Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In Chapter 81, Chapter 82 , the sexual functions of the male and female are described to the point of fertilization of the ovum. If the ovum becomes fertilized, a new sequence of events called gestation or pregnancy takes place, and the fertilized ovum eventually develops into a full-term fetus. The purpose of this chapter is to discuss the early stages of ovum development after fertilization and then to discuss the physiology of pregnancy. In Chapter 84 , some special aspects of fetal and early childhood physiology are discussed.
While still in the ovary, the ovum is in the primary oocyte stage. Shortly before it is released from the ovarian follicle, its nucleus divides by meiosis and a first polar body is expelled from the nucleus of the oocyte (see Figure 82-3 ). The primary oocyte then becomes the secondary oocyte. In this process, each of the 23 pairs of chromosomes loses one of its partners, which becomes incorporated in a polar body that is expelled. This leaves 23 unpaired chromosomes in the secondary oocyte. It is at this time that the ovum, which is still in the secondary oocyte stage, is ovulated into the abdominal cavity. Then, almost immediately, it enters the fimbriated end of one of the fallopian tubes.
When ovulation occurs, the ovum, along with a hundred or more attached granulosa cells that constitute the corona radiata, is expelled directly into the peritoneal cavity and must then enter one of the fallopian tubes (also called uterine tubes ) to reach the cavity of the uterus. The fimbriated ends of each fallopian tube fall naturally around the ovaries. The inner surfaces of the fimbriated tentacles are lined with ciliated epithelium, and the cilia are activated by estrogen from the ovaries, which causes the cilia to beat toward the opening, or ostium, of the involved fallopian tube. One can actually see a slow fluid current flowing toward the ostium. By this means, the ovum enters one of the fallopian tubes.
Although one might suspect that many ova fail to enter the fallopian tubes, conception studies suggest that up to 98% of ova succeed in this task. Indeed, in some recorded cases, women with one ovary removed and the opposite fallopian tube removed have had several children with relative ease of conception, thus demonstrating that ova can even enter the opposite fallopian tube.
After the male ejaculates semen into the vagina during intercourse, a few sperm are transported within 5 to 10 minutes upward from the vagina and through the uterus and fallopian tubes to the ampullae of the fallopian tubes near the ovarian ends of the tubes. This transport of the sperm is aided by contractions of the uterus and fallopian tubes stimulated by prostaglandins in the male seminal fluid and also by oxytocin released from the posterior pituitary gland of the female during her orgasm. Of the almost half a billion sperm deposited in the vagina, a few thousand succeed in reaching each ampulla.
Fertilization of the ovum ( Figure 83-1 ) normally takes place in the ampulla of one of the fallopian tubes soon after both the sperm and the ovum enter the ampulla. Before a sperm can enter the ovum, however, it must first penetrate the multiple layers of granulosa cells attached to the outside of the ovum (the corona radiata ) and then bind to and penetrate the zona pellucida surrounding the ovum. The mechanisms used by the sperm for these purposes are presented in Chapter 81 .
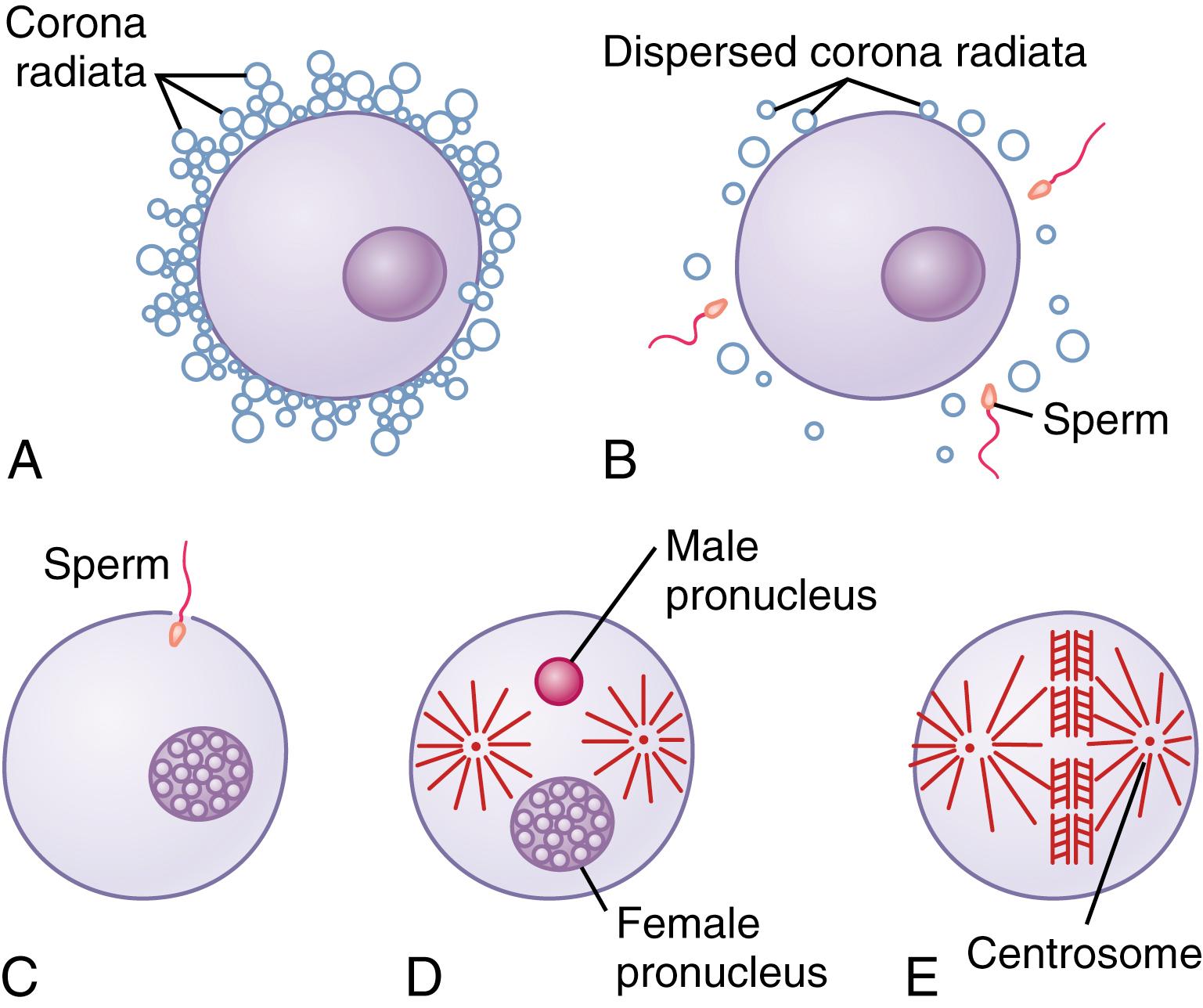
Once a sperm has entered the ovum (which is still in the secondary oocyte stage of development), the oocyte divides again to form the mature ovum plus a second polar body that is expelled (see Figure 82-3 ). The mature ovum still carries in its nucleus (now called the female pronucleus ) 23 chromosomes. One of these chromosomes is the female chromosome, known as the X chromosome.
