Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
| Adjusted odds ratio | aOR |
| Advisory Committee on Immunization Practices | ACIP |
| American College of Obstetricians and Gynecologists | ACOG |
| Artificial reproductive technology | ART |
| Azidothymidine | AZT |
| Bisphenol A | BPA |
| Body mass index | BMI |
| Casarean delivery | CD |
| Centers for Disease Control and Prevention | CDC |
| Confidence intervals | CI |
| Cytomegalovirus | CMV |
| Electronic medical record | EMR |
| Fetal alcohol syndrome | FAS |
| Group B Streptococcus | GBS |
| Human chorionic gonadotropin | hCG |
| Human immunodeficiency virus | HIV |
| In vitro fertilization | IVF |
| Intrauterine device | IUD |
| Intrauterine growth restriction | IUGR |
| Last menstrual period | LMP |
| Low birthweight | LBW |
| Maternal serum alpha-fetoprotein | MSAFP |
| National Intimate Partner and Sexual Violence Surveys | NISVS |
| Neonatal intensive care unit | NICU |
| Neural tube defect | NTD |
| Odds ratio | OR |
| Peripherally inserted central catheter | PICC |
| Postpartum hemorrhage | PPH |
| Premature rupture of the membranes | PROM |
| Preterm birth | PTB |
| Rhesus immune globulin | RhIG |
| Sexually transmitted infections | STIs |
| Small for gestational age | SGA |
| Tolerance-annoyance, cut-down, eye-opener | T-ACE |
| Toxoplasmosis, other infections, rubella, cytomegalovirus, herpes | TORCH |
| Trial of labor after cesarean | TOLAC |
| U.S. Preventive Services Task Force | USPSTF |
| United States | US |
Preconception and prenatal care should be considered in the context of women's health throughout the life span. This chapter reviews pertinent considerations for prenatal care using the broader definitions espoused by the United States (US) Public Health Service and the American College of Obstetricians and Gynecologists (ACOG). Specifically, prenatal care should consist of a series of interactions with caretakers that includes the following: (1) early and continuing risk assessment, (2) health promotion, and (3) medical and psychosocial interventions and follow-up. The overarching objective of prenatal care is to promote the health and well-being not only of the pregnant woman, fetus, and newborn but also of the family. Hence, the breadth of prenatal care does not end with delivery but rather includes preconception care and postpartum care that extends up to 1 year after the infant's birth. Importantly, this introduces the concept of interconception care and the notion that all health care interactions with reproductive-age women (and men) are opportunities to assess risk; promote healthy lifestyle behaviors; and identify, treat, and optimize medical and psychosocial issues that could impact pregnancy and the lifetime health of the mother and child.
National and international societies have recognized the importance of the continuum of preconception, prenatal, and interconception care as a comprehensive public health priority across the life span, beginning as early as adolescence. The aim of preconception care is to promote the health of women before conception in order to reduce preventable adverse pregnancy outcomes by facilitating risk screening, health promotion, and effective interventions as part of routine health care, and by emphasizing those factors requiring action before conception or early in pregnancy to have maximal impact. Interconception care is defined as care provided between delivery and the beginning of the woman's next pregnancy. The term interconception health was coined by the Centers for Disease Control and Prevention (CDC) as a strategy to optimize parental health between pregnancies by addressing disease processes, health behaviors, and environmental hazards causally associated with infant mortality and other adverse pregnancy outcomes. During the interconception period, intensive interventions are provided to women who have had a previous pregnancy that ended in an adverse outcome (i.e., fetal loss, preterm birth [PTB], low birthweight [LBW], birth defects, or infant death); however for the purpose of this discussion, and for clinical application, preconception and interconception care are essentially interchangable. Many medical conditions among reproductive-age women frequently become apparent during pregnancy and may contribute to negative birth outcomes in the infant, or long-term health consequences for the mother.
The evidence and rationale for providing these services are multiple. First, increasing evidence suggests that human health status in adulthood is dictated by microenvironmental and macroenvironmental conditions around the time of conception (fetal programming of adult disease). Hence, the first prenatal visit may be too late to address modifiable behaviors that could optimize not only pregnancy outcome but the health of the child and future adult. A second significant contribution to adverse pregnancy outcome is related to congenital anomalies, PTB, and LBW. Children born with these conditions contribute significantly to neonatal and infant mortality as well as to family and society health care costs. Patients who present at their first prenatal visit, even as early as the first trimester, are often too late to initiate behaviors or therapeutic interventions to prevent developmental abnormalities or mitigate risk for LBW and potential preterm delivery. Third, almost half of pregnancies are mistimed, unplanned, or unwanted such that women may not be at optimal health or practicing ideal health behaviors at the time of conception, and this is particularly true for adolescents and/or low-income women. Fourth, the proportion of women who delay childbearing or get pregnant with significant medical conditions is increasing, and specific opportunities exist to optimize fertility and pregnancy outcomes as it relates to medication management for those planning pregnancy. Specifically, for those planning pregnancy, preconception/interconception visits provide an opportunity for teachable moments. Data suggest couples planning pregnancy are more likely to change behaviors. Hence, although the functional set of services provided during preconception care, prenatal care, and interconception care are distinct and should be individualized for the patient, operationally, these clinical visits should be viewed as a continuum of comprehensive women's health services provided across a woman's life span, from menarche extending past menopause or sterilization. Finally, national surveys reveal that 84% of reproductive-age women (18 to 44 years) have had a health care visit within the past year, which suggests significant opportunity to provide preconception counseling, yet data indicate this is not being done. Although primary care settings and the well-woman visit are an ideal time to provide these services, all health care practitioners—including but not limited to nutritionists, pharmacists, nurses, midwives, physicians in family practice, obstetrician-gynecologists, and medical subspecialists—should approach every health care encounter with a reproductive-age woman as an opportunity to maximize her health and that of her future offspring by asking two simple questions: (1) Are you pregnant or planning to become pregnant? (2) If not, what are you doing to keep from becoming pregnant?
