . As a biomarker for detecting CS-AKI, urine levels of IL-18 perform similarly to both KIM-1 and NGAL. In ICU patients, urinary IL-18 levels may be useful for the early diagnosis of AKI and may predict mortality risk in patients with acute respiratory distress syndrome (ARDS). In a broader ICU population, urinary IL-18 was not reliable in predicting subsequent AKI but was associated with poor clinical outcomes such as death and dialysis. Urinary IL-18 has not been widely adopted in clinical practice.
The sources of postoperative renal insult are extensively reviewed elsewhere. Two primary mechanisms that contribute to perioperative AKI are ischemia-reperfusion and nephrotoxic effects, with three notable sources of insult common to many surgical procedures where postoperative renal dysfunction is prevalent: hypoperfusion, inflammation, and atheroembolism. Several less common sources of renal insult may contribute in selected patients (e.g., rhabdomyolysis, specific drug-related, etc.).
Abnormalities of renal perfusion that exceed the autoregulatory reserve of the renal circulation, such as cardiopulmonary bypass, are believed to be a major source of perioperative ischemia-reperfusion injury. Other conditions, including low output states, hypovolemic shock ( Fig. 17.1 ), vasoconstrictor use, and circulatory arrest, may all contribute to the renal ischemic burden of specific surgical procedures. As described earlier, the normally low medullary pO 2 (10–20 mmHg) makes this tissue particularly vulnerable to hypoperfusion. Paracrine systems, such as the renin-angiotensin system and nitric oxide synthase, are key to regulation of renal blood flow and modulation of microvascular function and oxygen delivery in the renal medulla. Autonomic influences are also important, with the alpha-1 adrenergic receptor-mediated vasoconstriction and alpha-2 adrenergic receptor-mediated vasodilation contributing to modulation of renal perfusion. Lastly, it is increasingly recognized that venous congestion caused by elevated central venous pressure may contribute to postsurgical AKI pathophysiology.
Both systemically and locally in the kidney, surgery provides a consistent trigger for the generation of proinflammatory cytokines (e.g., tumor necrosis factor alpha [TNFα] and interleukin 6 [IL-6]) and an inflammatory response caused by the disruption of tissues and the potential for perioperative endotoxemia. Preexisting infection may further predispose surgical patients to postoperative renal dysfunction. Other postoperative factors, such as infectious and septic complications, transfusion, and interventions such as cardiopulmonary bypass, contribute to the generation of cytokines that have major effects on the renal microcirculation and may lead to tubular dysfunction through a common path of apoptosis.
Showers of emboli are common during certain surgical procedures. Although some types of emboli do not appear to have an important role in postoperative AKI (e.g., air) others are significant contributors to renal injury. Atheroembolism is common during some surgical procedures, particularly those involving operative aortic manipulation, and is highly associated with AKI. The strong association of intra-aortic balloon counterpulsation with AKI may also be related to the dislodgement of aortic plaque. Thromboembolism and infectious emboli from endocarditis may also contribute to renal insult ( Figs. 17.3 and 17.4 ). Atheroembolism can produce all degrees of renal injury, ranging from the obstruction of major renal vessels from large fragments of plaque to the occlusion of multiple small renal vessels by cholesterol microcrystals.
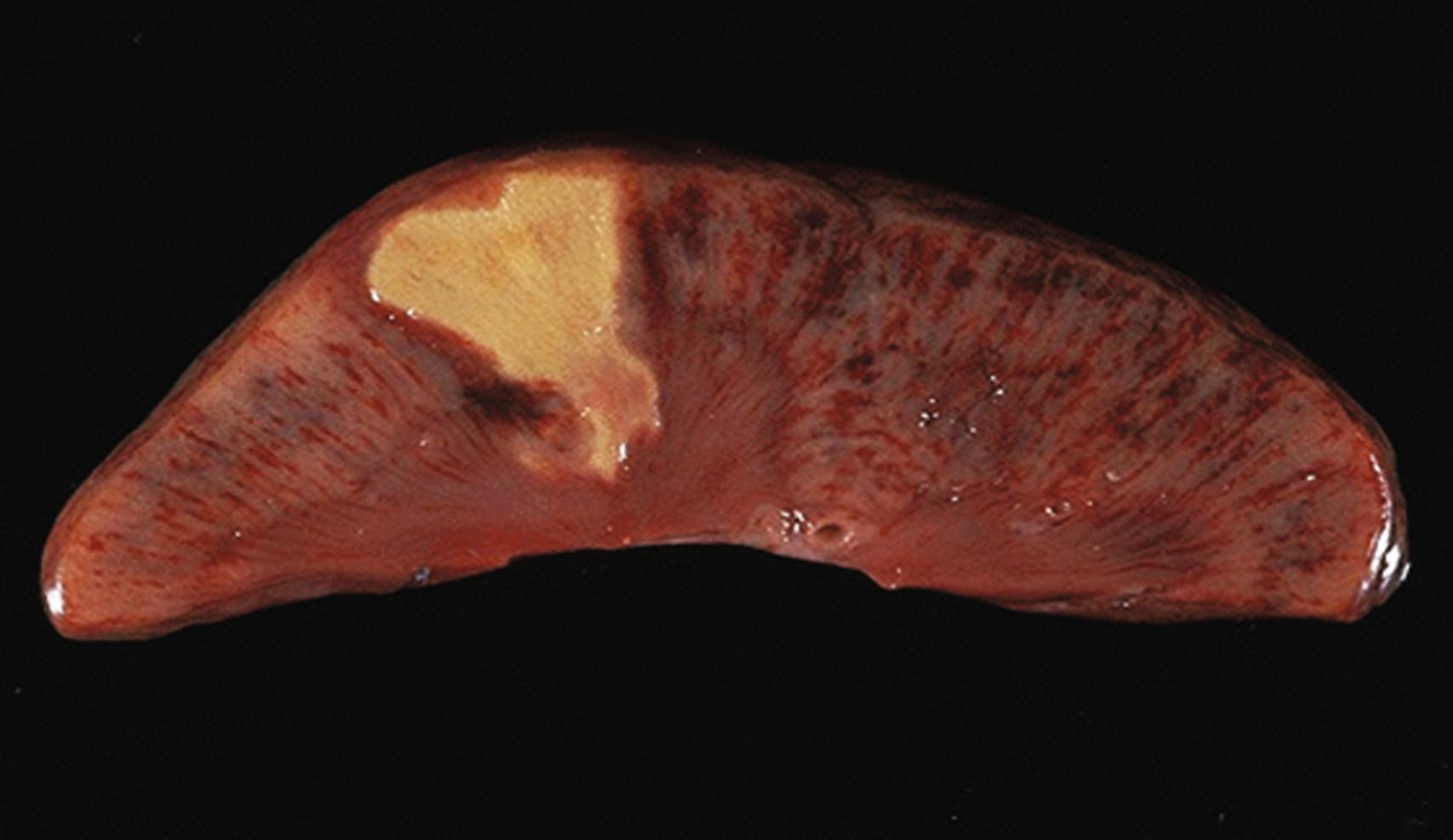

Hemoglobinuria occurs when intravascular hemolysis releases sufficient hemoglobin to exceed the adsorptive capacity of circulating haptoglobin and renal tubular reuptake mechanisms. Only approximately 25% of circulating free hemoglobin is in a form readily filtered by the kidney. Myoglobinuria occurs with muscle cell necrosis in conditions such as ischemic limb reperfusion and major trauma (crush syndrome). The mechanisms underlying myoglobin- and hemoglobin-related AKI are similar. Deterioration in kidney function comes from the combined effects of renal vasoconstriction, tubular obstruction by casts, and direct cytotoxicity. The nitric oxide scavenging potential of these pigments contributes to renal vasoconstriction.