In the meantime, the fertilizing sperm has also changed. On entering the ovum, its head swells to form a male pronucleus, shown in Figure 83-1 D . Later, the 23 unpaired chromosomes of the male pronucleus and the 23 unpaired chromosomes of the female pronucleus align themselves to re-form a complete complement of 46 chromosomes (23 pairs) in the fertilized ovum or zygote (see Figure 83-1 E ).
Half of the mature sperm carry in their genome an X chromosome (the female chromosome) and half carry a Y chromosome (the male chromosome). Therefore, if an X chromosome from a sperm combines with an X chromosome from an ovum, giving an XX combination, a female child will be born, as explained in Chapter 81 . If a Y chromosome from a sperm is paired with an X chromosome from an ovum, giving an XY combination, a male child will be born.
After fertilization has occurred, an additional 3 to 5 days is normally required for transport of the fertilized ovum through the remainder of the fallopian tube into the cavity of the uterus ( Figure 83-2 ). This transport is effected mainly by a feeble fluid current in the tube resulting from epithelial secretion plus action of the ciliated epithelium that lines the tube; the cilia always beat toward the uterus. Weak contractions of the fallopian tube may also aid passage of the ovum.
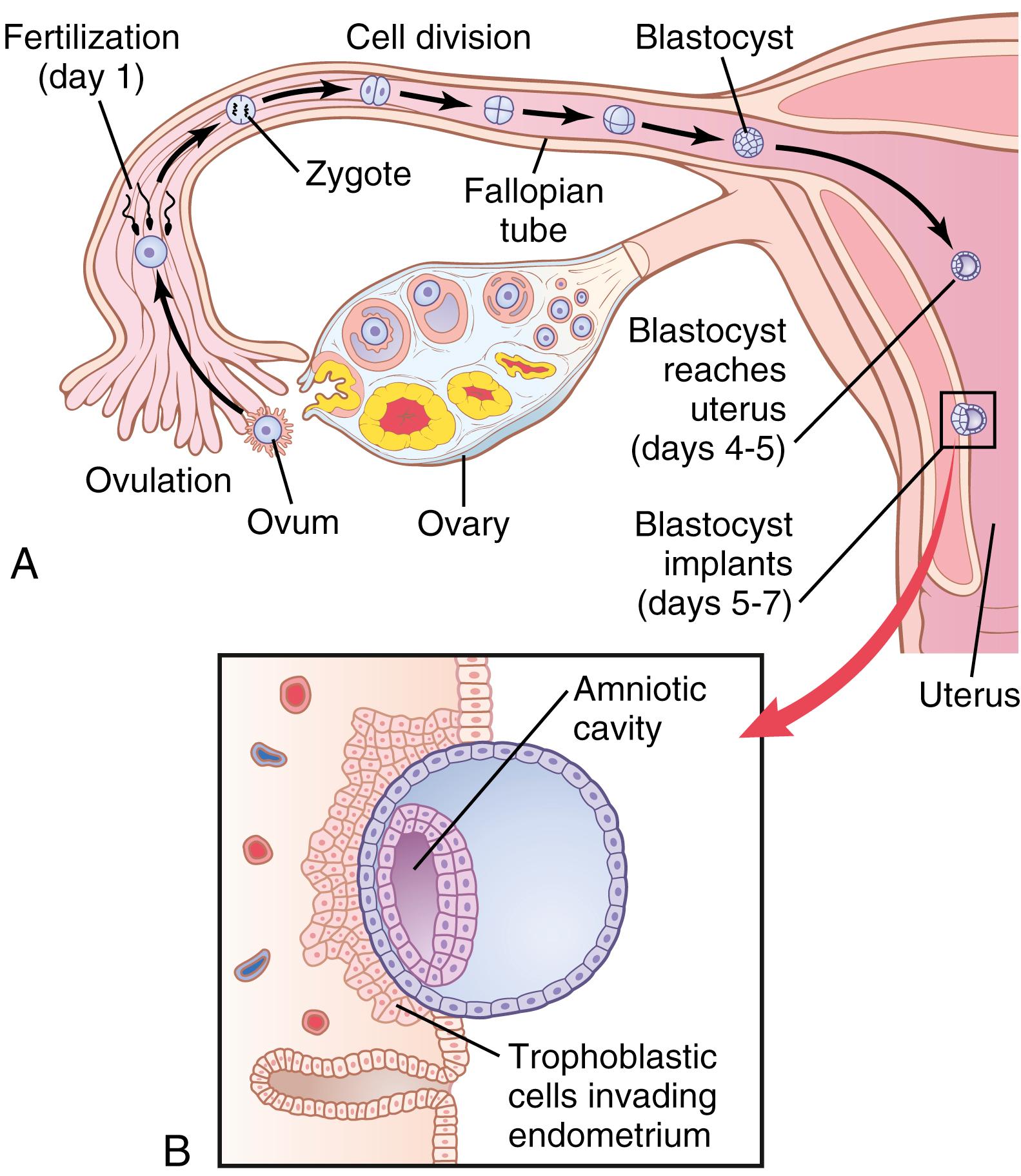
The fallopian tubes are lined with a rugged cryptoid surface that impedes passage of the ovum despite the fluid current. Also, the isthmus of the fallopian tube (the last 2 centimeters before the tube enters the uterus) remains spastically contracted for about the first 3 days after ovulation. After this time, the rapidly increasing progesterone secreted by the ovarian corpus luteum first promotes increasing progesterone receptors on the fallopian tube smooth muscle cells; then the progesterone activates the receptors, relaxing the tubules and allowing entry of the ovum into the uterus.