Collectively, these questions are a great segue to the ultimate question: What is your reproductive life plan? The answers to these questions will guide the subsequent health care interaction and appropriate preconception or interconception counseling and any interventions.
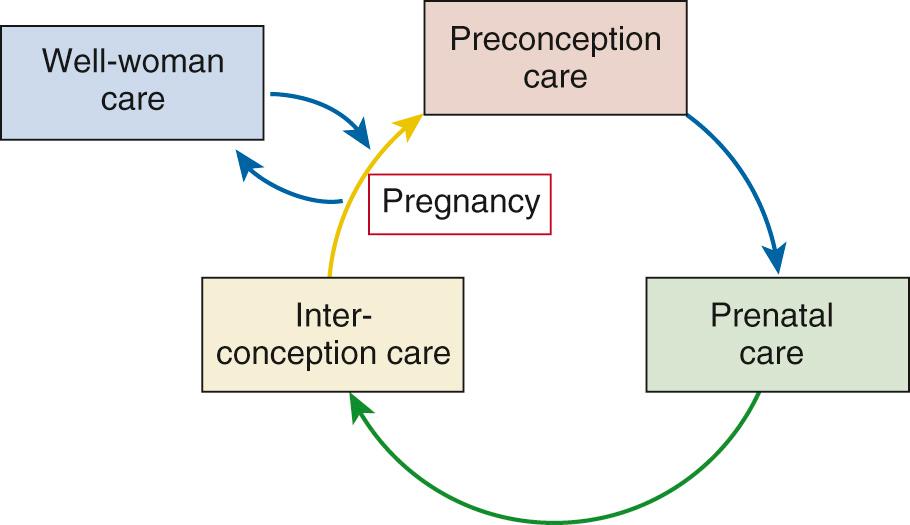
A pregnancy or the desire to become pregnant is the sentinel event in differentiating preconception, interconception, and well-woman care ( Fig. 5.1 ). Preconception care is included as a preventive health service in well-woman visits covered by the Patient Protection and Affordable Care Act . Barriers to more widespread utilization of preconception care include lack of provider knowledge and training about essential components of preconception care across all specialties. Although multiple checklists and online assessments exist, a detailed description provided by the Select Panel on Preconception Care has also been published, along with validated tools. The strategy is similar to most health care interactions: the provider asks screening questions in regard to personal and family history and exposures, undertakes health promotion (counseling for risk reduction), and provides treatment if specific conditions are identified. Table 5.1 lists representative examples of topic areas pertinent for a preconception care visit, and it gives examples of medical conditions that could be optimized prior to conception, assuming pregnancy is planned. Ideally, a checklist or questionnaire should be completed prior to seeing the clinician. Online and interactive modules are available, and inclusion in electronic medical records (EMRs) and sharing across clinical sites should be encouraged. Perhaps the most essential component of the preconception or well-woman visit that needs widespread implementation and dissemination is the development and documentation of an individual's reproductive life plan.
| Clinical Condition | Comment |
|---|---|
| General Health | |
| Age | <18 years: Teenage pregnancy is associated with adverse maternal and familial consequences and increased risk of preterm birth. |
| 18–34 years: This is the ideal age group, especially if part of the reproductive life plan. | |
| >35 years: Increased genetic risks; increase in complications, risk of cesarean delivery, obstetric morbidity, and mortality; general health, not age, should guide recommendations for pregnancy. | |
| Weight | Underweight: Advise weight gain before conceiving and/or greater weight gain with pregnancy. |
| Overweight: Advise weight loss before conceiving; increased BMI is associated with multiple adverse outcomes that include pregnancy loss, stillbirth, diabetes, preeclampsia, and cesarean delivery. | |
| Psychiatric/Neurologic | |
| Depression, anxiety | Adjust medications to those most favorable to pregnancy at the lowest possible dose; counsel about fetal echocardiography and neonatal withdrawal syndrome for some medications; reassure that risk/benefit profile favors treatment. |
| Seizure disorders | Start folic acid 4 mg when considering pregnancy to decrease risk of NTD; if no seizure in 2 years, consider trial off medication; adjust medications to those most favorable to pregnancy to avoid risk of dysmorphic structural malformation syndromes; close serum monitoring is required during pregnancy; reassure that risk/benefit profile favors treatment. |
| Migraines | Migraine pattern can change with pregnancy. Most migraine-specific medications are not contraindicated. |
| Cardiac | |
| Congenital cardiac disease or valve disease | Coordinate with cardiologist; pregnancy may be contraindicated with some conditions depending on severity (NYHA classification) or medications needed. |
| Coronary artery disease | Coordinate with cardiologist. |
| Hypertension | Adjust medications to optimize blood pressure. Discontinue ACE inhibitors and ARBs; these drugs are associated with congenital abnormalities. |
| Respiratory | |
| Asthma | Optimize treatment regimen per stepped protocol; if steroid dependent, use early ultrasound to evaluate for fetal cleft; advise patients at increased risk for gestational diabetes that medications, including steroids, are not contraindicated; emphasize that benefits of treatment exceed risks. |
| Gastrointestinal | |
| Inflammatory bowel disease | Optimize treatment regimen, advise that it is ideal to conceive while in remission; some medications have absolute versus relative contraindications. |
| Genitourinary | |
| Uterine malformations | Coordinate with reproductive endocrinologist if indicated. |
| Metabolic/Endocrine | |
| Diabetes | Achieve euglycemia before conception (hemoglobin A <7%); dose-dependent relationship regarding risk of congenital anomalies with medications; with type 1 and longstanding type 2 diabetes, insulin therapy is best; sulfonylureas are usually reserved for gestational diabetes mellitus. |
| Hematologic | |
| Sickle cell/thalassemia | Genetic counseling; advise sickle cell patient that crises can be exacerbated by pregnancy, and a risk of preterm birth/low birthweight is present. |
| History of DVT/PE, known hereditary thrombophilias | Risk of recurrent DVT/PE requires prophylaxis during pregnancy |
| Infectious | |
| STIs, TORCH, parvovirus | Establish risk factors, counsel to avoid infection, and treat as appropriate. |
| Rheumatologic | |
