Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The primary determinants of a cardiac operation's success are events in the operating room (OR), but even patients who are seriously ill when they leave the OR can survive and have a good long-term result when postoperative care is appropriate and intensive. Conversely, ill-advised or overly energetic interventions early after operation can put a patient at risk who would otherwise convalesce normally.
Normal convalescence is not normal physiology. For instance, care early after open intracardiac operations is complicated by the whole body inflammatory response to cardiopulmonary bypass (CPB). Currently, the major issue relating to abnormalities of postoperative convalescence is the degree of preoperative morbidity in terms of both circulatory derangements and comorbid subsystem abnormalities. Failure to realize that a patient undergoing CPB is, for a time afterward, in a special biological situation to which the knowledge and rules applicable to other humans may or may not apply can lead to conceptual and management errors as well as unnecessary tests and interventions.
Fortunately, despite these problems and thanks to expanding knowledge, postoperative care can and should be simple for many patients undergoing cardiac operations. A normal, uncomplicated convalescence devoid of findings or events that increase the probability of hospital death, complications, or a suboptimal late result is what patients can now expect. Generally, they have adequate function of all subsystems, as determined by standard criteria. So long as this pattern of normal convalescence continues, testing and intervention can be safely minimized. In these situations, expeditious discharge from the intensive care unit (ICU) can be accomplished and a short subsequent hospital stay anticipated.
But alertness to deviations from the pattern of an uncomplicated convalescence is mandatory; deviations are an indication for closer observation and possibly more intensive testing and treatment. Analysis of early convalescence can place the patient into one of three categories: optimal, suboptimal but in control, and critically ill. Each category carries therapeutic implications.
Optimal: routine care; no change or important modification is currently necessary or foreseeable.
Suboptimal but in control: careful consideration is given to a change in therapy, and a new modality is likely (e.g., additional catecholamine support for low cardiac output or lidocaine drip for frequent premature ventricular contractions [PVCs]).
Critically ill: a modification, change, or new intervention is necessary and urgent (e.g., treatment of oliguria or metabolic acidosis; return to the OR for bleeding).
Both the suboptimal and critically ill categories define abnormal convalescence.
The patient convalescing normally and without complications after cardiac surgery usually appears at a glance to be doing well. Although there is always pain, varying in intensity from patient to patient, there is no restlessness, agitation, or anxiety. Eyes and skin look normal, and the pulse is full but may be rapid. Breathing is neither labored nor excessively rapid. The patient is oriented and lucid and—whether a neonate, infant, or adult—exhibits generally appropriate behavior. Few tests and interventions are needed.
When convalescence is abnormal, observations and interventions must be intensive and at times complex. In these situations in particular, care must be well organized and follow specific patient-management protocols that allow all members of the intensive care team to be clear about details of management.
Use of protocols is facilitated by considering the patient to be a complex, integrated system composed of a number of separate but interrelated subsystems (i.e., cardiovascular, pulmonary, renal, nervous, gastrointestinal). Care of such a patient can be accomplished effectively using a “subsystems analysis” approach. This analysis begins in the OR as CPB is discontinued (see Chapter 2, Chapter 4 ) and continues into the early and late postoperative period. This is not to say that care can be carried out in an automatic fashion. Optimal postoperative care requires overall direction by a knowledgeable and experienced physician using, when indicated, specialized methods of securing information and the skills of personnel in a dedicated cardiovascular ICU.
Management of patients after cardiac surgery has become in some institutions a specialty of its own. Literature on the subject abounds. Around the world, numerous institutions with extensive experience in the surgery of both congenital and acquired heart disease have developed their own protocols and specific systems of management. These include “fast track” protocols and critical pathways that integrate the goals of all caregivers and other interested parties. Within the context of these developments, this chapter discusses general principles, along with enough specific details to be helpful to those desiring to change or develop their own protocols.
Cardiac reserve is the capacity to increase (or at least maintain) cardiac output as a response to a variety of stressful sudden developments, including increased total body oxygen consumption ( ![]() ), increased ventricular afterload, and decreased ventricular preload. Providing that capacity are all the cardiac and extracardiac mechanisms for maintaining and increasing the force of ventricular contraction and cardiac output. Most of these reside in myocardial contractility and coronary blood flow. 1 In patients convalescing from cardiac surgery, adequacy of cardiac performance alone is insufficient for a high probability of normal convalescence and survival. There must, in addition, be adequacy of cardiac reserve.
), increased ventricular afterload, and decreased ventricular preload. Providing that capacity are all the cardiac and extracardiac mechanisms for maintaining and increasing the force of ventricular contraction and cardiac output. Most of these reside in myocardial contractility and coronary blood flow. 1 In patients convalescing from cardiac surgery, adequacy of cardiac performance alone is insufficient for a high probability of normal convalescence and survival. There must, in addition, be adequacy of cardiac reserve.
1 The familiar exercise stress test is a test for cardiac reserve.
Inadequacy of cardiac reserve may become apparent only during periods of increased
![]() (from struggling or hyperthermia), suddenly increased ventricular afterload (from paroxysmal pulmonary arterial hypertension in a neonate), or acute reduction in ventricular preload (from sudden blood loss). Such inadequacies of cardiac reserve probably explain “sudden death” occurring early after cardiac surgery.
(from struggling or hyperthermia), suddenly increased ventricular afterload (from paroxysmal pulmonary arterial hypertension in a neonate), or acute reduction in ventricular preload (from sudden blood loss). Such inadequacies of cardiac reserve probably explain “sudden death” occurring early after cardiac surgery.
Cardiac reserve is highly dependent on the preoperative condition of the patient. When, because of disease, reserves are being nearly fully utilized to maintain adequate cardiac performance in nonstressful situations, that which remains may be insufficient to successfully meet the stresses of the intraoperative and postoperative period. Reserves probably cannot be increased before the operation unless they are acutely impaired by a reduced myocardial energy charge. Energy charge may be increased by the cardioplegic technique used in the OR (see “Cold Cardioplegia, Controlled Aortic Root Reperfusion, and [When Needed] Warm Cardioplegic Induction” under Methods of Myocardial Management during Cardiac Surgery in Chapter 3 ).
Limited cardiac reserves are specifically compensated for by many features of early postoperative care.