This delayed transport of the fertilized ovum through the fallopian tube allows several stages of cell division to occur before the dividing ovum—now called a blastocyst, with about 100 cells—enters the uterus. During this time, the fallopian tube secretory cells produce large quantities of secretions used for nutrition of the developing blastocyst.
After reaching the uterus, the developing blastocyst usually remains in the uterine cavity an additional 1 to 3 days before it implants in the endometrium; thus, implantation ordinarily occurs on about the fifth to seventh day after ovulation. Before implantation, the blastocyst obtains its nutrition from the uterine endometrial secretions, called “uterine milk.”
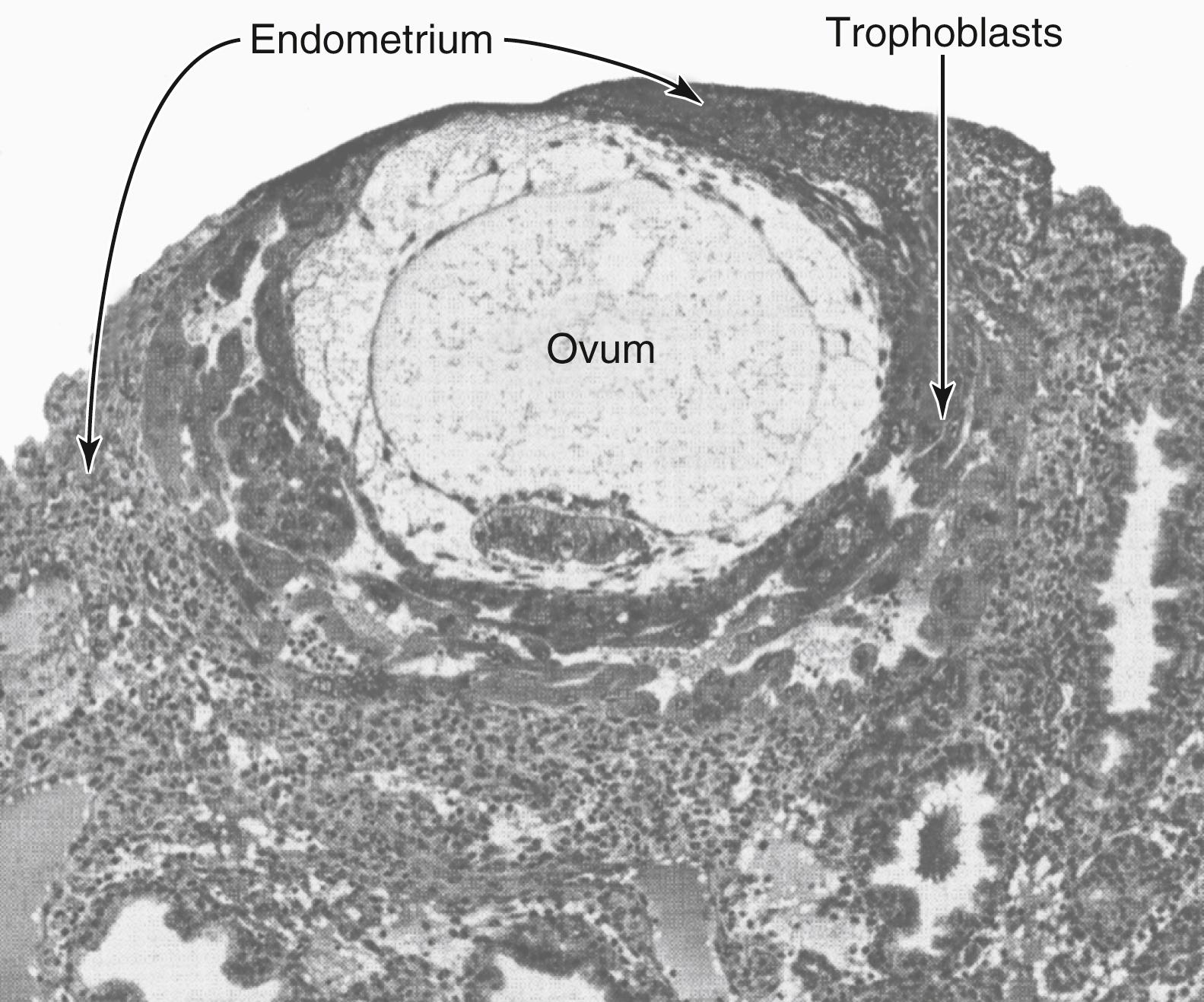
Implantation results from the action of trophoblast cells that develop over the surface of the blastocyst. These cells secrete proteolytic enzymes that digest and liquefy the adjacent cells of the uterine endometrium. Some of the fluid and nutrients released are actively transported by the same trophoblast cells into the blastocyst, adding more sustenance for growth. Figure 83-3 shows an early implanted human blastocyst with a small embryo. Once implantation has taken place, the trophoblast cells and other adjacent cells (from the blastocyst and the uterine endometrium) proliferate rapidly, forming the placenta and the various membranes of pregnancy.
In Chapter 82 , we pointed out that the progesterone secreted by the ovarian corpus luteum during the latter half of each monthly sexual cycle has an effect on the uterine endometrium, converting the endometrial stromal cells into large swollen cells containing extra quantities of glycogen, proteins, lipids, and even some minerals necessary for development of the conceptus (the embryo and its adjacent parts or associated membranes). Then, when the conceptus implants in the endometrium, continued secretion of progesterone causes the endometrial cells to swell further and to store even more nutrients. These cells are now called decidual cells, and the total mass of cells is called the decidua.