| SLE | It is ideal to conceive while SLE is in remission; some medications may be contraindicated. |
| Genetic | |
| Known genetic disorder in patient or partner | Genetic counseling, medical records to confirm diagnosis, and evaluation are warranted for prenatal diagnosis or assisted reproduction to avoid inheritance risk based on parents’ preferences and values. |
Files and associates have defined a reproductive life plan as a “set of personal goals regarding the conscious decision about whether or not to bear children” and outline a strategy to achieve those goals. Key elements to consider when developing or discussing a reproductive life plan include: (1) the desire or lack of desire to have children; (2) parental ages; (3) maternal health and coexisting medical conditions; (4) the desired number of children and anticipated spacing of children, taking into consideration ideal birth-spacing intervals, maternal age, and likelihood of fertility; (5) risk tolerance for genetic or medical/obstetric complications; (6) family history; and (7) life context (age, school, career, partner, readiness for childbearing). Importantly, reproductive life plans should be individualized, iterative, and addressed initially at menarche, confirmed or modified at subsequent health encounters by all care providers, and retired with menopause or sterilization. Files and colleagues have provided an algorithm and guidelines for developing a reproductive life plan, and convenient online tools have been designed for women contemplating pregnancy. Unfortunately, even when women have achieved the pregnancy intention stage of readiness, many are still indulging in habits or behaviors associated with poor pregnancy outcomes, such as poor diet (overweight or underweight), smoking, drug abuse, and binge drinking. This suggests a greater need for both one-on-one provider-patient interactions and more pervasive social media messages. Research has demonstrated that patients want to be told this information from their providers, and many will respond favorably. Further, sharing this information in groups, community settings, and in the presence of the partner has been shown to increase effectiveness. Importantly, reproductive life plans should not be limited to reproductive-age women—the concept is pertinent for adolescent boys and men as well.
If conception is not anticipated within the year, an obvious discussion/intervention includes effective contraception options. Likewise, when specific conditions are detected and pregnancy is not recommended or intended, reliable contraception should be prescribed, and the importance of compliance should be strongly reinforced. Data suggest that many women with complex medical problems who are advised against pregnancy conceive unintentionally and/or do not use contraception because of low perceived risk of conceiving. If pregnancy is desired, in addition to screening and encouraging healthy behaviors, interventions to optimize the medical condition and adjust medications to profiles favorable for pregnancy should occur (see Table 5.1 for medical conditions and interventions common for preconception care and pregnancy).
The average maternal age at first birth has increased steadily over the last 4 decades in developed countries. In 1970, the average age of first childbirth was 24.3, compared with 30 in 2015, the most recent year for which comparable international data are available .
Among the Organization for Economic Cooperation and Development countries, Mexico has the lowest mean age of women at childbirth, (26.2) Switzerland, Spain, and Korea have the highest at 31.8, 31.9 and 32.4 respectively while the mean age of women at childbirth in the US is 28. In middle- and low-income countries, maternal age over 35 years is a contributing factor to maternal near miss, maternal death, and severe maternal outcome, and adverse perinatal outcomes, including PTB (<37 weeks of gestation), stillbirths, early neonatal mortality, perinatal mortality, LBW (<2500 g), and neonatal intensive care unit (NICU) admission. In high-income countries, maternal age over 35 is also associated with pregnancy loss, fetal anomalies, stillbirth, and obstetric complications but better access to perinatal care reduces the overall mortality risks.
With advancing maternal age comes an increased likelihood of preexisting chronic medical diseases such as arthritis, hypertension, and diabetes. The national Maternity Experiences Survey of the Canadian Perinatal Surveillance System found that nulliparous women aged 35 years and over are significantly more likely to have had a miscarriage or infertility treatment, to request or be offered a cesarean delivery (CD), and to have a higher rate of CD than primiparous women aged 20 to 29 years, but they are not at higher risk for PTB, LBW, or small-for-gestational-age (SGA) infants. A recent systematic review and meta-analysis study found that age over 35 increased the risk of stillbirth (OR, 1.75; 95% CI, 1.62–1.89), preeclampsia (OR 1.99; 95% CI, 1.65–2.36), neonatal death (OR, 1.48; 95% CI, 1.30–1.67), NICU admission (OR, 1.49; 95% CI, 1.34–1.66), and gestational diabetes mellitus (GDM) (OR, 2.85; 95% CI, 2.46–3.32 ) . These risks increase with advancing maternal age and the rates of preeclampsia and GDM almost double in women aged 50 years or more, and the vast majority of them can expect to deliver via CD.