Although not often conceptualized and not specifically measurable, adequacy of blood flow (cardiac output) in meeting the patient's needs during recovery from cardiac surgery is the central issue with respect to the cardiovascular subsystem. Arteries and veins are infrequently the primary limiting factors, so emphasis is on adequacy of performance of the heart itself in providing adequate blood flow to the body.
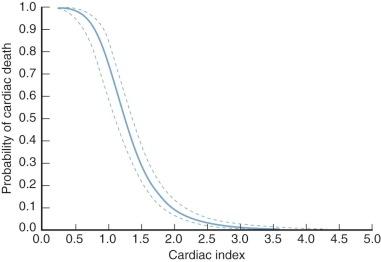
Cardiac index (cardiac output expressed as L · min −1 · m −2 ) is one measure of adequacy of the cardiovascular subsystem, as evidenced by the oft-demonstrated relation between cardiac index and survival (described by Dietzman and colleagues in 1969 ). In adults, a cardiac index of at least 2.0 L · min −1 · m −2 during the first few hours in the ICU and one of at least 2.4 on the morning after operation are required for normal convalescence ( Fig. 5-1 ). This is at the lower end of the range of normal, which is 2.2 to 4.4. Infants and small children appear, in general, to require a somewhat higher cardiac index for normal convalescence ( Fig. 5-2 ). Also, in young patients, cardiac index tends to be lower about 4 hours after operation than it was soon after discontinuing CPB, and then begins to rise after 9 to 12 hours.
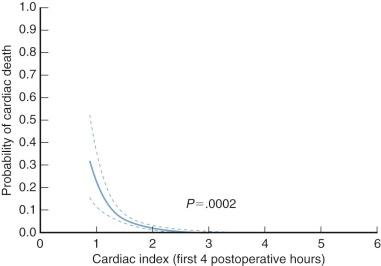
Cardiac indices below these values are usually inadequate for maintaining a normal convalescence; this can be formalized in the inverse relation between cardiac index early postoperatively and the probability of hospital death. This relation can be refined by considering not only cardiac output but also mixed venous oxygen levels, with lower levels worsening prognosis at any given value of cardiac output. 2
2 Although dye dilution or thermodilution is the standard for measuring cardiac output, caution is required in interpreting measurement values. In small individuals, confidence intervals around the measurement may be large; low core temperature may diminish accuracy; and tricuspid regurgitation, absence of an adequate mixing chamber, lack of steady state, and intracardiac shunts (as originally articulated by Stewart and Hamilton ) invalidate the measurement.
Arterial blood pressure is an insensitive method of estimating adequacy of cardiac output early postoperatively, primarily because systemic vascular resistance (Rs) is usually elevated. This may be related to increased levels of circulating catecholamines, plasma renin, angiotensin II, or other mechanisms. This high resistance may result in a normal or high arterial blood pressure even when cardiac output is low.
Some patients tend early postoperatively to have low Rs and arterial blood pressure, even when cardiac performance is good. This may occur more frequently in children with cyanotic heart disease, adults with diabetes, and patients with sepsis or drug interactions (especially preoperative use of angiotensin-converting enzyme [ACE] inhibitors). Arterial hypotension is an indication for thoughtful evaluation. Children cannot be considered to be convalescing normally when mean arterial blood pressure is lower than about 10% below normal for the patient's age ( Table 5-1 ). For adults, particularly the elderly, arterial blood pressure may mandate maintenance at or above commonly accepted normal values to ensure adequate perfusion of various organs like the brain, viscera, and kidneys.
| Age | Systolic Pressure/ Diastolic Pressure (mmHg) | Mean a (mmHg) | 10% > Mean Normal Value (mmHg) | 10% < Mean Normal Value (mmHg) | |
|---|---|---|---|---|---|
| ≤Years | <Years | ||||
| 0.5 | 80/46 | 57 | 63 | 51 b | |
| 0.5 | 1.0 | 89/60 | 70 | 77 | 63 |
| 1.0 | 2.0 | 99/64 | 76 | 84 | 68 |
| 2.0 | 4.0 | 100/65 | 77 | 85 | 69 |
| 4.0 | 12.0 | 105/65 | 78 | 86 | 70 |
| 12.0 | 15.0 | 118/68 | 85 | 94 | 74 |
| 15.0 | 120/70 | 87 | 96 | 78 | |
a Mean arterial blood pressure has been calculated as the diastolic pressure plus one third of the pulse pressure.
Simple observation of pedal pulses is a commonly used, useful, but not infallible method of estimating adequacy of cardiac output in children and young adults. Normal (grade 4) pedal pulses early postoperatively are highly but not perfectly correlated with adequate cardiac output and a high probability of survival. In older adults, estimation of the adequacy of perfusion by amplitude of pedal pulses is often confounded by the presence of peripheral arterial occlusive disease.
Skin temperature in the foot is another indirect but reasonably reliable estimator of adequacy of cardiac output. A study of cardiac surgery in infants younger than 3 months of age indicated that pedal pulses and skin temperature predicted probability of hospital death from cardiac causes and thus were reasonably good estimators of adequacy of cardiac output. As with assessment of pedal pulse amplitude, in older adults skin temperature offers guidance but not solid evidence for adequacy of perfusion.