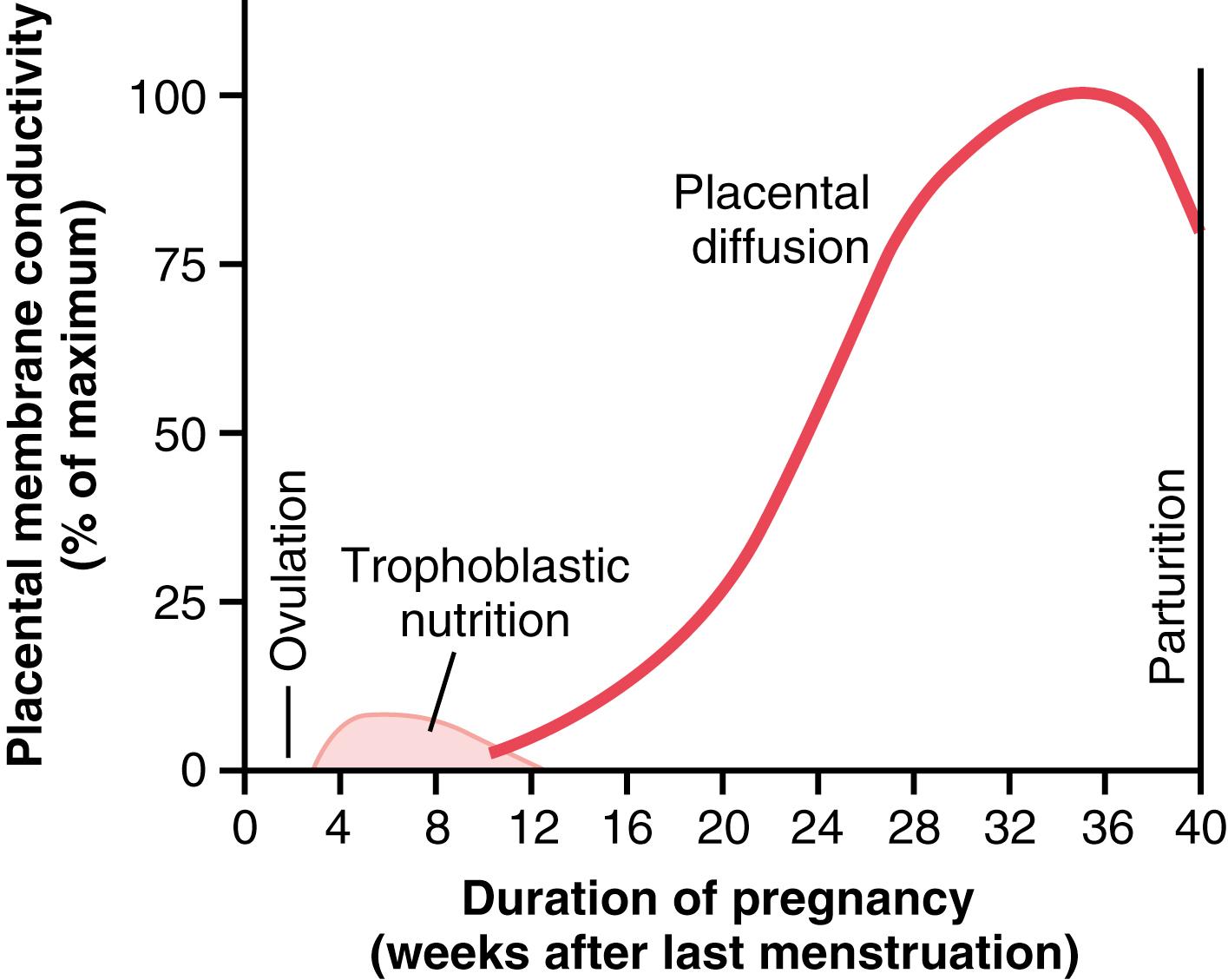
As the trophoblast cells invade the decidua, digesting and imbibing it, the stored nutrients in the decidua are used by the embryo for growth and development. During the first week after implantation, this is the only means by which the embryo can obtain nutrients; the embryo continues to obtain at least some of its nutrition in this way for up to 8 weeks, although the placenta also begins to provide nutrition after about the 16th day beyond fertilization (a little more than 1 week after implantation). Figure 83-4 shows this trophoblastic period of nutrition, which gradually gives way to placental nutrition.
While the trophoblastic cords from the blastocyst are attaching to the uterus, blood capillaries grow into the cords from the vascular system of the newly forming embryo. About 21 days after fertilization, blood also begins to be pumped by the heart of the human embryo. Simultaneously, blood sinuses supplied with blood from the mother develop around the outsides of the trophoblastic cords. The trophoblast cells send out more and more projections, which become placental villi into which fetal capillaries grow. Thus, the villi, carrying fetal blood, are surrounded by sinuses that contain maternal blood.
The final structure of the placenta is shown in Figure 83-5 . Note that the blood of the fetus flows through two umbilical arteries, then into the capillaries of the villi, and finally back through a single umbilical vein into the fetus. At the same time, the mother’s blood flows from her uterine arteries into large maternal sinuses that surround the villi and then back into the uterine veins of the mother. The lower part of Figure 83-5 shows the relationship between the fetal blood of each fetal placental villus and the blood of the mother surrounding the outsides of the villus in the fully developed placenta.
