Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Nowhere else in the realm of liver transplantation have both the advances made in the field and the realities of the surgery been more evident than in the intensive care unit (ICU). The impact of the Model for End-Stage Liver Disease (MELD) system, with increasing MELD scores and waiting times, means sicker recipients before the transplant—more renal dysfunction, malnutrition, and moribund state. In response to this increased allograft demand, the donor pool has been expanded to use older, fattier, and more marginal donor allografts. The net effect of this combination is a greater requirement for posttransplant ICU care. Despite this, the improvements in ICU techniques and development of advanced technologies, along with evidence-based protocols, have better positioned the clinician to be able to address these increased ICU demands. In short, the world of transplant has changed, and this is not your grandfather’s transplant ICU anymore.
Intensive care management of the liver transplant recipients represents an area where evidence-based approaches can have significant impacts on the costs and outcomes associated with transplantation. The availability of ICU beds is a common rate-limiting factor, even in those programs that have their own dedicated ICU resources. The efficient use of intensive care resources can have significant cost-saving benefits for a transplant program, at a time of strained financial funding.
Upon arrival of the liver transplant recipient in the ICU, the clinician must first assess and stabilize the hemodynamic status of the recipient. Patient hemodynamics are affected by volume status, cardiac function, and systemic vascular resistance (SVR). Successful management requires an accurate and timely assessment of these parameters using monitoring technologies.
Electrocardiogram (ECG) monitoring serves two main purposes: First, it allows identification of cardiac disturbances such as atrial fibrillation and flutter, enabling appropriate and prompt therapy. Second, it can assess the clinical impact of any major electrolyte changes. Abnormal electrolyte levels are common in liver recipients, especially those with renal dysfunction, and ECG can identify the clinically significant changes.
Invasive arterial blood pressure (BP) monitoring is essential, and initial posttransplant recipients can experience significant and rapid BP changes because of blood loss or a hyperdynamic circulation requiring pressors. In addition, invasive arterial BP monitoring provides easy access to obtain specimens for determination of arterial blood gas levels, serial hemoglobin levels, and coagulation tests.
The volume status of a liver recipient is a continually dynamic parameter throughout the perioperative and initial postoperative course, and volume status can be simultaneously affected by contrary factors such as blood loss and overload from renal dysfunction. An accurate gauge of the volume status in posttransplant patients is crucial, although debate exists as to the best way to assess intravascular volume status.
The most frequently used tool for hemodynamic monitoring is the Swan-Ganz, or pulmonary artery, catheter, which measures filling pressures in the heart and pulmonary artery. These filling pressures are intended to reflect the cardiac filling volumes. Pulmonary artery catheter use is controversial, because filling pressures have been shown to not always accurately define cardiac preload. In contrast, some believe that the data derived from these pressures have little positive predictive value in guiding hemodynamic management for improving liver perfusion and determining the appropriate resuscitative efforts. Some transplant programs use a modified pulmonary artery catheter equipped with a rapid-response thermistor that continuously measures right ventricular end-diastolic volume by calculating it from right ventricular ejection fraction and the stroke volume index. The drawbacks of this catheter are that it is more expensive and can have trouble consistently calculating an accurate end-diastolic volume, because of catheter positioning and arrhythmias that alter the R wave.
Another method for assessing volume status is pulse dye densitometry. It is a safe, noninvasive, method using indocyanine green (ICG) to measure systemic hemodynamic parameters. It measures cardiac output (CO) by analyzing the pulsatile change in ICG concentration in peripheral arterial blood without actually directly sampling the blood. It correlates well with the thermodilution technique from pulmonary artery catheters. The main limitation is that ICG is cleared by the liver and in patients with hepatic dysfunction there is a limit to the frequency of ICG administration and subsequent measurement.
Transesophageal echocardiography (TEE) can be used to directly measure cardiac function and assess intravascular volume. It allows rapid visualization of left ventricular function and determination of the left ventricular end-diastolic area index, which calculates left ventricular filling by measuring ventricular diameter and correlating it with changes in stroke volume while intravascular volume is being adjusted. TEE can differentiate the cause of hypotension as a low intravascular volume, a myocardial problem, an extracardiac pathological condition (such as effusion), or a combination of those three factors. TEE offers several advantages, such as the fact that it can be positioned more easily than a pulmonary catheter in an emergent setting. Nonetheless there are drawbacks and complications with TEE, including esophageal variceal bleeding and esophageal tears with probe placement.