Whole body ![]() is infrequently calculated, but knowledge of it is useful; in some circumstances, it is a better basis for prognostic and therapeutic inferences than cardiac output or mixed venous oxygen levels. Whole body
is infrequently calculated, but knowledge of it is useful; in some circumstances, it is a better basis for prognostic and therapeutic inferences than cardiac output or mixed venous oxygen levels. Whole body ![]() can be calculated by a rearranged Fick equation, 3 which states:
can be calculated by a rearranged Fick equation, 3 which states:
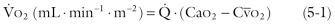
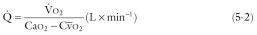
where ![]() is cardiac output in L · min −1 ,
is cardiac output in L · min −1 , ![]() is oxygen consumption in mL · min −1 , Ca o 2 is arterial O 2 content in mL · L −1 , and
is oxygen consumption in mL · min −1 , Ca o 2 is arterial O 2 content in mL · L −1 , and ![]() is mixed venous O 2 content in mL · L −1 . The oxygen content of blood consists of combined plus dissolved oxygen:
is mixed venous O 2 content in mL · L −1 . The oxygen content of blood consists of combined plus dissolved oxygen:
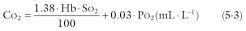
where Hb is hemoglobin concentration in g · L −1 , S o 2 is O 2 saturation as a percentage of capacity, P o 2 is O 2 tension in mmHg, 1.38 is effective O 2 capacity of 1 g of hemoglobin in mL · g −1 , and 0.03 is solubility of O 2 in blood at 37°C in mL · L −1 · mmHg −1 . Rearranging equation 5-2 and substituting for Ca o 2 from equation 5-3,
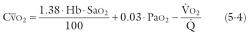
where Sa o 2 is arterial O 2 saturation as a percentage of capacity, and Pa o 2 is arterial O 2 tension in mmHg. For a given ![]() , equation 5-4 indicates that to increase
, equation 5-4 indicates that to increase ![]() , there must be an increase in Sa o 2 , Pa o 2 , Hb, or
, there must be an increase in Sa o 2 , Pa o 2 , Hb, or ![]() .
. ![]() varies directly with
varies directly with ![]() , and
, and ![]() varies directly with cellular P o 2 , unless there is shunting in the peripheral circulation. Thus, to maximize tissue oxygen supply, there must be an increase of one or more of the following: Pa o 2 (and thus Sa o 2 ), Hb, or
varies directly with cellular P o 2 , unless there is shunting in the peripheral circulation. Thus, to maximize tissue oxygen supply, there must be an increase of one or more of the following: Pa o 2 (and thus Sa o 2 ), Hb, or ![]() .
.
The normal value for
![]() at 37°C is 155 mL · min −1 · m −2 . The value for whole body
at 37°C is 155 mL · min −1 · m −2 . The value for whole body
![]() in the patient recovering from cardiac surgery must be interpreted in light of his or her body temperature; residual hypothermia is the most common explanation for the somewhat low
in the patient recovering from cardiac surgery must be interpreted in light of his or her body temperature; residual hypothermia is the most common explanation for the somewhat low
![]() usually present within the first few hours after open heart surgery. This reduced
usually present within the first few hours after open heart surgery. This reduced
![]() is in part due to reduced capillary density (reduced area of capillary flow) and increased heterogeneity of capillary flow through the muscle mass and other tissues of the body in the early hours after CPB. Normally convalescing patients operated on with hypothermic CPB generally require 4 to 8 hours for this to disappear and their peripheral perfusion to return to normal.
is in part due to reduced capillary density (reduced area of capillary flow) and increased heterogeneity of capillary flow through the muscle mass and other tissues of the body in the early hours after CPB. Normally convalescing patients operated on with hypothermic CPB generally require 4 to 8 hours for this to disappear and their peripheral perfusion to return to normal.
When ![]() is appreciably reduced below the normal level for the existing body temperature, a hazardous condition exists; indeed, one useful definition of shock is “a condition characterized by an acute reduction in
is appreciably reduced below the normal level for the existing body temperature, a hazardous condition exists; indeed, one useful definition of shock is “a condition characterized by an acute reduction in ![]() .” Abnormally low
.” Abnormally low ![]() may result from reduction or extreme heterogeneity of capillary flow (of which “no reflow” is an extreme example) in one or more organs of the body (sometimes termed a reduction in capillary density ), lengthening of the diffusion path between capillaries and cells, or intracellular metabolic derangement. One or all of these may exist in patients early after cardiac surgery. When important reduction in
may result from reduction or extreme heterogeneity of capillary flow (of which “no reflow” is an extreme example) in one or more organs of the body (sometimes termed a reduction in capillary density ), lengthening of the diffusion path between capillaries and cells, or intracellular metabolic derangement. One or all of these may exist in patients early after cardiac surgery. When important reduction in ![]() , considering the temperature, persists for more than a few hours, probability of death increases.
, considering the temperature, persists for more than a few hours, probability of death increases.
Mixed venous oxygen level, generally expressed as oxygen tension ( ![]() ) or saturation (
) or saturation ( ![]() ), is a useful index of circulatory adequacy, because it reflects to some extent mean tissue oxygen levels. When
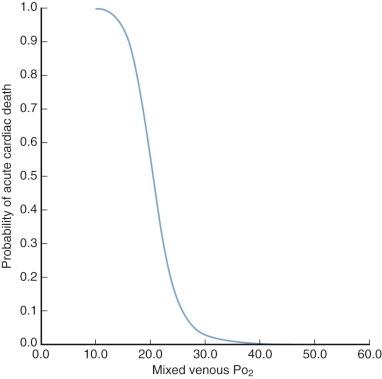
), is a useful index of circulatory adequacy, because it reflects to some extent mean tissue oxygen levels. When ![]() is less than 30 mmHg, cardiac output is likely to be inadequate; when it is below about 23 mmHg, the inadequacy is apt to be severe ( Fig. 5-3 ). However, normal or near-normal venous oxygen levels are not reassuring as to the adequacy of cardiac output, unless it is known that
is less than 30 mmHg, cardiac output is likely to be inadequate; when it is below about 23 mmHg, the inadequacy is apt to be severe ( Fig. 5-3 ). However, normal or near-normal venous oxygen levels are not reassuring as to the adequacy of cardiac output, unless it is known that ![]() is approximately normal for the existing body temperature.
is approximately normal for the existing body temperature.