Many nulliparous women aged 40 and over require the use of artificial reproductive technologies (ART) to become pregnant. Women over 35 who conceived with ART are more likely to proceed with an elective CD and are at increased risk of retained placenta compared with women over 35 who conceive spontaneously. In older women, ART techniques may require oocyte donation, which has been associated with increased risks of preeclampsia and premature delivery. A recent systematic review and meta-analysis of 35 studies has found that the risk of preeclampsia as evaluated by adjusted odds ratio (aOR) was 2.11 (95% CI, 1.42–3.15) in singleton and 3.31 (95% CI, 1.61–6.80) in multiple pregnancies. The risks of PTB and LBW in singletons were aOR 1.75 (95% CI, 1.39–2.20) and 1.53 (95% CI, 1.16–2.01), respectively. Furthermore, as a result of ART, the twin birth rate to women aged 40–44 nearly doubled between 1990 and 2001, and that of triplets increased fourfold in women 35 years and older between 1975 and 1998. Within this context, in particular in older women with a preexisting medical condition, reproductive medicine subspecialists, maternal fetal medicine subspecialists, and obstetrician/gynecologists should promote more realistic views of the evidence-based realities of pregnancy in women over 35.
The true prevalence of teen pregnancy worldwide is difficult to determine because of incomplete data but estimates suggest it occurs in 13% of the US population and 25% worldwide. In a comprehensive review by Sedgh et al., there were 21 countries with complete statistics revealing that the pregnancy rate among 15- to 19-year-olds was highest in the US (57/1000), and lowest in Switzerland (8/1000). It is believed that rates are higher in Mexico and sub-Saharan Africa but there is incomplete reporting. A Swedish survey found that adolescents are more likely to be delivered vaginally than older women and that the risks of placenta previa, postpartum hemorrhage (PPH), and perineal lacerations are lower for adolescents than for adult women. However, adolescents have increased risk of preeclampsia, PTB, LBW, and pregnancy loss including neonatal and infant mortality, stillbirths, intrapartum deaths, and miscarriages. The younger the patient, the greater the risk for both mom and baby. Globally, complications from childbirth are the second leading cause of mortality in the 15- to 19-year-old age group.
Adolescent pregnancy is often unplanned (80%) and has a negative impact on the physical, emotional, educational, and economic condition of the pregnant teenager. This high rate of unintended pregnancy is associated with a high abortion rate. Approximately 50% of teen pregnancies lead to abortions. Adolescent parenthood is associated with a range of adverse outcomes for young mothers, including mental health problems such as depression, substance abuse, and posttraumatic stress disorder. Adolescents are also more likely to smoke (36% vs. 7%), drink alcohol or use drugs (1.1% vs. 0.2%), as well as have higher levels of emotional stress, partner violence and abandonment, and unstable or unsafe home environments compared to adults. Teen mothers are more likely to be impoverished and to reside in communities and families that are socially and economically disadvantaged, which likely is a contributing factor to their higher risk of delivering prematurely.
In middle- and low-income countries, higher risks of preterm delivery have also been demonstrated for adolescent mothers aged 10 to 19 years. In addition, their risk of eclampsia, puerperal endometritis, emergency CD, PPH, and systemic infections is higher compared with mothers aged 20 to 24 years. These worldwide consistent trends stress the important role of health care workers as health policy advocates to ensure that all teenagers receive sound sex education in school programs and that family planning agencies are permitted to counsel teenagers and provide contraception services that include medications and long-acting reversible contraception devices. The importance of family planning and access to contraception for teens cannot be overstated, as there is a high rate of rapid repeat pregnancy within 1 to 2 years.
Abnormal maternal weight is an increasingly common complication worldwide. Approximately 50% of reproductive age women are overweight or obese. As such, maternal obesity has become a global issue associated with obstetric, surgical, and anesthetic risks and increased risk for acute and chronic diseases, both in the mother and in the child. It also affects the economic productivity of individuals in the society and creates an additional cost burden on the health care system (see Chapter 46 ). Anorexia and bulimia nervosa, once thought to be rare eating disorders, have also been increasing because of cultural pressure on the drive for thinness in developed countries in contrast to longstanding food deprivation in developing countries.
Weight gain during pregnancy has been shown to be an important predictor of pregnancy outcome (see Chapter 6 ). Maternal weight gain correlates with fetal weight gain and is, therefore, closely monitored. Too little weight gain should lead to an evaluation of nutritional factors and an assessment of associated fetal growth. Excess weight gain is one of the first signs of fluid retention, but it may also reflect increased dietary intake or decreased physical activity. The trajectory of weight gain in the second and third trimester is an important predictor of birthweight.
In the US, the total weight gain recommended in pregnancy is 11 to 16 kg (25–35 lb) for women at a healthy weight. Underweight women can gain up to 18 kg (40 lb), but overweight women should limit weight gain to 7 kg (15 lb), although they do not need to gain any weight if they are morbidly obese.
At term, the typical woman gains about 3 to 4 kg (7–9 lb) from increased tissue fluid volume and fat, 1.5 to 2 kg (3–4 lb) from increased blood volume, 0.5 to 1 kg (1–2 lb) from breast enlargement, 1 kg (2 lb) from enlargement of the uterus, 1 kg or 1 L (2 lb) from amniotic fluid, 2.7 to 3.6 kg (6–8 lb) for the fetus, and 0.5 to 1 kg (1–2 lb) of placental weight. Usually, 1.4 to 2.7 kg (3–6 lb) are gained in the first trimester, and 0.2 to 0.6 kg (0.5–1 lb) per week are gained during the last two trimesters of pregnancy.