Immediately posttransplant, multiple factors simultaneously affect the recipient hemodynamics, and a good understanding of volume status is crucial for patient and graft survival. Both hypovolemia and hypervolemia can be detrimental to the allograft, so one must correctly identify the circumstance and direct the treatment appropriately.
Hypovolemia results from inadequate intraoperative resuscitation, postoperative bleeding worsened by coagulopathy, or third spacing and edema. The reduced preload leads to low CO and underperfusion of the recently transplanted allograft, causing damage and preservation injury. The treatment of hypovolemia is intravascular fluid replacement using one of several options. Blood products are a useful fluid for resuscitation in the initial posttransplant setting because they stay in the intravascular space, preventing allograft edema, and also reduce the existing edema through their hyperoncotic impact. Although the literature and conventional teaching recommend minimizing transfusion of blood products in the nontransplant patient because of an increased mortality risk, the transplant patient represents a different population than the one studied in these trials. The vascular barrier permeability of the allograft is inevitably compromised by the ischemia-reperfusion (IR) injury occurring from the transplant process, and the injured allograft is less sensitive to fluid and protein shifts using blood products, compared to crystalloids or colloids. Additionally, compared to typical nontransplant ICU patients, transplant recipients are already immunocompromised and are therefore less affected by the immunosuppressing effects of transfusions. Using blood products as the initial resuscitation fluid in the fresh posttransplant recipient yields the hyperoncotic benefits of this fluid, without its detrimental effects.
Regarding other potential fluid resuscitation options for hypovolemia, intravenous (IV) saline is readily available in the ICU and is cost-effective. However, these crystalloids quickly exit the intravascular space, causing third spacing and edema that can congest the sinusoids of the allograft, while also increasing intra-abdominal compartment pressure from bowel edema. Volume expanders such as 25% albumin are commonly used; however, the largest prospective double-blind trial of nontransplant ICU patients failed to show any benefit of resuscitation with albumin versus crystalloids, likely because 60% of the albumin is taken up by the extravascular space. Further large multicenter trials showed that resuscitation with albumin for shock leads to increased risk for renal failure and death in ICU patients. Finally, colloid starch solutions such as hetastarch (Hespan) and pentastarch (Pentaspan) increase the risk for acute kidney injury and mortality and should be used judiciously as a fluid for resuscitation, if at all.
Hypervolemia occurs from overresuscitation or more commonly in recipients with renal dysfunction. Hypervolemia can lead to third spacing, capillary leak syndrome, and graft congestion, particularly because the vascular barrier permeability of the allograft is compromised from the IR injury from the transplant process. Allografts with significant preservation injury or steatosis and allografts from elderly donors are especially sensitive to the sinusoidal congestion from hypervolemia. It is important to lower the central venous pressure (CVP) to create a suitable pressure gradient between the systemic circulation and portal circulation that can facilitate blood through the allograft. Hypervolemia must be aggressively managed with IV diuresis, including infusions of loop diuretics, furosemide (Lasix 40 to 100 mg/hr), or bumetanide (Bumex 1 to 2 mg/hr), or by continuous renal replacement therapy (CRRT), which should be initiated if the urine output on diuretic infusion is inadequate or if renal failure is present.
Successful management of the volume status in the initial posttransplant period requires a clear understanding of the altered cardiovascular hemodynamics that exist in the pretransplant cirrhotic patient. Cirrhotic patients are hyperdynamic with a high CO because the faulty hepatic metabolism causes a buildup of vasodilators such as nitric oxide and endothelial cannaboids that reduce SVR. After the transplant this systemic vasodilation and compensatory hyperdynamic state persist until the allograft begins to function and metabolize all of the excess vasodilatory factors. This initial posttransplant vasodilation puts a greater demand in having adequate preload, and treatment includes vasopressors that increase vasoconstriction and SVR such as norepinephrine (Levophed 1 to 20 mcg/min), vasopressin (Pitressin 0.04 units/min), or phenylephrine (Neo-Synephrine 10 to 180 mcg/min).