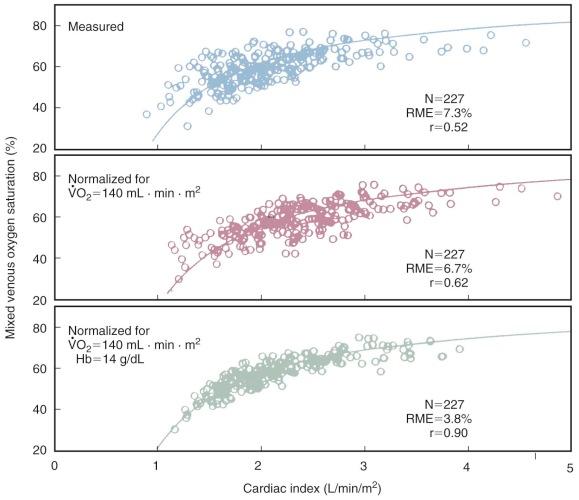
In a nonsurgical but critically ill ICU population, Jain and colleagues found a weak relationship between ![]() and cardiac index. There was considerable variability among all patients in various conditions, but when normalized for
and cardiac index. There was considerable variability among all patients in various conditions, but when normalized for ![]() and hemoglobin concentration, the correlation coefficient improved ( Fig. 5-4 ). Using indwelling fiberoptic reflectance oximetry, other investigators have found no or only a very weak relationship between
and hemoglobin concentration, the correlation coefficient improved ( Fig. 5-4 ). Using indwelling fiberoptic reflectance oximetry, other investigators have found no or only a very weak relationship between ![]() or
or ![]() and measured cardiac index. Following CPB, changes in
and measured cardiac index. Following CPB, changes in ![]() may be useful in detecting low cardiac output, one of many causes of decreased oxygen delivery. Identifying decreases may be of particular value in patients coming to surgery with a high severity of illness index. Online continuous oximetry is useful in critically ill patients because it reflects unanticipated events or occasionally the usefulness (or nonusefulness) of therapeutic maneuvers. These aspects of postoperative care illustrate the need for uniformity in monitoring techniques and highlight the conflict between overreliance on devices and the ability to form accurate estimates (in this case, of cardiac performance) based on previous correlations. However, many surgeons rely on continuous
may be useful in detecting low cardiac output, one of many causes of decreased oxygen delivery. Identifying decreases may be of particular value in patients coming to surgery with a high severity of illness index. Online continuous oximetry is useful in critically ill patients because it reflects unanticipated events or occasionally the usefulness (or nonusefulness) of therapeutic maneuvers. These aspects of postoperative care illustrate the need for uniformity in monitoring techniques and highlight the conflict between overreliance on devices and the ability to form accurate estimates (in this case, of cardiac performance) based on previous correlations. However, many surgeons rely on continuous ![]() monitoring to detect deviations from normal convalescence early postoperatively. Catheters that allow measurement of both
monitoring to detect deviations from normal convalescence early postoperatively. Catheters that allow measurement of both ![]() and cardiac output are optimal.
and cardiac output are optimal.
Urine flow and serum potassium levels are useful indirect guides to the adequacy of cardiac output. Early postoperative oliguria suggests inadequate cardiac output and thus is often an indication for treatment of the cardiovascular subsystem. Hyperkalemia rising over a 4-hour period (with sampling every 2 hours) to a level of about 5 mEq · L −1 is a sensitive indicator of a low or falling cardiac output in neonates and infants, and hence an indication for intensifying treatment. Hyperkalemia is usually accompanied by a fall in pedal skin temperature and a rise in esophageal temperature, but it often precedes the appearance of a base deficit or of arterial hypotension.
A frequently used but somewhat nonspecific and insensitive indicator of the adequacy of cardiac output is the acid-base status of blood. Metabolic acidosis during and after cardiac surgery is almost always a result of lactic acidemia. Lactate production is a byproduct of anaerobic metabolism, which most often occurs under conditions in which cardiac output and oxygen consumption are suboptimal. Occasionally, excess lactate may occur with high measured cardiac output under conditions of high metabolic rate, diabetes, sepsis, or intestinal ischemia.
Concentration of lactic acid in blood may be measured directly. Normally, little or none is present, normal values in plasma being 0.7 to 2.1 mEq · L −1 . A concentration of about 5 mEq · L −1 correlates in general with moderate metabolic acidosis, and one of 10 mEq · L −1 with severe metabolic acidosis and usually markedly reduced cardiac output. Moderate elevation of lactic acid concentration is a common finding early after cardiac surgery, but in the normally convalescing patient, lactic acid gradually declines to normal values within 12 to 24 hours.
When arterial pH is less than about 7.4, acidosis is present but may be the result of retention of carbon dioxide; this is reflected in an arterial Pa co 2 greater than 40 mmHg. Alternatively, acidosis may be “metabolic” and due primarily to accumulation of lactic acid. Quantification of metabolic acidosis is expressed by a derived value obtained from an equation after measuring arterial pH and Pa co 2 . Either the buffer base deficit or the standard bicarbonate is calculated. Most commercially available equipment calculates the buffer base deficit or excess (which takes account of the buffering capacity of blood as well as that of bicarbonate) after measuring Pa o 2 , Pa co 2 , and pH of whole blood. The normal buffer base is about 48 mEq · L −1 , and the normal base excess or deficit is 0.
The cardiac index in normally convalescing adults is often 2.5 to 3.5 L · min −1 · m −2 after cardiac surgery performed with modern methods of myocardial management. It is generally higher 4 to 6 hours after operation than it is in the OR and still higher the next day, although exceptions occur. Even in patients who convalesce well, some variability in cardiac output occurs.
Risk factors for low cardiac output seem primarily to be those that affect cardiac output in the OR, which in turn is strongly correlated with cardiac output 4 to 6 hours later and the next day. Cardiac output after operations using CPB is usually correlated with age of the patient (older patients have lower output), cardiac condition, functional state of the patient just before operation (the higher the New York Heart Association [NYHA] class, the lower the output), duration of CPB, and duration of global myocardial ischemia. During the early postoperative period, a heart rate within usual ranges correlates directly with cardiac output, and arterial blood pressure within usual ranges correlates inversely with it. Within the usual ranges, the higher the atrial pressures, the higher the cardiac output ( Fig. 5-5 ).
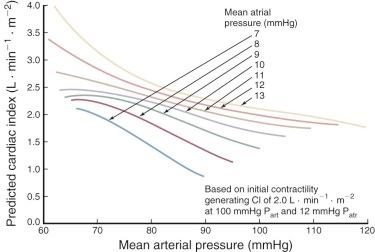
Determinants of cardiac output are ventricular preload, afterload, myocardial contractility, and heart rate. Most normally convalescing patients require no special measures to adjust these fundamental determinants; patients with impaired or inadequate cardiac performance require at least adjustment of preload and afterload, and at times adjustment of heart rate and/or pharmacologic or interventional augmentation of contractility. In many patients who have undergone cardiac surgery, it is specifically either the left (LV) or the right ventricle (RV) that limits cardiac output, less commonly both (see “ Relative Performance of Left and Right Ventricles ” later in this section).