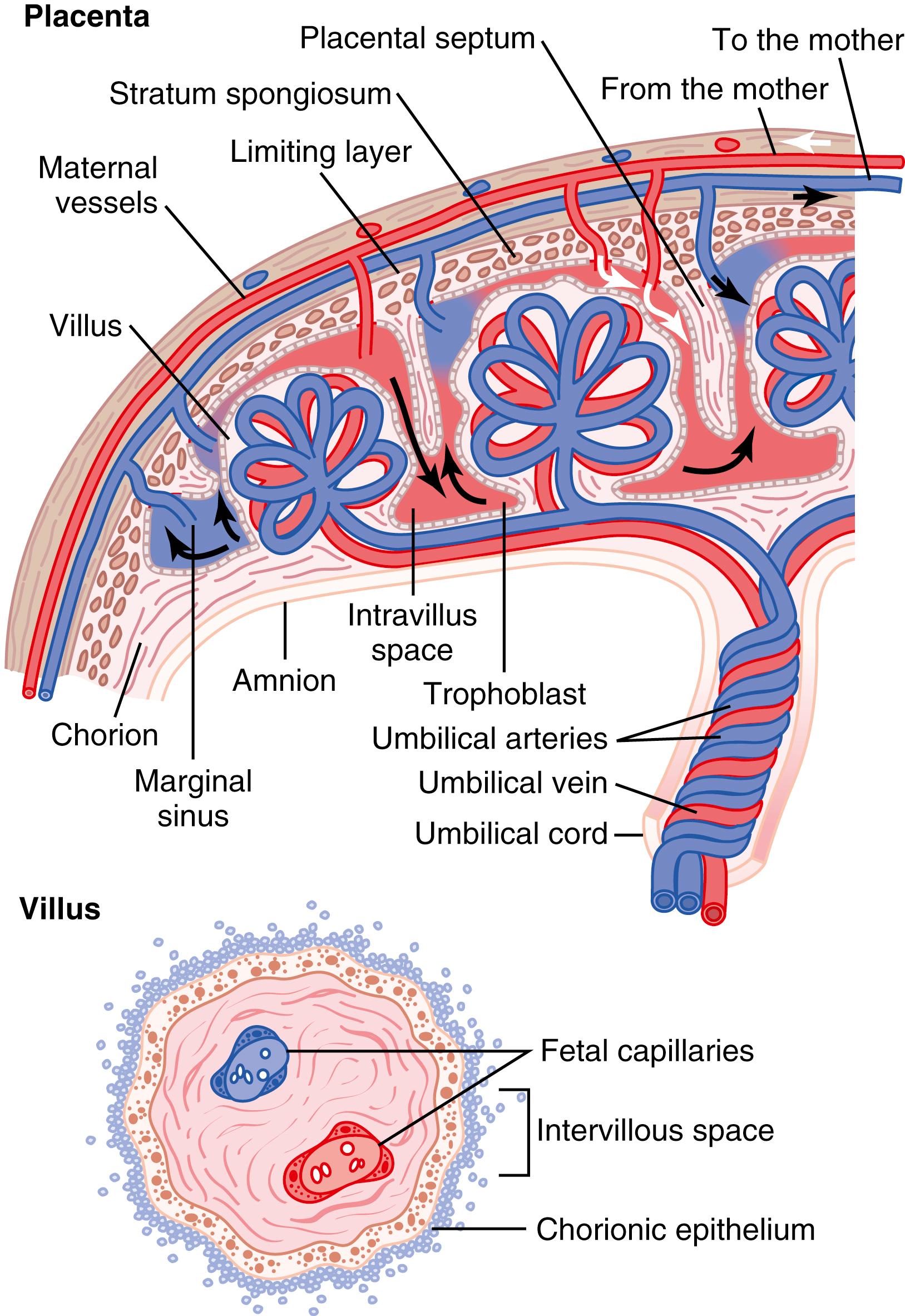
The total surface area of all the villi of the mature placenta is only a few square meters—many times less than the area of the pulmonary membrane in the lungs. Nevertheless, nutrients and other substances pass through this placental membrane mainly by diffusion in much the same manner that diffusion occurs through the alveolar membranes of the lungs and the capillary membranes elsewhere in the body.
The major function of the placenta is to provide for diffusion of foodstuffs and oxygen from the mother’s blood into the fetus’s blood and diffusion of excretory products from the fetus back into the mother.
In the early months of pregnancy, the placental membrane is still thick because it is not fully developed. Therefore, its permeability is low. Further, the surface area is small because the placenta has not grown significantly. Therefore, the total diffusion conductance is minuscule at first. In later pregnancy, the permeability increases because of thinning of the membrane diffusion layers and because the surface area expands many times over, thus giving the tremendous increase in placental diffusion shown in Figure 83-4 .
Rarely, “breaks” occur in the placental membrane, which allows fetal blood cells to pass into the mother or, even less commonly, the mother’s cells to pass into the fetus. Fortunately, it is rare for the fetus to bleed severely into the mother’s circulation because of a ruptured placental membrane.
Almost the same principles for diffusion of oxygen through the pulmonary membrane (discussed in detail in Chapter 40 ) are applicable for diffusion of oxygen through the placental membrane. The dissolved oxygen in the blood of the large maternal sinuses passes into the fetal blood by simple diffusion, driven by an oxygen pressure gradient from the mother’s blood to the fetus’s blood. Near the end of pregnancy, the mean partial pressure of oxygen (P o 2 ) of the mother’s blood in the placental sinuses is about 50 mm Hg, and the mean P o 2 in the fetal blood after it becomes oxygenated in the placenta is about 30 mm Hg. Therefore, the mean pressure gradient for diffusion of oxygen through the placental membrane is about 20 mm Hg.
One might wonder how it is possible for a fetus to obtain sufficient oxygen when the fetal blood leaving the placenta has a P o 2 of only 30 mm Hg. There are three reasons why even this low P o 2 is capable of allowing the fetal blood to transport almost as much oxygen to the fetal tissues as is transported by the mother’s blood to her tissues.