One strategy for fluid management in the ICU uses goal-directed therapy (GDT), which is defined as the monitoring and manipulation of hemodynamic variables to bring them to normal or supranormal values by fluids and inotropes. GDT is a bundle of care that includes ICU volume monitoring (i.e., pulmonary artery catheter, TEE) along with blood transfusion and inotropes to increase oxygen delivery, and these protocols typically use CO and SVR (or similar parameters) to guide fluid resuscitation and inotropic therapy. In a typical GDT protocol, a bolus of IV fluid is given to achieve a rise in stroke volume and upon reassessment of the parameter, fluid challenges are repeated if the target has not been met or has decreased. In many cases, supernormal values (i.e., hemoglobin level) occur while achieving these target parameters. In randomized trials of GDT protocols, greater oxygen delivery resulted in fewer complications and a shorter hospital stay, and in GDT protocols for sepsis, substantial efficacy was seen when GDT was started early, but not after organ failure was established.
For the liver allograft in particular, it is important to consider how the splanchnic vasculature (the blood supply to the liver) is different from—and does not always correlate to—the systemic vasculature. Although a normal person can tolerate a 25% to 30% decrease in blood volume without a change in systemic blood pressure or heart rate, the splanchnic perfusion becomes compromised after only a 10% to 15% reduction in intravascular volume. During times of hemodynamic stress, the systemic BP is maintained by the renin-angiotensin system, which selectively vasoconstricts mesenteric arterioles, maintaining BP at the expense of splanchnic hypoperfusion. To ensure splanchnic perfusion to the new liver allograft, the goal should be to aim for normovolemia in the systemic vasculature, using packed red blood cells (PRBC) transfusion with aggressive diuresis to maintain intravascular normovolemia and avoiding third spacing and graft edema.
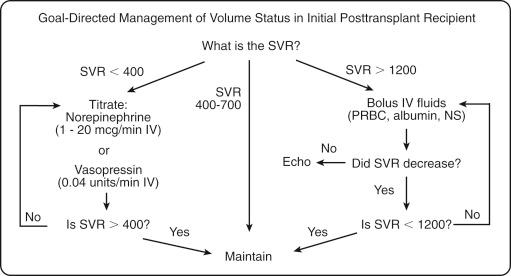
Employing GDT principles, along with understanding of the nature of the splanchnic vasculature response, the following protocol is useful for managing volume status in the initial posttransplant patient. Using data from a pulmonary artery catheter, central venous catheter, and arterial catheter, the CO, cardiac index, CVP, and BP are measured. The SVR can be calculated from these parameters, and it is a useful instrument for a goal-directed management of initial posttransplant hemodynamics. The normal SVR range is 800 to 1200 dynes-sec/m 2 , and in the vasodilated initial posttransplant recipient, the SVR is typically reduced to 400 to 700 dynes-sec/m 2 with the usual compensatory increase in CO found in a hyperdynamic cirrhotic patient ( Fig. 69-1 ). For recipients with SVR of less than 400 dynes-sec/m 2 and hypotension, the BP should be maintained with vasopressors such as norepinephrine (Levophed 1 to 20 mcg/min) or vasopressin (Pitressin 0.04 units/min), and the CVP minimized to avoid hypervolemia and limit allograft congestion. For those recipients with SVR of greater 1200 dynes-sec/m 2 , any hypotension is likely from decreased CO from reduced preload, and these patients need increased fluid resuscitation. Transfusion of blood products, with concomitant diuresis or CRRT to remove fluid, allows for increasing the preload to increase splanchnic perfusion, while minimizing sinusoidal edema and allograft congestion from third spacing. After the clinician initiates therapy, the SVR should be reassessed and further treatment plans should be guided by these new results, continuing with resuscitation until the target SVR is less than 1200 dynes-sec/m 2 . If the SVR is greater than 1200 dynes-sec/m 2 and does not decrease with fluid resuscitation, an echocardiogram should be performed to consider cardiac dysfunction as the cause, and inotropic support with dobutamine (Dobutrex 2.5 to 10 mcg/kg/min IV), milrinone (Primacor 0.25 to 1.0 mcg/kg/min IV) or epinephrine (0 to 10 mcg/min IV) should be initiated.