If the patient does not show a 4.5 kg (10 lb) weight gain by mid pregnancy, her nutritional status should be reviewed. Inadequate weight gain is associated with an increased risk of an LBW infant. Inadequate weight gain seems to have its greatest effect in woman at a healthy weight or those who are underweight before pregnancy. Underweight mothers must gain more weight during pregnancy to produce infants of normal weight. In overweight and obese women, weight loss or gain of 11 lb (5 kg) or less is associated with increased risk of an SGA infant and decreased neonatal fat mass, lean mass, and head circumference.
When excess weight gain is noted, patients should be counseled to avoid foods that are high in fats and carbohydrates, to limit sugar intake, and to increase their physical activity. Several small studies suggest that monitoring weight gain, quantity of food consumed, and physical activity combined with behavioral counseling can limit weight gain during pregnancy and promote postpartum weight loss.
Dietary and lifestyle interventions in pregnancy can reduce maternal gestational weight gain and improve outcomes for both mother and baby. Among the interventions, those based on diet are the most effective and are associated with reductions in maternal gestational weight gain and improved obstetric outcomes. Most obstetric providers are aware of these recommendations, but many do not feel comfortable making treatment recommendations.
Weight gain and weight retention after pregnancy is a risk factor for subsequent obesity. Thus postpartum weight loss should be encouraged. Women who resumed their prepregnancy weight by 6 months postpartum gained only 2.4 kg (5 lb) over the next 10 years compared with 8.3 kg (18 lb) for women who retained weight after delivery. It has been estimated that 40% to 60% of normal and overweight women gain more than the Institute of Medicine recommendations making postpartum weight loss difficult. Data suggest that 75% of women are heavier than their prepregnancy weight 1 year postpartum, and weight retention between the first and second pregnancy is associated with an increased risk for perinatal complications, even in underweight and normal-weight women.
Stabilizing interpregnancy weight appears to be an important target to avoid adverse perinatal outcomes in a second pregnancy. Although clinicians have focused on teaching women that appropriate weight gain is important for pregnancy, the concomitant importance of postpartum weight loss has not been given equal attention. A meta-analysis on the effect of diet, exercise, or both for weight reduction in women after childbirth has found that both diet and exercise together and diet alone help women lose weight after childbirth.
Seventy percent of American adults are overweight (body mass index [BMI] ≥25 kg/m 2 ) or obese (BMI ≥30 kg/m 2 ). Pregestational weight gain or obesity and excessive gestational weight gain are now well-established independent risk factors for maternal-fetal complications and long-term risks in adult life for the child. The selected risks include increased miscarriage, congenital anomalies, hypertensive disorders, GDM, macrosomia, and delivery complications that include instrumental delivery, shoulder dystocia, emergency CD, PPH, venous thromboembolism, anesthetic complications, and wound infections. In overweight and obese women, the OR for macrosomia is 1.95 (CI, 95% 1.79–2.11) and aOR for CD is 2.04 (95% CI, 1.41–2.95) for an interpregnancy weight retention of two or more BMI units. Being overweight or obese before pregnancy is also associated with a higher risk of fetal loss, and losing 4 kg or more before pregnancy is associated with a lower risk of fetal loss.
Maternal obesity is a potential risk factor for childhood obesity and asthma. The children of women who are overweight or obese during pregnancy are also at increased risk for cognitive deficits, externalizing problems (particularly attention-deficit/hyperactivity disorder), and internalizing psychopathology in childhood and adolescence. These findings should be interpreted with caution due to measurement issues in gestational weight gain and potential confounding effects of shared familial characteristics (i.e., genetics and maternal and child's lifestyle factors).
Emerging evidence supports the role of first microbial contacts in promoting and maintaining a balanced immune response in early life, and recent findings suggest that microbial contact begins prior to birth and is shaped by the maternal microbiota, which is related to maternal BMI. Although the mechanisms remain unclear, postnatal maturation of immune regulation seems to be largely driven by exposure to microbes, and the gastrointestinal tract is the largest source of microbial exposure. Early exposures that impact the intestinal microbiota are associated with the development of childhood diseases that may persist into adulthood such as asthma, allergic disorders (atopic dermatitis, rhinitis), chronic immune-mediated inflammatory diseases, type 1 diabetes, obesity, and eczema. Breast milk samples from obese mothers tend to contain a different and less diverse bacterial microbiota compared with milk from those at a healthy weight. Alterations in the bacterial composition of the mother have been shown to affect the development and function of the gastrointestinal tract of her offspring. Thus prepregnancy strategies to modify the microbiota of future mothers may prove to be a safe and effective target for interventions to decrease the risk of allergic and noncommunicable diseases in future generations.
Being underweight before pregnancy, or having insufficient gestational weight gain have been considered individual risk factors for the occurrence of miscarriage, PTB, SGA, and hypertensive disorders . Gestational weight gain below the recommendations is associated with higher risk of SGA (OR, 1.53; 95% CI, 1.44 to1.64) and PTB (OR, 1.70; 95% CI, 1.32–2.20) and no significant difference in CD rates (OR, 0.98; 95% CI, 0.96–1.02). A systematic review and meta-analysis has shown that the birthweight of children of mothers with anorexia nervosa is lower by 0.19 kg compared with children of mothers at a healthy weight. A population study from the same authors has also shown that eating disorders are associated with increased odds of receiving fertility treatment and subsequent twin births. Women with anorexia nervosa were more likely to have an unplanned pregnancy and to have mixed feelings about the unplanned pregnancy.