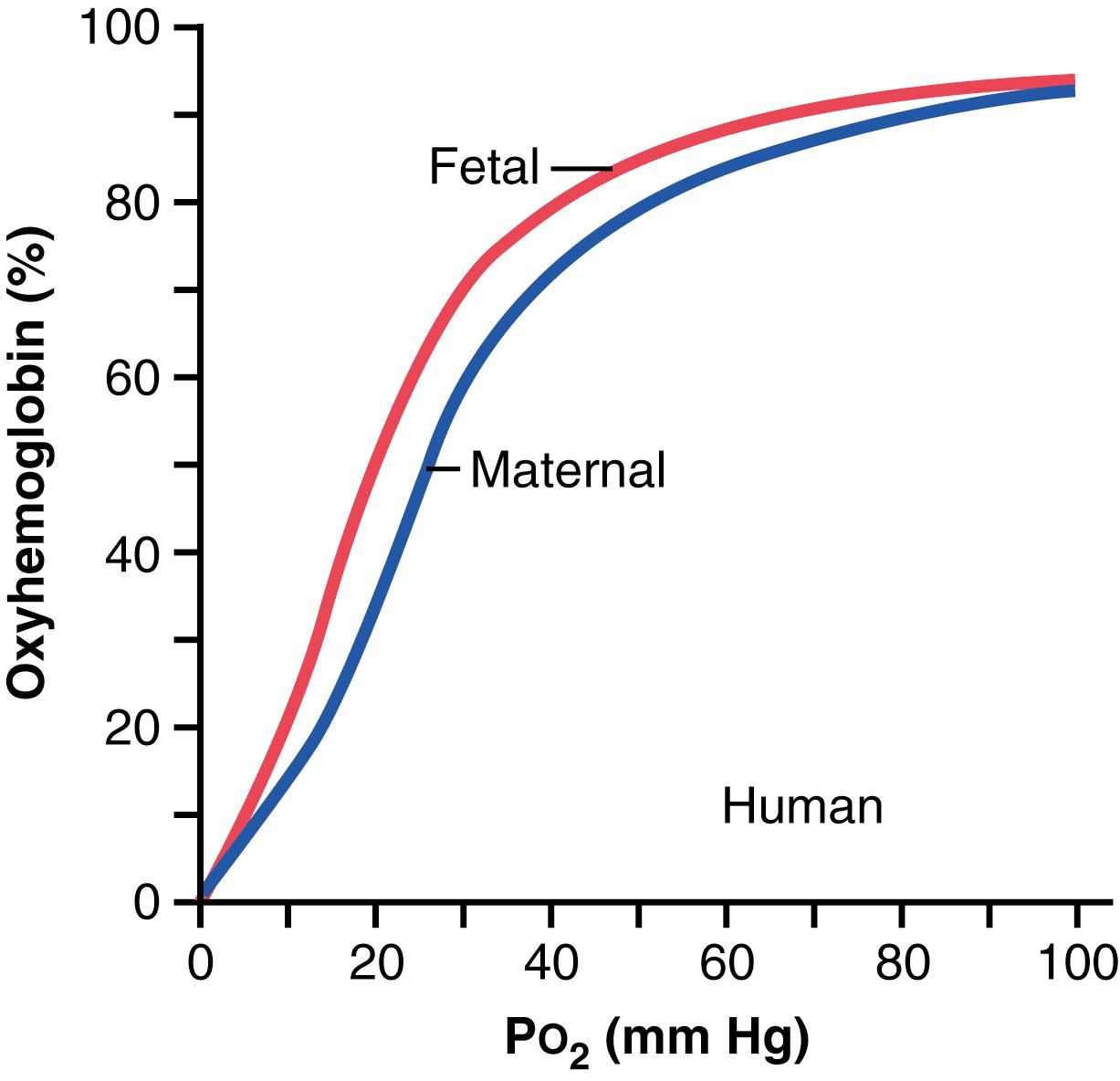
First, the hemoglobin of the fetus is mainly fetal hemoglobin, which is a type of hemoglobin synthesized in the fetus before birth. Figure 83-6 shows the comparative oxygen dissociation curves for maternal hemoglobin and fetal hemoglobin, demonstrating that the curve for fetal hemoglobin is shifted to the left of that for maternal hemoglobin. This means that at the low P o 2 levels in fetal blood, the fetal hemoglobin can carry 20% to 50% more oxygen than can maternal hemoglobin.
Second, the hemoglobin concentration of fetal blood is about 50% greater than that of the mother, which is an even more important factor in enhancing the amount of oxygen transported to the fetal tissues.
Third, the Bohr effect, which is explained in relation to the exchange of carbon dioxide and oxygen in the lung in Chapter 41 , provides another mechanism to enhance the transport of oxygen by fetal blood. That is, hemoglobin can carry more oxygen at a low P co 2 than it can at a high P co 2 . The fetal blood entering the placenta carries large amounts of carbon dioxide, but much of this carbon dioxide diffuses from the fetal blood into the maternal blood. Loss of the carbon dioxide makes the fetal blood more alkaline, whereas the increased carbon dioxide in the maternal blood makes it more acidic.
These changes increase the capacity of fetal blood to combine with oxygen and decrease oxygen binding of maternal blood, which forces still more oxygen from the maternal blood while enhancing oxygen uptake by the fetal blood. Thus, the Bohr shift operates in one direction in the maternal blood and in the other direction in the fetal blood. These two effects make the Bohr shift twice as important here as it is for oxygen exchange in the lungs; therefore, it is called the double Bohr effect.
By these three means, the fetus is capable of receiving more than adequate oxygen through the placental membrane, despite the fact that the fetal blood leaving the placenta has a P o 2 of only 30 mm Hg.
The total diffusing capacity of the entire placenta for oxygen at term is about 1.2 ml of oxygen per minute per mm Hg oxygen pressure difference across the membrane, which compares favorably with that of the lungs of the newborn baby.
Carbon dioxide is continually formed in the fetal tissues in the same way that it is formed in maternal tissues, and the only means for excreting the carbon dioxide from the fetus is through the placenta into the mother’s blood. The partial pressure of carbon dioxide (P co 2 ) of the fetal blood is 2 to 3 mm Hg higher than that of the maternal blood. This small pressure gradient for carbon dioxide across the membrane is more than sufficient to allow adequate diffusion of carbon dioxide because the extreme solubility of carbon dioxide in the placental membrane allows carbon dioxide to diffuse about 20 times as rapidly as oxygen.
Other metabolic substrates needed by the fetus diffuse into the fetal blood in the same manner as oxygen. For example, in the late stages of pregnancy, the fetus often uses as much glucose as is used by the entire body of the mother. To provide this much glucose, the trophoblast cells lining the placental villi provide for facilitated diffusion of glucose through the placental membrane—that is, the glucose is transported by carrier molecules in the trophoblast cells of the membrane. Even so, the glucose level in fetal blood is 20% to 30% lower than that in maternal blood.