Several well-known nephrotoxins are relevant to the perioperative period. Aminoglycoside antibiotics have potent nephrotoxic effects. Non-steroidal anti-inflammatory drugs (NSAIDs), such as aspirin and indomethacin, are nephrotoxic, and cause renal vasoconstriction through inhibition of endogenous locally synthesized vasodilator prostaglandins. Perioperative treatment with cyclosporin as part of transplant surgery can have acute effects on renal function in addition to the well-known long-term adverse effects.
Although more controversial, intravenous contrast has been associated with AKI. With the use of less toxic contrast agents and lower dosing strategies, evidence suggests that use of intravenous contrast for diagnostic imaging studies (i.e., computed tomography [CT] scans) is not associated with AKI. However, it is still unclear whether higher doses of intravenous contrast agents (i.e., cardiac catheterization, interventional radiology procedures) cause contrast-induced nephropathy. The judicious use of intravenous contrast agents in these settings, particularly in those with preexisting renal dysfunction, is still warranted.
There is good evidence to support the role of perioperative clinical management decisions in affecting important renal outcomes. A vast majority of these best practices involve kidney insult reduction or avoidance at all stages throughout the perioperative period. Notably, there is little evidence to support significant value in therapeutic interventions to accelerate recovery once AKI has occurred (short of temporary RRT); rather management of patients with established kidney injury involves mostly minimizing further AKI to facilitate normal recovery. Potential interventions and best practices are outlined later and discussed by time period (preoperative, intraoperative and postoperative), and topics are also organized for practical purposes into three tables by their value ( Table 17.2 : recommended practices to avoid; Table 17.3 : limited value probably no harm; and Table 17.4 : recommended practices).
| Class III: Risk > Benefit | |||
|---|---|---|---|
| Preoperative/Procedural Planning | Intraoperative | Postoperative | |
| Level A (consistent evidence) |
|
|
|
| Level B (some evidence) |
|
|
|
| Level C (limited evidence) |
|
||
| Preoperative/Procedural Planning | Intraoperative | Postoperative | |
|---|---|---|---|
| Level A (consistent evidence) |
|
|
|
| Level B (some evidence) |
|
|
|
| Level C (limited evidence) |
|
|
|
| Class I: Benefit >> Risk | |||
|---|---|---|---|
| Preoperative/Procedural Planning | Intraoperative | Postoperative | |
| Level A (consistent evidence) |
|
|
|
| Level B (some evidence) |
|
|
|
| Level C (limited evidence) |
|
|
|
Individualized for specific patient level concerns, the context to apply this outlined “optimal renal practices” approach to clinical management can be gained from consideration of renal risk stratification, as outlined elsewhere in this textbook , , , However, AKI prediction beyond knowledge of the risk associated with specific procedures, and the greater likelihood of need for dialysis with chronic kidney disease is notoriously poor, particularly at pre-identifying the victim of severe AKI. This inability to pre-identify at-risk patients with confidence further highlights the importance of developing a safe “renal best practices” approach to reduce the overall burden of AKI as part of routine patient management.
Renal preservation strategies with the goal of avoiding, preventing, or attenuating renal insult before it occurs include the use of preoperative risk stratification to influence procedure selection, procedure modification, and management of chronic medication administration. Procedure modifications with lowered AKI risk have already been part of the justification for transitions in routine care such as stent grafting (versus open surgical procedures) to treat aortic aneurysmal disease. Potential future tools also include preoperative genetic screening to identify subgroups at high renal risk. The following section focuses on procedural choice, nonpharmacologic interventions, and pharmacologic agents.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here