The pulmonary artery catheter can also guide fluid resuscitation using mixed venous oxygen saturation (SvO 2 ), which provides information on CO and tissue perfusion. SvO 2 is the percentage of oxygen in the blood that returns to the right side of the heart, and it uses blood drawn from the tip of the pulmonary artery catheter before its reoxygenation in the pulmonary capillaries. A normal SvO 2 is 60% to 80%, and if the SvO 2 is decreased, it means that the peripheral tissue is extracting a higher percentage of blood from the tissue as a compensation for poor CO, and increased fluid resuscitation is needed.
An accurate gauge of the volume status is crucial. Debate exists as to the best approach, but options include Swan-Ganz catheters, pulse dye densitometry, and transesophageal echocardiography.
Transfusion of packed red blood cells is an excellent resuscitation solution because they stay in the intravascular space and limit the allograft edema. Colloid solutions such as hetastarch and pentastarch should be avoided.
The splanchnic vasculature is different from, and does not always correlate to, the systemic vasculature. A normal person can tolerate a 25% to 30% decrease in blood volume without a change in systemic blood pressure, but the splanchnic system becomes compromised after only a 10% to 15% reduction in intravascular volume due to vascular redistribution from the renin-angiotensin system.
Upon arrival of the liver transplant recipient in the ICU, clinicians assess the liver allograft using a combination of clinical parameters, laboratory values, and radiological studies. The clinical factors that indicate a functioning allograft include stable body temperature, improving urine output, improving mentation and wakefulness, normalization of respiratory effort, and if drains are present, a change from sanguinous/serosanguineous output to ascites.
The liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are wrongly termed “liver function tests,” because they do not measure liver function, but rather reflect hepatocyte cell death. Nonetheless, despite not measuring liver function, these liver enzymes can be useful in identifying the potential for allograft problems. During a standard posttransplant ICU course, the transaminase levels rise and peak during the first 2 days before declining. Several factors (size mismatch, increased steatosis, prolonged ischemia time) will significantly elevate AST and ALT levels, and if these levels resolve quickly, they are not a concern; however, liver enzyme levels that fail to decline after 2 days warrant further investigation. The canalicular enzymes γ-glutamyl transferase and alkaline phosphatase begin their rise approximately the fourth day after transplant and typically rise up to five times normal before declining.
The liver has synthetic, excretory, and metabolic functions, each of which can be assessed by particular laboratory values.
The allograft synthetic function is assessed by the prothrombin time or international normalized ratio (INR), which measures the extrinsic pathway of coagulation and the synthesis of coagulation factors by the liver. Albumin levels can also be used to assess synthetic function; however, its utility is limited because albumin levels are also affected by nutritional status, volume overload, and the fact albumin is a negative phase reactant.
The excretory and clearance function of the liver is measured by the bilirubin level, and stratification into direct and indirect levels provides further information.
The metabolic function of the liver is assessed by the serum glucose level, because the liver maintains blood glucose levels through glycogenolysis and gluconeogenesis, and very low levels of glucose refractory to treatment should raise concern about a nonfunctioning liver. The metabolic function can also be measured by lactate level, because lactate is converted to pyruvate in the liver via the Cori cycle. Elevations of lactate can indicate poor liver function; however, serum lactate is a balance between lactate metabolism and lactate production, so an elevated lactate level can also reflect increased production from peripheral tissue hypoxia, which would require a different intervention such as improving CO.
Radiological studies are integral to evaluating allograft function ( Fig. 69-2 ). Duplex ultrasonography (DUS) can assess the hepatic artery and portal vein inflow and the hepatic vein outflow. DUS is cost-effective, noninvasive, and can be performed at the bedside in the ICU; its main drawback is that it is operator dependent and is limited by the body shape. DUS is best considered as a screening tool, rather than a diagnostic study.