Ventricular preload, which is correlated directly with the force of contraction, is equated with sarcomere length at end-diastole, and thus with change in ventricular volume between end-systole and end-diastole. This volume change is determined by transmural pressure during diastole, compliance and thickness of the ventricular wall, and curvature of the wall (La Place effect). Transmural geometric arrangement of fibers also plays a role but changes little during the postoperative period.
Transmural pressure is determined by intraventricular pressure and intrapericardial pressure. Intraventricular pressure at end-diastole (which is a determinant of the force of contraction) is related to phasic changes in atrial pressure, and these are affected by blood volume and systemic venous capacitance. The latter is decreased early after CPB. Because transmural pressure is affected by intrapericardial pressure, it is affected by closure of the pericardium and sternum, both of which increase intrapericardial pressure and decrease transmural pressure. Daughters and colleagues have demonstrated that pericardial closure in the setting of cardiac surgery, both itself and independent of sternal closure, increases intrapericardial pressure, decreases transmural pressure, and unfavorably affects cardiac performance. Changes in myocardial compliance during and after cardiac operations are due primarily to changes in myocardial water content.
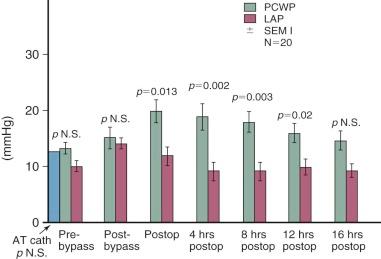
After cardiac surgery in patients with normal atrioventricular (AV) valves, most acute changes in preload are equated with acute changes in mean left (in the case of the LV) or right (in the case of the RV) atrial pressure. This is because in this setting, and when the atria are functioning normally as reservoirs, ventricular end-diastolic pressure is similar to the mean pressure in the corresponding atrium. Therefore, mean atrial pressure is measured in cardiac surgical patients to deduce ventricular end-diastolic pressure. Right atrial pressure is usually measured using a fine polyvinyl catheter introduced through the right atrial appendage or internal jugular vein. Left atrial pressure is measured through a fine catheter introduced through the right superior pulmonary vein (or the left atrial appendage in neonates and young infants). In the absence of pulmonary vascular disease and important pulmonary congestion or edema, pulmonary artery diastolic pressure is a reasonable approximation of left atrial pressure. In most adult patients following CPB, pulmonary capillary wedge pressure exceeds left atrial pressure, and this discrepancy increases through the twelfth postoperative hour. It is thought this difference is due to accumulation of interstitial lung water ( Fig. 5-6 ).
In the intact ventricle, afterload is defined as systolic wall stress. This is the analog of the load that resists shortening in the isolated papillary muscle. Other things being equal, increased afterload results in decreased stroke volume. In the intact ventricle, afterload is related to (1) ventricular transmural pressure during systole, (2) ventricular wall curvature as determined by ventricular volume (La Place effect), (3) ventricular wall thickness, and (4) shape of the ventricle.
Ventricular wall determinants of afterload change little during and early after operations. Instead, acute changes in afterloads of the LV and RV are usually produced by changes in intraventricular pressures during systole. These changes are equated with changes in proximal aortic and pulmonary arterial systolic pressures. During and early after operation, proximal pulmonary arterial pressures may be monitored directly, but proximal aortic pressures are not. They must be inferred from measured radial (or femoral) artery pressures. Because of the many determinants of the magnitude of systolic amplification, systolic blood pressure at the radial artery is usually higher than in the ascending aorta, except in the situation of peripheral vasoconstriction secondary to low cardiac output or high-dose α-adrenergic agents. In most instances, systolic pressure variability between the aorta and peripheral arteries is not clinically important, but an awareness of it is advantageous in some situations. Mean pressures are similar in the two areas.
A tendency toward arterial hypertension is present in many adult patients early postoperatively, related to increased systemic arteriolar resistance. This complication (1) increases ventricular afterload and thereby decreases stroke volume, (2) increases aortic wall tension and thereby increases the likelihood of tearing the aortic purse-string sutures and suture lines, and (3) increases LV metabolic demands that exacerbate any latent myocardial ischemia. An appropriate criterion for treatment to lower arterial blood pressure in this setting is a mean arterial blood pressure 10% above the normal value. Mean arterial blood pressure, not systolic pressure, is monitored for this purpose because of the interrelations between peripheral and central arterial pressures discussed earlier. However, the patient's preoperative blood pressure must be taken into account, and to avoid cerebral complications, markedly hypertensive patients must not be rendered hypotensive. In the ICU, sodium nitroprusside is generally used for this purpose (see Appendix 5A ), but nitroglycerin may be preferred when myocardial ischemia is present, because it decreases coronary resistance. Negative intrathoracic pressure also increases LV load resisting shortening by increasing LV transmural pressure. Positive-pressure ventilation negates this effect, but labored spontaneous ventilation may augment afterload, and this may decrease cardiac output.
When a change in stroke volume cannot be explained by a change in end-diastolic fiber length (preload) or load resisting shortening (afterload), it is considered to result from a change in the contractile state. Contractility in a given ventricle can be acutely depressed or increased. When an attempt is made to compare ventricular contractility from patient to patient, and from time to time in the same patient, problems arise. A papillary muscle that is twice as thick as others might appear to have twice the contractility when studied in the usual way. In the ventricle, and at least theoretically in papillary muscle, data interpreted in terms of contractility must be normalized according to muscle thickness and length.
In vivo assessment of myocardial contractility and the resultant quantification of ventricular pump function are desirable goals postoperatively. The simplest representation of the capacity of the heart as a pump is a determination of any of several modifications of the Frank-Starling mechanism. For instance, the measured change in cardiac output (or stroke volume) with aliquot infusions of blood or blood substitute serves as a surrogate for assessment of contractile function. Clearly this is not reflective of intrinsic contractile properties of the myocardium, because the pressure-volume relationship is affected not only by preload but also by load resisting shortening, myocardial compliance, and intact vagal and sympathetic reflex activity. Changes in the instantaneous ventricular pressure (or aortic pressure) over time, dp/dt, may reflect myocardial contractility, but this quantity is exquisitely sensitive to afterload and preload and cannot be assumed to be an index of contractility that can be transferred from one patient to another or within the same patient over a period of time.