Because of the high solubility of fatty acids in cell membranes, these fatty acids also diffuse from the maternal blood into the fetal blood, but more slowly than glucose, so glucose is used more easily by the fetus for nutrition. Also, such substances as ketone bodies and potassium, sodium, and chloride ions diffuse with relative ease from the maternal blood into the fetal blood.
In the same manner that carbon dioxide diffuses from the fetal blood into the maternal blood, other excretory products formed in the fetus also diffuse through the placental membrane into the maternal blood and are then excreted along with the excretory products of the mother. These products include especially the nonprotein nitrogens such as urea, uric acid, and creatinine. The level of urea in fetal blood is only slightly greater than that in maternal blood because urea diffuses through the placental membrane with great ease. However, creatinine, which does not diffuse as easily, has a fetal blood concentration considerably higher than that in the mother’s blood. Therefore, excretion from the fetus depends mainly, if not entirely, on the diffusion gradients across the placental membrane and its permeability and surface area. Because there are higher concentrations of the excretory products in the fetal blood than in the maternal blood, there is continual diffusion of these substances from the fetal blood to the maternal blood.
In pregnancy, the placenta forms especially large quantities of human chorionic gonadotropin, estrogens, progesterone, and human chorionic somatomammotropin, the first three of which, and probably the fourth as well, are all essential to a normal pregnancy.
Menstruation normally occurs in a nonpregnant woman about 14 days after ovulation, at which time most of the endometrium of the uterus sloughs away from the uterine wall and is expelled to the exterior. If this happens after an ovum has implanted, the pregnancy will terminate. However, this sloughing is prevented by secretion of human chorionic gonadotropin by the newly developing embryonic tissues.
Coincidental with the development of the trophoblast cells from the early fertilized ovum, human chorionic gonadotropin is secreted by the syncytial trophoblast cells into the fluids of the mother, as shown in Figure 83-7 . Secretion of this hormone can first be measured in the blood 8 to 9 days after ovulation, shortly after the blastocyst implants in the endometrium. Then, the secretion rate rises rapidly to reach a maximum at about 10 to 12 weeks of pregnancy and decreases back to a lower value by 16 to 20 weeks. It continues at this level for the remainder of the pregnancy.
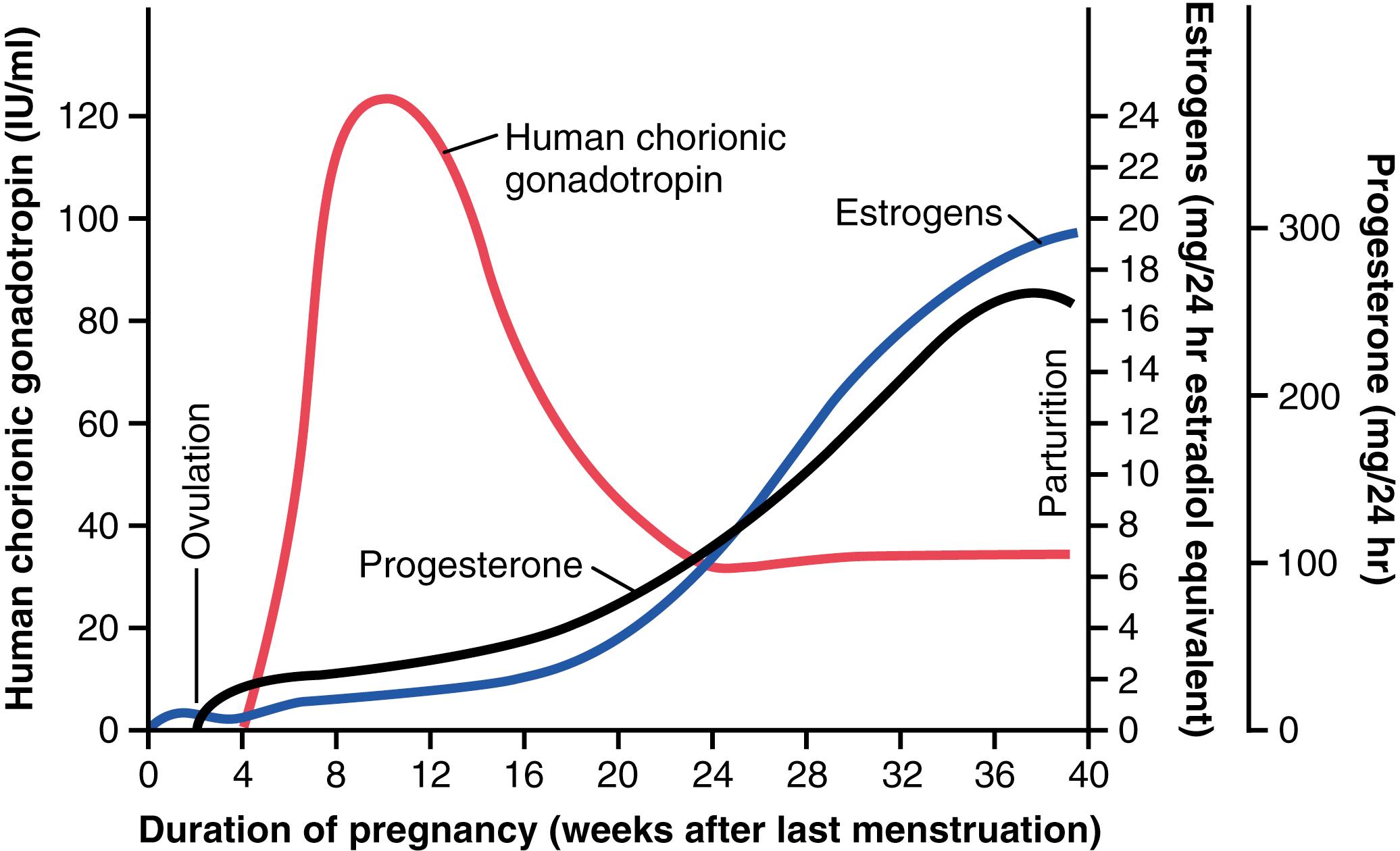
Human chorionic gonadotropin is a glycoprotein having a molecular weight of about 39,000 and much the same molecular structure and function as luteinizing hormone secreted by the pituitary gland. The most important function of human chorionic gonadotropin is to prevent involution of the corpus luteum at the end of the monthly female sexual cycle. Instead, it causes the corpus luteum to secrete even larger quantities of its sex hormones—progesterone and estrogens—for the next few months. These sex hormones prevent menstruation and cause the endometrium to continue to grow and store large amounts of nutrients rather than being shed in the menstruum. As a result, the decidua-like cells that develop in the endometrium during the normal female sexual cycle become actual decidual cells— greatly swollen and nutritious—at about the time that the blastocyst implants.