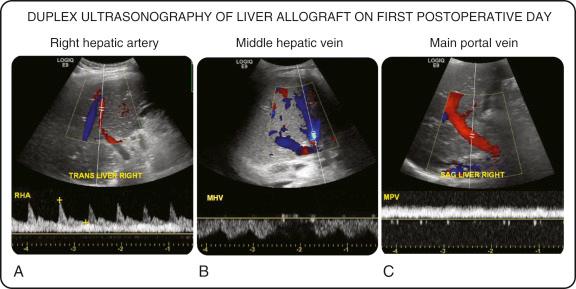
The resistive index (RI) assesses the ratio of the upstroke of the systolic wave in the hepatic artery to the end-diastolic flow rate, and normal initial posttransplant RI should be 0.6 to 0.9. DUS assesses the acceleration time (time from end-diastole to first systolic peak), which should be less than 0.08 seconds. Both left and right intrahepatic branches are evaluated because a normal waveform in the porta hepatis does not rule out an obstruction. An RI of less than 0.6 or an acceleration time of greater than 0.08 seconds is suggestive of insufficient hepatic arterial inflow (i.e., from hepatic artery stenosis, steal syndrome, or arcuate ligament syndrome) or hepatic artery thrombosis, and a very low RI of less than 0.4 or an absent hepatic artery Doppler signal is suggestive of hepatic artery thrombosis. A low RI should be investigated with an angiogram or surgical reexploration. An RI of greater than 0.9 is common in the early posttransplant period, due to increased hepatic artery resistance from allograft edema related to IR injury, and this should normalize in a few days as the edema resolves.
The portal vein is assessed by a color Doppler scan showing hepatopetal monophasic flow that varies with respiration, and portal vein complications will present as a lack of color Doppler signal. Portal vein flow velocity should be greater than 25 cm/sec, and low portal flows on DUS are predictive of portal vein complications. In adults the portal vein luminal diameter should be greater than 25 mm, without any acceleration of flow at any points of narrowing.
The duplex velocity waveform of the hepatic veins should show a vein flow velocity of greater than 10 cm/sec with a triphasic waveform (a large antegrade systolic and diastolic waveform with retrograde wave due to the backward transmission from right atrial pressure changes during the cardiac cycle). A loss of the triphasic waveform with persistent monophasic pattern is associated with hepatic vein complications such as stenosis. A flat waveform, with hepatic outflow velocity of less than 10 cm/sec along with dilation of the veins is associated with outflow obstruction.
In managing the initial allograft function upon a recipient’s arrival in the ICU, the clinician must consider the following elements: (1) pretransplant comorbidities, (2) donor quality, (3) operative course, (4) graft inflow hemodynamics, and (5) graft outflow hemodynamics. Most allograft complications are a function of one of these parameters, and they need to be either screened and predicted in advance or identified and monitored in the ICU ( Fig. 69-3 ).
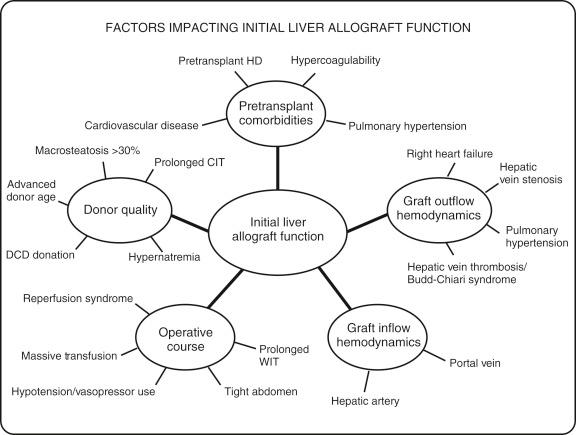
Pulmonary hypertension (PH), pretransplant hemodialysis, hypercoagulable state, and cardiovascular disease, are all preexisting factors that can negatively affect the initial allograft outcome. Knowing these comorbidities can prepare the clinician for potential complications and guide the studies and interventions required.
PH must be screened for, identified, and managed before transplant. If questions exist before transplant, then the pulmonary artery pressure needs to be measured before starting. The case should not proceed if the pressure is above 35 mm Hg and cannot be reduced with therapy, because there is a 36% intraoperative and early postoperative mortality risk due to right heart failure and allograft failure. The posttransplant ICU management of recipients with PH includes IV prostacyclins such as epoprostenol (Flolan), and care must be taken because epoprostenol can cause systemic hypotension. Posttransplant ICU management also requires aggressive fluid management, keeping the CVP low (<5 mm Hg) with a loop diuretic infusion or CRRT to get the CVP down to the required levels quickly.