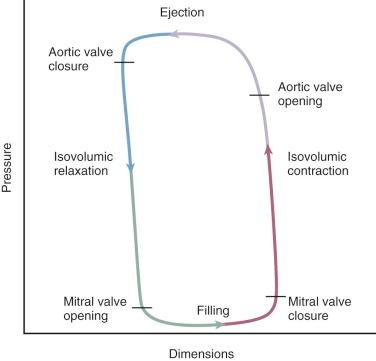
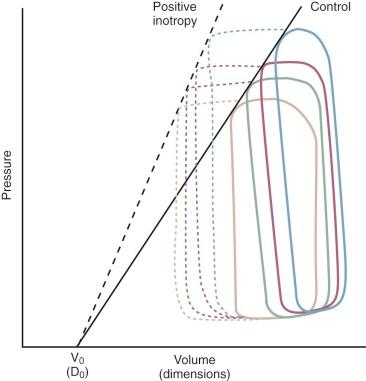
The relationship between ventricular pressure and volume (pressure-volume loop) is currently the nearest approximation to an in vivo assessment of contractility. Additionally, the area within the loop represents stroke work. The end-systolic pressure and the pressure at end-diastole of several different loops allow an expression of contractility and stiffness, respectively. The loops are composed of four segments: isovolumic contraction, ejection, isovolumic relaxation, and filling ( Fig. 5-7 ). When ventricular volume or resistance is altered, a group of points at end-systole fall along a line, the slope of which Suga and colleagues called Emax . Emax is an index of contractility ( Fig. 5-8 ). Changes of the slope in a steeper direction reflect increased inotropy. A shift in the rightward direction represents negative inotropy. With the use of catheters to measure LV pressure and transesophageal echocardiography (TEE) for instantaneous border detection, pressure-volume loops and Emax (contractility at zero volume) can be interpreted online in the ICU. It is paradoxical that the least clearly and directly defined determinant of the force of cardiac contraction is the one most discussed and treated. Its specific treatment is by the administration of inotropic drugs, usually catecholamines (see “ Treatment of Low Cardiac Output ” later in this section).
During and early after cardiac operations, one of the two ventricles is usually the factor limiting cardiac performance, not both. It is usually advantageous to consider and treat patients with this concept clearly in mind. The clue of greatest importance in this regard, when the AV valves are normal, is the relation between the left and right atrial pressures, because they represent the closest approximation available to ventricular end-diastolic pressure and, by implication, sarcomere length. When the cardiac valves are normal, the ventricle with the highest corresponding atrial pressure is the one limiting cardiac performance. Echocardiography can often provide supportive information.
Sinus rhythm is optimal postoperatively, and with this rhythm a wide range of heart rates at various ages is compatible with survival ( Table 5-2 ). The normal compensatory response to increased O 2 demand is increased heart rate. Often in the elderly and also in patients with diseased myocardium, this response is absent. It is prudent to manipulate heart rate in otherwise normally convalescing patients with slow sinus (or junctional) rhythm to improve cardiac output. For this, atrial pacing via two temporary atrial leads placed at operation is used. In these situations, atrial pacing is also helpful to suppress premature beats (both atrial and ventricular) and may limit the onset of an established arrhythmia.
| Age | Heart Rate (beats · min −1 ) | |
|---|---|---|
| ≤Years | <Years | |
| 1/12 | 120-190 | |
| 1/12 | 6/12 | 110-180 |
| 6/12 | 12/12 | 100-170 |
| 1 | 3 | 90-160 |
| 3 | 6 | 80-150 |
| 6 | 15 | 80-140 |
| 15 | 70-130 | |
Disturbances of cardiac rhythm may also contribute to low cardiac output. Junctional (AV nodal) rhythm reduces cardiac output by 10% to 15%. Junctional rhythm is less efficient than sinus rhythm because the atrial contribution to ventricular filling is absent in the former. Because junctional rhythm is usually transient and its effects are easily overcome by atrial pacing (unless the rate is rapid), its presence does not connote an added immediate risk.
Bradyarrhythmias due to damage to the AV node or His bundle, hypoxemia, or drugs can result in low cardiac output. Tachyarrhythmias in the form of atrial fibrillation or flutter or paroxysmal atrial tachycardia may result in hypotension. Risk of tachyarrhythmias increases during infusion of catecholamines. A complete discussion of postoperative rhythm disturbances and their treatment is found later in this section under “Cardiac Arrhythmias.”
The surgeon's responsibility for obtaining an adequate operation demands that he or she continue to search for evidence of this postoperatively, particularly when the patient has low cardiac output. Using the methods described in this and other chapters, a search is made for residual intra- or extracardiac shunting, pathway obstructions, valvar regurgitation, graft or conduit dysfunction, or cardiac compression. If the operation is found to be inadequate in any of these regards, prompt reoperation is usually indicated.
Myocardial dysfunction was once thought to explain low cardiac output after cardiac surgery when atrial pressures were elevated above the usual postoperative values in the absence of any other explanation. The availability of two-dimensional echocardiography in the OR and ICU, particularly TEE, makes possible both direct demonstration of ventricular wall motion and assessment of end-diastolic and end-systolic volumes. These studies can lead more directly to the inference that low cardiac output is due to myocardial necrosis or stunning or to impaired cardiac reserve in the face of increased stress. This inference can be supported by the finding of increased creatine kinase (CK)-MB isoenzyme or troponin in the serum.
The most common cause of reduced preload is overlooked hypovolemia. This may be a relative intravascular loss secondary to vasodilatation, bleeding into undrained cavities (pleural spaces, retroperitoneum, or free peritoneal space), or uncharted chest tube drainage. The most obvious cause of hypovolemia, of course, is bleeding associated with cardiotomy or CPB, reflected by excessive chest tube output. Low cardiac output or low arterial blood pressure associated with low filling pressures (left or right atrial pressure, central venous pressure, or pulmonary capillary wedge pressure) is the sine qua non of hypovolemia. Echocardiography showing vigorous wall motion and small chamber size is simply confirmatory.