Pregnancy after bariatric surgery appears to effectively reduce the risk of complications such as fetal macrosomia, GDM, and hypertensive disorders of pregnancy (see Chapters 31 and 46 ). A study using the Swedish Medical Birth Register from 2006 through 2011 found 670 women who had previously undergone bariatric surgery. Compared with matched control pregnancies, pregnancy after bariatric surgery is associated with lower risks of gestational diabetes (1.9% vs. 6.8%; OR, 0.25; 95% CI, 0.13–0.47) and large-for-gestational-age infants (8.6% vs. 22.4%; OR, 0.33; 95% CI, 0.24–0.44), higher risk of SGA infants (15.6% vs. 7.6%; OR, 2.20; 95% CI, 1.64–2.95) and shorter gestation (273 vs. 277 days; mean difference −4.5 days; 95% CI, −2.9 to −6.0). A recent systematic review and meta-analysis of 20 cohort studies including 8364 women who had bariatric surgery showed reduced rates of GDM, macrosomia, gestational hypertension, PPH, and CD rates. A group of patients showed an increase in SGA and PTB when compared with control subjects matched for pre-surgery body mass index.
Women who become pregnant after bariatric surgery constitute a unique obstetric population with an increased risk of severe micronutrient deficiencies. As such, screening for micronutrients should be considered in the preconception period or at the first prenatal visit and should include the following: complete blood count, iron, ferritin, folate, calcium, zinc, magnesium, iodine, and vitamins A, B12, D, and K. Additional screening and supplementation should occur every trimester and postpartum. Women should follow Institute of Medicine weight gain recommendations and serial evaluation of fetal growth should be performed to monitor for intrauterine growth restriction (IUGR).
Primary care, preventative health, and well-women visits are ideal settings in which to screen and counsel women about sexually transmitted infections (STIs) such as syphilis, gonorrhea, chlamydia, and human immunodeficiency virus (HIV) in addition to toxoplasmosis, other infections, rubella, cytomegalovirus (CMV), and herpes (TORCH) infections. It is also an ideal time to confirm and update immunization status (see Chapter 57, Chapter 58 ). Patients who are sexually active should be counseled about the importance of condoms to prevent STIs (irrespective of other contraceptive methods), and they should be screened for STIs based on age and geographic prevalence as per national guidelines.
Toxoplasmosis screening based on risk factors may be indicated at this time because approximately 11% of the U.S. population is infected. Patients should be screened at the beginning of pregnancy, and those who have negative screens are at risk for congenital toxoplasmosis and should be counseled to avoid risks such as contact with infected cats and ingestion of raw or undercooked meat. Immunocompetent patients who screen positive can be reassured of low risk with regard to fetal loss or stillbirth, although rare reports of congenital infection after previous infection have been described. A prospective analysis of the population risks and benefits to substantiate routine screening for and education about toxoplasmosis has not been done in the US. However, proponents arguing a theoretic benefit based on treatment availability extrapolated epidemiologic data from some European countries (France, Belgium, Austria) where screening is widespread, and the prevalence of congenital infection was comparable to other congenital diseases that are currently screened for by mandate, such as phenylketonuria and congenital hypothyroidism. In the US, treatment of the mother with spiramycin for an acute confirmed toxoplasmosis infection is difficult and requires assistance from the Food and Drug Administration. Studies suggest maternal treatment does not prevent fetal infection but may reduce congenital disease severity.
Approximately 50% to 80% of reproductive-age women have evidence of prior CMV infection, and susceptible women (e.g., child care workers and women with small children in day care) should be counseled about the importance of hand hygiene after contact with toys, saliva, and urine as a preventive measure. Risk of vertical transmission is more likely after primary infection, and current treatment options are limited. Routinely screening for CMV to enhance awareness and encourage prevention practices in at-risk mothers is performed in only a few European countries (France, Belgium) but is currently not endorsed in the US. Likewise, women with primary or recurrent herpes should be informed about the benefits of prophylactic antiviral suppression in the third trimester to decrease risk of vertical transmission and the need for CD.
All reproductive-age women should be current with immunizations recommended by the Advisory Committee on Immunization Practices (ACIP) and the CDC. This is the time to draw and document protective titers for rubella, varicella, and hepatitis B and to immunize the susceptible patient. Readers are referred to the CDC website for most current recommendations for immunization in pregnancy ( www.cdc.gov/vaccines/pregnancy ). If the woman conceives and is expected to deliver during flu season, she should receive the influenza vaccine to decrease her disease severity. She should be given the tetanus, diphtheria, and pertussis (Tdap) vaccine in late pregnancy (27–36 weeks) to provide passive immunity for the newborn. Influenza and pertussis vaccination is now recommended by health care authorities in most European countries and Australia.
The time to screen appropriate populations for genetic disease-carrier status and multifactorial congenital malformations or familial diseases with major genetic components is before pregnancy . If patients screen positive, referral for genetic counseling is indicated, and consideration of additional preconception options may be warranted including donor egg or sperm, ART after preimplantation selection, prenatal genetic testing after conception, or adoption. Certain diseases may be related to race/ethnicity or geographic origin. Patients of African, Asian, or Mediterranean descent should be screened for the various heritable hemoglobinopathies (sickle cell disease, α- and β-thalassemia). Patients of Jewish and French-Canadian heritage should be screened for Tay-Sachs disease, Canavan disease, and cystic fibrosis. In the US, it has been suggested that cystic fibrosis screening be offered to all couples planning a pregnancy or seeking prenatal testing. Resolution of these issues during the preconception period is much easier and less hurried without the time limits placed by an advancing pregnancy. The age of the father is also important because genetic, structural, behavioral, or cognitive risks to the child may exist when the father is older; this emphasizes the importance of a reproductive life plan for men as well as women.