Pretransplant hemodialysis (HD) is a predictor of primary nonfunction (PNF), and the aggressive use of intraoperative and postoperative CRRT should be initiated in the ICU to decrease the intravascular volume, decrease right heart pressure, improve the hepatic venous gradient flow, and ultimately improve perfusion to the liver.
It is rare for liver recipients to be hypercoagulable, but when the situation exists, appropriate prophylaxis should be given in the ICU to ensure no thrombosis occurs in the allograft. Consideration must be given to recipients with hepatocellular carcinoma (HCC) MELD upgrades, who often have a normal INR and normal platelets who can be hypercoagulable due to their malignancy. Treatment for the hypercoagulable recipient is aimed at preventing graft thrombosis using a heparin infusion, and carefully titrating the level of anticoagulation to the risk for postoperative bleeding based on the recipient's postoperative course.
Coronary artery disease, congestive heart failure, and valvular heart disease should be screened before transplant. Recipients having this preoperative history, along with the operative stress and ischemia from the surgery, have a potential for decreased CO after transplant that can limit perfusion of the allograft.
Donor quality has the greatest impact on allograft function, and in an age of organ supply shortages, marginal donors are routine. Although the donor risk index predicts overall survival based on organ quality, it is not as relevant for predicting the initial allograft outcomes in the ICU, because several parameters that strongly affect initial allograft function such as cold ischemia time and macrosteatosis are not part of the donor risk index calculation.
Cold ischemic time (CIT) of more than 12 hours is an independent predictor of preservation injury and PNF, with an incidence of 8% when CIT exceeds 12 hours. Donors with prolonged CIT suffer from increased IR injury particularly to the sinusoidal endothelial cells of the liver, and extracellular matrix, both of which are most sensitive to cold ischemia (hepatocytes are more sensitive to warm ischemia). They suffer damage upon reperfusion that contributes to microcirculatory disruption that can injure the hepatocytes and lead to PNF or initial poor function of the allograft. In addition, the IR injury can trigger apoptosis of the hepatocytes, which leads to necrosis of the liver. Many experimental therapies to limit IR injury have been studied in animals, but no clear therapy has been delineated. Prostaglandin E1 (Prostin 0.5 mcg/kg/hr) may be of some benefit in reducing IR injury in select cases with prolonged CIT, although randomized controlled trials in the general liver transplant population have not shown a clear benefit. N -acetylcysteine therapy (Mucomyst loading dose 140 mg/kg then 70 mg/kg for 17 doses) has also shown benefits to limit IR injury, improve liver function, and decrease the incidence of PNF in some reports, although other studies have shown no benefit. Although the benefit is not clear, N -acetylcysteine therapy is safe, and in select populations of prolonged CIT it may be warranted.
Macrosteatosis decreases the tolerance of the liver to IR injury, and livers with macrosteatosis of greater than 30% are at an increased risk for impaired function, rejection, and PNF. Fatty livers have reduced hepatic microcirculation and reduced sinusoid space, which limits perfusion of the graft and potentiates IR injury. Fatty livers synthesize less adenosine triphosphate (ATP) after reperfusion, and the ATP depletion contributes to proteolytic events, which lead to massive necrosis after reperfusion. Lower graft perfusion in steatotic livers contributes to poor outcomes. Steatotic livers will typically have higher peak transaminase levels that take longer to resolve. Treatment of the recipient of a fatty donor in the ICU involves keeping the CVP low (<5 mm Hg) with a loop diuretic infusion, or CRRT, to prevent graft congestion and to improve the hepatic venous gradient and maximize allograft perfusion.
Donor age has been steadily increasing over the last 25 years as the organ supply-demand issues have worsened. Allografts from donors who are more than 60 years old have increased risk for PNF and can also show a greater degree of delayed function from a cholestatic pattern. ICU management of the older donor allograft involves keeping the CVP low (<5 mm Hg) with a loop diuretic infusion, or CRRT, to prevent graft congestion and improve the hepatic venous gradient and maximize allograft perfusion.
Donation after cardiac death (DCD) donor livers have an additional level of ischemia—beyond the typical cold ischemia from storage and warm ischemia during implantation—due to the increased warm ischemia from the donor procurement. Hepatocytes are sensitive to warm ischemia (as opposed to the sinusoidal endothelial cells, which are more susceptible to cold ischemia), and so DCD livers can have an increased risk for PNF. The DCD donor liver also has an increased risk for graft thrombosis stemming from inadequate donor anticoagulation at procurement.