Occasionally there is a sympathetic response that supports blood pressure, but often this is blunted early after anesthesia. There may be reflex tachycardia, but ultimately cardiac output suffers.
Infrequently, excessive diuresis leads to relative hypovolemia and lowering of cardiac output. In this instance, the picture may be complicated by hypokalemia leading to arrhythmias.
In the presence of LV hypertrophy, fibrosis, or myocardial edema, filling pressures do not reflect ventricular volume. In this situation, ventricular compliance is diminished. The root problem is inadequate resting (diastolic) sarcomere length. In these situations, echocardiography is especially useful. The picture is characterized by a small ventricular chamber in the presence of high filling pressure, tachycardia, small stroke volume, low arterial blood pressure, and low cardiac output. Appropriate interventions should be aimed at decreasing heart rate, initiating β-blockade, and subsequent volume infusion. Some inotropic agents may be detrimental in this situation.
Acute pericardial tamponade (with its resultant acute decrease in ventricular preload in the face of elevated atrial pressures) must always be considered when low cardiac output is present early postoperatively. Undrained intrapericardial bleeding may cause acute cardiac tamponade. It may also occur as a result of marked myocardial edema and chamber dilatation inside the closed chest, because the pericardium can be constricting under these circumstances even when it has not been resutured. Acute dilatation of the RV during an acute pulmonary hypertensive crisis may result in acute atypical tamponade in neonates and infants. It is these phenomena that explain the advantage of leaving the sternum open and covering the mediastinum with an impermeable sheet sutured to the skin edges in critically ill patients, or of opening it in the ICU when this form of cardiac tamponade is limiting cardiac output.
After an early period of adequate and stable cardiac output, cardiac tamponade is a likely cause of rapid deterioration that cannot be easily explained otherwise. It is usually associated with rapidly rising right and left atrial pressures that often, but not always, equalize. Often, drainage from chest tubes is initially brisk and then ceases, and serial chest radiographs show progressive widening of the cardiac and superior mediastinal shadows. Arterial pressure falls, and a paradoxical pulse may be replaced by a narrow pulse pressure. Characteristically, arterial pressure shows a minimal response to a bolus injection of an inotrope. TEE examination is indicated as soon as cardiac tamponade from retained intrapericardial blood is suspected, and is often diagnostic.
Cardiac tamponade can also manifest in multiple atypical presentations that must always be considered when acute low cardiac output develops. For example, right and left atrial pressures may differ widely in the setting of impacted clot adjacent to the right atrium. Neither TEE nor transthoracic echocardiography (TTE) is reliable for detecting this impacted clot, creating the potential for misdiagnosing tamponade as acute RV failure secondary to other causes such as pulmonary hypertension. Therefore, when the diagnosis of cardiac tamponade is considered as a possible etiology of low cardiac output that does not promptly respond to nonsurgical intervention, emergent reoperation is advisable (or reopening the sternum at bedside, especially in infants).
Increased RV afterload may appear quickly as a result of a sudden rise in pulmonary artery pressure and vascular resistance. Consequently, during an episode of paroxysmal pulmonary hypertension (often provoked by intratracheal suctioning), cardiac output may fall rapidly and apparently “sudden” death may occur, particularly in neonates and infants. These outcomes are probably not purely the result of increased RV afterload, because they reflect impaired RV reserve as well.
Increased LV afterload may result from a sudden elevation of systemic arterial pressure, such as may occur during suctioning, restlessness, or hypoxia. These result in a sudden increase in LV afterload that, combined with impaired LV reserves, can result in low cardiac output early postoperatively. Sustained increase in systemic vascular resistance and LV afterload is present early after cardiac operations in at least half the adult patients operated on for acquired heart disease.
Often, disturbances of afterload (afterload mismatch) and preload (preload reserve) are neither independent nor isolated events. Increased RV afterload leads to decreased LV preload. Similarly, but not as important, increased LV afterload leads to decreased RV preload. A common situation in which a corrective operation leads to increased LV afterload is restoration of mitral valve competence or closure of a ventricular septal defect (VSD). In a physics analogy, mitral regurgitation or left-to-right flow through a VSD represents a pair of resistors in a parallel circuit in which R T = 1/r 1 + 1/r 2 , where r 1 and r 2 represent resistances in the two outflow streams and R T is total resistance. Closure of one outflow (r) increases downstream resistance to ventricular shortening; by inference, wall tension and myocardial oxygen consumption ( ![]() ) increase.
) increase.
A number of circumstances increase the probability of low cardiac output after cardiac surgery. These have been determined for the most part by numerous multivariable analyses of outcomes after surgery for specific conditions (see Chapter 7, Chapter 8, Chapter 9, Chapter 10, Chapter 11, Chapter 12, Chapter 13, Chapter 14, Chapter 15, Chapter 16, Chapter 17, Chapter 18, Chapter 19, Chapter 20, Chapter 21, Chapter 22, Chapter 23, Chapter 24, Chapter 25, Chapter 26, Chapter 27, Chapter 28, Chapter 29, Chapter 30, Chapter 31, Chapter 32, Chapter 33, Chapter 34, Chapter 35, Chapter 36, Chapter 37, Chapter 38, Chapter 39, Chapter 40, Chapter 41, Chapter 42, Chapter 43, Chapter 44, Chapter 45, Chapter 46, Chapter 47, Chapter 48, Chapter 49, Chapter 50, Chapter 51, Chapter 52, Chapter 53, Chapter 54, Chapter 55, Chapter 56, Chapter 57, Chapter 58 ).
Chronic impairment of ventricular preload, afterload, and/or contractility by any mechanism (ventricular hypertrophy, stiffness, chronic heart failure) increases the risk of low cardiac output after the cardiac surgical procedure. These are for the most part immutable risk factors for low cardiac output, because they do not change quickly after the operation. However, when a patient who had been alive and ambulatory preoperatively has inadequate cardiac output postoperatively, the assumption must be that intraoperative damage has been superimposed on the chronic state. On the other hand, certain surgical procedures, such as ablation of the regurgitant volume of aortic regurgitation or closure of a defect with a large left-to-right shunt, have an immediately favorable impact on cardiac output. Thus, the cardiac surgical procedure itself often increases cardiac output; it is myocardial damage during the procedure that decreases it.