The impact of the increasing availability of direct to consumer testing, and private pay, provider-ordered tests has yet to be determined. A strategy to monitor impact from the individual patient perspective, as well as the societal, health economic, and public policy perspective is warranted and will need to consider the availability of local and national resources.
Intimate partner violence (IPV)—also called domestic violence, battering, or spouse abuse—is a pervasive problem across women's lifespans. More than one in three women in the US have experienced rape, physical violence, or stalking either in person or electronically through mobile devices and social media. Although IPV cuts across gender, racial, ethnic, and socioeconomic boundaries, racial differences do exist ( Fig. 5.2 ). Data from National Intimate Partner and Sexual Violence Surveys (NISVS) indicate that the prevalence is highest among women who are multiracial (57%) and lowest among women who are of Asian-Pacific Island heritage (18%). Risk factors for being an IPV victim include but are not limited to age (adolescence and young adulthood), low income, low educational attainment, unemployment, and childhood physical or sexual victimization.

Pregnancy and the postpartum period are often when IPV begins. Every year, approximately 324,000 pregnant women are abused in the US. However, the true prevalence of IPV is unknown because many victims are afraid to disclose their personal experiences of violence. The consequences of IPV in pregnancy impact not only the mother but the child as well ( Fig. 5.3 ).

Maternal problems can be categorized into physical and mental. Physical complications include substance abuse, smoking, late entry into prenatal care, and preterm delivery. Poor maternal bonding, depression, anxiety, increased risk for suicide, and being a victim of homicide are some of the negative maternal results. The impacts of IPV on the infant are lifelong and range from LBW to developmental delay, emotional problems, sleep problems, and the potential to perpetrate IPV.
Because prenatal care often provides regular contact with a health care provider, the perinatal period is an ideal “window of opportunity” to address IPV. The US Department of Health and Human Services recommend that IPV screening and counseling should be a core part of women's preventive health visits. Screening for IPV should be done at the first prenatal visit, at least once per trimester, and at the postpartum check. The screening question in pregnancy can be “Since you became pregnant, have you been physically hurt by anyone?” If positive, IPV support and prevention and referral options should be part of the screening. The referral list should include the national 24-hour toll-free hotline. Even if abuse is not acknowledged, normalizing the IPV screening in a caring manner and having educational materials readily accessible is helpful.
In the general population, fetal and neonatal exposures to drugs and other toxins are often the consequence of the lifestyle choices of the parents, with exposure to tobacco smoke and alcohol among the most pervasive and easily documented. Smoking and use of alcohol and drugs by pregnant women are all harmful to the developing fetus (see Chapter 8 ). Overall, the risks to the neonate include IUGR, birth defects, altered neuropsychologic behavior, and, for some drugs, withdrawal symptoms.
In many countries, smoking has replaced poverty as the most important risk factor for PTB, IUGR, and sudden infant death syndrome. Cigarette smoke contains scores of toxins that exert a direct effect on placental and fetal cell proliferation and differentiation. The use of or exposure to tobacco products by pregnant women is associated more specifically with placenta previa, placental abruption, placenta accreta, pregnancy bleeding of unknown origin, and preterm premature rupture of membranes. Smoking is linked to a reduction of weight, fat mass, and most anthropometric parameters in the fetus. Epidemiologic studies support a relationship between maternal smoking during pregnancy and adverse neurobehavioral effects for her offspring later in life. The long-term effects of passive and active smoking during pregnancy on childhood or adulthood diseases, including respiratory and cardiovascular disease and cancer, are only starting to emerge from large epidemiologic studies.
In spite of these well-established negative consequences, epidemiologic studies have shown that, depending on the patient population, 20% to 50% of pregnant women smoke or are exposed to passive smoking . In many industrialized countries, prevalence rates of women actively smoking appear to have peaked and have begun to decline, whereas in other countries, smoking is becoming increasingly common among young women. Smoking during pregnancy has been recognized as the most important modifiable risk factor associated with adverse perinatal outcomes. Because most of the placental and fetal damage is done in the first trimester of pregnancy, helping women to quit smoking before conceiving should be a primary objective in prepregnancy counseling or as part of ongoing prenatal care.
Alcohol is a well-established teratogen, and alcohol used during pregnancy can lead to fetal alcohol syndrome (FAS), which includes specific morphologic features such as microcephaly and long-term abnormal neuropsychologic outcomes (see Chapter 8 ). Worldwide, many national departments of health have policy statements that support total alcohol abstinence during pregnancy. Women planning pregnancy should be encouraged to reduce or quit alcohol consumption prior to conceiving. Regular screening for alcohol use should be carried out using such tools as the Tolerance-Annoyance, Cut-Down, Eye-Opener (T-ACE) questionnaire or other simple screening tools, and therapy should be made available to those women who screen positive.
Substance abuse in pregnancy (see Chapters 8 and 59 ) has increased over the past three decades, and it has been recently estimated that approximately 225,000 infants yearly in the US are exposed prenatally to illicit substances.
Cannabis is the most commonly used illicit substance in the US, predominantly for its pleasurable physical and psychotropic effects. Several states and the District of Columbia have made recreational use of cannabis legal, and this topic has gained national attention. Studies regarding its impact on pregnancy have been conflicting, but it has been shown that after adjustment for coexisting risk factors, cannabis use is associated with increased risk for preterm delivery, can lead to IUGR and withdrawal symptoms in the neonate, has increased risk for neonatal morbidity and mortality, and has been associated with negative effects on intellectual outcome.