Under the influence of human chorionic gonadotropin, the corpus luteum in the mother’s ovary grows to about twice its initial size by a month or so after pregnancy begins. Its continued secretion of estrogens and progesterone maintains the decidual nature of the uterine endometrium, which is necessary for early development of the fetus.
If the corpus luteum is removed before approximately the seventh week of pregnancy, spontaneous abortion almost always occurs, sometimes even up to the 12th week. After that time, the placenta secretes sufficient quantities of progesterone and estrogens to maintain pregnancy for the remainder of the gestation period. The corpus luteum involutes slowly after the 13th to 17th week of gestation.
Human chorionic gonadotropin also exerts an interstitial cell– stimulating effect on the testes of the male fetus, resulting in production of testosterone in male fetuses until the time of birth. This small secretion of testosterone during gestation is what causes the fetus to grow male sex organs instead of female organs. Near the end of pregnancy, testosterone secreted by the fetal testes also causes the testes to descend into the scrotum.
The placenta, like the corpus luteum, secretes estrogens and progesterone. Histochemical and physiological studies show that these two hormones, like most other placental hormones, are secreted by the syncytial trophoblast cells of the placenta.
Figure 83-7 shows that toward the end of pregnancy, the daily production of placental estrogens increases to about 30 times the mother’s normal level of production. However, secretion of estrogens by the placenta is quite different from secretion by the ovaries. Most important, the estrogens secreted by the placenta are not synthesized de novo from basic substrates in the placenta. Instead, they are formed almost entirely from androgenic steroid compounds, dehydroepiandrosterone and 16-hydroxyde-hydroepiandrosterone, which are formed in the mother’s adrenal glands and in the fetus’s adrenal glands. These weak androgens are transported by the blood to the placenta and converted by the trophoblast cells into estradiol, estrone, and estriol. The cortices of the fetal adrenal glands are extremely large, and about 80% consists of a so-called fetal zone, the primary function of which seems to be to secrete dehydroepiandrosterone during pregnancy.
In Chapter 82 , we pointed out that estrogens exert mainly a proliferative function on most reproductive and associated organs of the mother. During pregnancy, the extreme quantities of estrogens cause (1) enlargement of the mother’s uterus, (2) enlargement of the mother’s breasts and growth of the breast ductal structure, and (3) enlargement of the mother’s female external genitalia.
The estrogens also relax the pelvic ligaments of the mother, so the sacroiliac joints become relatively limber, and the symphysis pubis becomes elastic. These changes allow easier passage of the fetus through the birth canal. There is reason to believe that estrogens also affect many general aspects of fetal development during pregnancy—for example, by affecting the rate of cell reproduction in the early embryo.
Progesterone is just as essential as estrogen for a successful pregnancy. In addition to being secreted in moderate quantities by the corpus luteum at the beginning of pregnancy, progesterone is secreted later in tremendous quantities by the placenta, as shown in Figure 83-7 .
The following special effects of progesterone are essential for the normal progression of pregnancy:
Progesterone causes decidual cells to develop in the uterine endometrium. These cells play an important role in nutrition of the early embryo.
Progesterone decreases contractility of the pregnant uterus, thus preventing uterine contractions from causing spontaneous abortion.
Progesterone contributes to development of the conceptus even before implantation because it specifically increases secretions of the mother’s fallopian tubes and uterus to provide appropriate nutrition for the developing morula (the spherical mass of 16 to 32 blastomeres formed before the blastula) and blastocyst. Progesterone may also affect cell cleavage in the early developing embryo.
The progesterone secreted during pregnancy helps estrogen prepare the mother’s breasts for lactation, which is discussed later in this chapter.
Human chorionic somatomammotropin, a protein hormone with a molecular weight of about 22,000, begins to be secreted by the placenta at about the fifth week of pregnancy. Secretion of this hormone increases progressively throughout the remainder of pregnancy in direct proportion to the weight of the placenta. Although the functions of chorionic somatomammotropin are uncertain, it is secreted in quantities several times greater than that of all the other pregnancy hormones combined. It has several possible important effects.
First, when administered to several types of animals, human chorionic somatomammotropin causes at least partial development of the animal’s breasts and in some cases causes lactation. Because this was the first function of the hormone that was discovered, it was first named human placental lactogen and was believed to have functions similar to those of prolactin. However, attempts to use it to promote lactation in humans have not been successful.
Second, this hormone has weak actions similar to those of growth hormone, causing formation of tissue proteins in the same way that growth hormone does. It also has a chemical structure similar to that of growth hormone, but 100 times as much human chorionic somatomammotropin as growth hormone is required to promote growth.
Third, human chorionic somatomammotropin causes decreased insulin sensitivity and decreased utilization of glucose in the mother, thereby making larger quantities of glucose available to the fetus. Because glucose is the major substrate used by the fetus to energize its growth, the possible importance of such a hormonal effect is obvious. Further, the hormone promotes the release of free fatty acids from fat stores of the mother, thus providing this alternative source of energy for the mother’s metabolism during pregnancy. Therefore, it appears that human chorionic somatomammotropin is a general metabolic hormone that has specific nutritional implications for the mother and the fetus.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here