Donor sodium level of greater than 160 mmol/L has been associated with increased incidence of PNF, although more recent data question the association.
The operative course is a useful predictor of overall allograft function. Significant blood loss requiring massive transfusion, reperfusion syndrome, prolonged warm ischemia time, hypotension requiring pressors, and a tight abdomen upon closure are all intraoperative events that can affect the allograft function in the ICU.
Significant blood loss requiring massive transfusion has been shown to be a strong predictor of PNF. Posttransplant ICU management includes correction of coagulopathy that occurs after massive transfusion using fresh frozen plasma, platelets, and cryoprecipitate as well as treatment of electrolyte abnormalities, particularly hypocalcemia.
Intraoperative reperfusion syndrome has been reported to be strongly associated with PNF. Methylene blue inhibits nitric oxide synthetase and is a free radical scavenger that limits the impact of IR injury on allograft function. The evidence of benefit is contradictory; however, several reports and a randomized trial have shown methylene blue infusion in patients after reperfusion syndrome improves overall hemodynamics as well as allograft function. Treatment in the ICU for recipients having an intraoperative reperfusion syndrome is an infusion of methylene blue 2 mg/kg IV if it was not given intraoperatively.
Prolonged warm ischemia time (WIT) leads to IR injury and is associated with increased rates of initial poor function, PNF, and biliary strictures in the long term. Treatment for allografts with prolonged WIT includes infusion of N -acetylcysteine (Mucomyst loading dose of 150 mg/kg IV over 15 minutes, then 100 mg/kg IV infused over 16 hours). Studies show N -acetylcysteine can limit IR injury, improve liver function, and decrease the incidence of PNF. Although there are also studies that show no benefit, therapy with N -acetylcysteine is very safe and cost-effective and should therefore still be considered in cases with prolonged WIT regardless of the lack of clear evidence. Prolonged WIT can also be a marker of intraoperative complications that may have an independent impact on donor allograft function.
Hypotension during the transplant that responds poorly to pressors can lead to initial poor function and PNF in the posttransplant period. Methylene blue 2 mg/kg IV infusion can sometimes be effective in reversing the catecholamine-resistant hypotension.
In those recipients whose abdomen is tight at the time of closure, consideration must be given to potential compartment syndrome with increased edema after transplant. This compartment syndrome can lead to liver necrosis and allograft failure from impaired liver perfusion. The best strategy is to leave the patient’s abdomen open, possibly with a temporary skin closure and a delayed secondary closure with or without a mesh once intra-abdominal edema has decreased. Aggressive fluid management with IV infusions of a loop diuretic (Lasix infusion 40 to 100 mg/hr) or initiation of CRRT, along with PRBC transfusions to increase the oncotic pressure, can significantly decrease abdominal wall and intestinal edema and limit intra-abdominal pressure.
The liver allograft has two main sources of inflow—the hepatic artery and the portal vein—which must be considered in managing the initial liver allograft function.
Hepatic artery thrombosis and stenosis can present with marked elevation of transaminase levels (i.e., AST > 5000 International Units /L) and are screened by DUS, which assesses arterial flow by the RIs. The initial RI should be 0.6 to 0.9, and if RI is less than 0.5, one should pursue a hepatic artery angiogram or immediate surgical reexploration to verify certainty of arterial flow. One should have a very low threshold to investigate and treat any hepatic artery concerns, because permanent ischemic damage can occur to the biliary tree in only a matter of hours. Hepatic artery thrombosis may require urgent listing of the recipient for a retransplant.
Portal vein issues present with a massive transaminase increase and often have significant ascites, gastric bleeding, and variceal bleeding in the case of portal vein thrombosis. DUS is the test of choice to screen for portal vein issues. Portal vein issues can lead to rapid deterioration and need to be recognized early.
Graft outflow hemodynamics are probably the least appreciated parameters and yet are crucial for allograft function. The liver allograft outflow is via the hepatic vein, and good function of the allograft depends on maintaining a gradient of perfusion across the liver from the portal vein to the vena cava. Any factor limiting outflow affects the perfusion of the allograft.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here