Cocaine use in pregnancy can lead to spontaneous abortion, PTB, placental abruption, and preeclampsia. Although fetal cocaine exposure has been linked to numerous abnormalities in arousal, attention, and neurologic and neurophysiologic function, most such effects appear to be self-limited and restricted to early infancy and childhood. Neonatal issues include poor feeding, lethargy, and seizures.
Poor obstetric outcomes can be up to six times higher in patients who abuse opiates such as heroin and methadone. Opiate exposure elicits a well-described withdrawal syndrome that affects central nervous, autonomic, and gastrointestinal systems; this effect is most severe among methadone-exposed infants. Amphetamine use can lead to congenital anomalies and other poor obstetric outcomes. Methamphetamine use in particular is an escalating problem worldwide because it is the only illegal substance that can be made from legally obtained over-the-counter cold medications.
Mothers who use illicit drugs require specialized prenatal care, and the neonate may need extra supportive care. Once recognized, a specialized multidisciplinary approach that includes access to rehabilitation centers can lead to improved maternal and neonatal outcomes.
Data regarding the accumulation of mercury in fish have led to warnings advising pregnant women to avoid or decrease fish consumption; however, the benefits of omega-3 fatty acids found in fish outweigh the risks . Mercury is neurotoxic and has been associated with a dose-dependent impact on neurologic development. Unfortunately, the harms of mercury are counterbalanced by the benefits of omega-3 fatty acids that include decreased LBW and PTB, an increase in visual acuity, and higher performance on developmental tests and higher IQ scores in offspring. It is unclear whether dietary supplements are similarly beneficial because the stability and bioavailability varies and has not been well characterized. The US Environmental Protection Agency and the FDA have attempted to clarify these mixed messages by recommending that reproductive-age women, pregnant women, and children eat a variety of fish two or three times a week. They recommend 8 to 12 oz of fish per week, and albacore tuna should be limited to not more than 6 oz. Pregnant women should specifically avoid eating fish with a high mercury content (king mackerel, shark, swordfish, and tilefish).
Occupational hazards should be identified. If a patient works in a laboratory with chemicals or in agriculture around a lot of pesticides, she should be advised to identify potential reproductive toxins and limit her exposure. This is an active area of research, and several online resources are available for information about potential environmental and occupational teratogens. Patients whose occupations require heavy physical exercise or excess stress should be informed that they may need to decrease such activity later in pregnancy because both have been associated with an increased risk of PTB and reduced fetal growth in observational studies.
A study based on the National Health and Nutrition Examination Survey demonstrated that all pregnant women are exposed to and have detectable levels of chemicals that can be harmful to reproduction or human development . Because exposure to environmental agents can be mitigated or prevented, it is important for women to be made aware of known toxic substances and to be informed as to how to access resources to gain additional information. Environmental contributors to reproductive health begin in utero and are influenced by social, physical, nutritional, and chemical agents. Lead has historical significance but more recent concerns are related to mercury, phthalates, perchlorates, pesticides, and bisphenol A (BPA). These agents are considered endocrine-disrupting chemicals that interfere with cellular proliferation or differentiation and result in altered metabolic, hormonal, or immunologic capabilities. For example, BPA is commonly found in plastics used for food and beverage products and packaging and has been associated with recurrent miscarriages and aggression and hyperactivity in girls. Primary care physicians can play an important role by providing guidance to women about how to avoid toxic exposures at home and in the community, and they can help educate patients by referring them to online resources.
Clear evidence shows that for some conditions—such as diabetes mellitus, phenylketonuria, and inflammatory bowel disease—medical disease management before conception can positively influence pregnancy outcome . Medical management to normalize the intrauterine biochemical environment should be discussed with the patient, and appropriate management plans should be outlined before conception; advice can also be given about avoiding specific medications in the first trimester (e.g., isotretinoin). Table 5.1 gives examples of general health and medical conditions by organ system that can be optimized by preventing pregnancy until it can be planned and then adjusting medication type or dose to minimize teratogenicity or impact on neonatal development.
Guidelines to address the content and efficacy of prenatal care have focused on the medical, psychosocial, and educational aspects of the prenatal care system. Prenatal care satisfies the definition of primary care from the Institute of Medicine as “integrated, accessible health care services by clinicians who are accountable for addressing a large majority of personal health care needs, developing a sustained partnership with patients, and practicing in the context of family and community.” Prenatal care is another opportunity to introduce and reinforce habits, knowledge, and life-long skills in self-care, health education, and wellness to inculcate principles of routine screening, immunization, and regular assessment for psychological, behavioral, and medical risk factors. Phelan argues that clinicians are not taking advantage of pregnancy as a “teachable moment”—a naturally occurring life transition that motivates people to spontaneously adopt risk-reducing behaviors. If health and habits are not optimized during the preconception period, pregnancy qualifies as a teachable moment because it meets the following criteria proposed by McBride and colleagues :
Perception of personal risk and outcome expectancies is increased.
The perceptions are associated with strong affective or emotional responses.
The event is associated with a redefinition of self-concept or social role.
Education about pregnancy, childbearing, and childrearing is an important part of prenatal care, as are detection and treatment of abnormalities. However, contemporary models of prenatal and childbirth education have been criticized because research has not shown a strong association between class attendance and childbirth experiences or parenting expectations.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here