Acute reduction in ventricular contractility preoperatively can sometimes be ameliorated by intraoperative maneuvers. For the most part, these maneuvers are directed toward increasing an acutely reduced energy charge (see “Cold Cardioplegia, Controlled Aortic Root Reperfusion, and [When Needed] Warm Cardioplegic Induction” under Methods of Myocardial Management during Cardiac Surgery in Chapter 3 ).
The most important intraoperative risk factor for low cardiac output early postoperatively, other than an incomplete operation, is a discrepancy between the duration of any global myocardial ischemia and the efficacy of the measures used for myocardial management (see complete discussion in Chapter 3 ). This is often reflected in the finding of long global myocardial ischemic time as a risk factor for low cardiac output and for death after operation. Coronary air embolization during CPB is said to adversely affect cardiac performance after the operation, but most air entering the coronary arteries while the heart is not supporting the circulation passes quickly into the coronary sinus and may have little deleterious effect.
The extensiveness of the “whole body inflammatory response” to CPB affects the heart as well and relates to the probability of low cardiac output after operation. This is one aspect of the association of duration of CPB with death after operation; another is that long CPB duration is sometimes the result, rather than the cause, of poor cardiac performance.
Low cardiac output can be the result of (1) an incomplete or inadequate operation; (2) acute myocardial ischemia, with or without necrosis, from impaired coronary blood flow resulting from failure of some part of a coronary artery bypass operation (CABG); (3) incomplete relief of ventricular inflow or outflow obstruction; (4) important residual or created AV valve or semilunar valve regurgitation that may increase the stroke volume requirements of the ventricle; and (5) residual VSD and large left-to-right shunt that may similarly increase LV stroke volume requirements.
One of the reasons for placing temporary fine polyvinyl catheters in the left atrium, right atrium, occasionally the pulmonary trunk via the RV and uncommonly the LV, and for placing temporary epicardial atrial and ventricular wires, is that they can be helpful in intraoperative and early postoperative efforts to identify an inadequate or incomplete operation as the cause of low cardiac output. TEE with color flow Doppler imaging is also important in identifying an incomplete or inadequate operation. The possibility of residual left-to-right shunting contributing to low cardiac output must always be considered after repair of congenital heart disease. With the materials now used as patches for repair of VSDs, an appreciable left-to-right shunt ( ![]() > 1.5) early postoperatively must be assumed to represent an incomplete repair or an overlooked defect. The shunt can be quantified by double indicator dilution, a method rarely used in the current era. Alternatively, the left-to-right shunt may be estimated in terms of the pulmonary (
> 1.5) early postoperatively must be assumed to represent an incomplete repair or an overlooked defect. The shunt can be quantified by double indicator dilution, a method rarely used in the current era. Alternatively, the left-to-right shunt may be estimated in terms of the pulmonary ( ![]() ) to systemic (
) to systemic ( ![]() ) flow ratio by simultaneously removing samples from the radial artery, right atrium, and pulmonary artery and solving the simplified shunt equation:
) flow ratio by simultaneously removing samples from the radial artery, right atrium, and pulmonary artery and solving the simplified shunt equation:
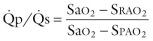
where Sa o 2 is the percent oxygen saturation of arterial blood, S rao 2 that of blood withdrawn from the right atrium, and S pao 2 that of blood withdrawn from the pulmonary artery.
TEE with color flow Doppler imaging now can sometimes settle the issue simply, but without the desirable quantification. Intraoperative TEE has become standard of care for intracardiac repairs of congenital heart disease for patients 3 kg or larger. In one study, intraoperative TEE during post-repair evaluation led to surgical revision in 4% of cases.
The heart is in an especially vulnerable position early postoperatively, because its poor function adversely affects coronary blood flow; this in turn further worsens cardiac function. This explains the observation that low cardiac output early after cardiac operations rarely resolves spontaneously. Aggressive treatment is indicated.
With treatment, most patients with low cardiac output early postoperatively recover, and unless it was produced by a large area of myocardial necrosis, most patients have no demonstrable ill effects from it late postoperatively.
Experience indicates that it is worthwhile to intensively treat patients with low or inadequate cardiac output early after cardiac surgery, because cardiac performance often improves after 1 or 2 days, followed by good recovery.
Many causes of low cardiac output are reversible. Investigating whether cardiac tamponade or compression is the cause is one of the first steps (see “ Acute Cardiac Tamponade ” earlier in this section). If tamponade is present and is caused by retained blood in the pericardium, emergency reoperation is indicated. If there is acute cardiac dilatation, such as may occur in a pulmonary hypertensive crisis, the sternum and pericardium should be rapidly opened (if they were closed). In patients at an increased risk of low cardiac output, this complication can be prevented by leaving the sternum open at operation and closing it 24 to 48 hours later. The pericardium should rarely be closed after cardiac operations, because this has a restrictive effect on the heart early postoperatively.
When cardiac constriction is believed not to be present, treatment is directed at increasing cardiac output by manipulating preload, afterload, contractile state, and heart rate and improving tissue oxygen levels. When these measures fail, use of devices to support the circulation must be considered (see “ Intraaortic Balloon Pump ” and “ Temporary Ventricular Assistance ” later in this section). All such devices have their own risks and imponderables; except for the intraaortic balloon pump, they are typically not used unless it seems likely the patient will not survive without them. The decision to use such devices is always made with concern for the possibility the patient may survive but be left seriously disabled, and with knowledge of the costs of such interventions.
When cardiac output is low, preload is manipulated by increasing blood volume with an appropriate fluid until the higher of the two atrial pressures is about 15 mmHg. If the wall thickness of the LV is unusually great or its contractility or compliance is decreased, it may be helpful to raise mean left atrial pressure to 20 mmHg. However, the tendency to pulmonary edema is increased when left atrial pressure is elevated to this level. When the RV is the limiting factor in cardiac performance, right atrial pressure usually can be raised advantageously only to about 18 mmHg. Above this, a descending limb on the Starling curve usually becomes apparent, and cardiac output falls. Also, the tendency to whole body fluid retention, pleural effusion, and ascites is increased by high right atrial pressure.
When LV performance is the limiting factor and systemic arterial blood pressure is more than 10% above normal (see Table 5-1 ), vasodilating agents should be used to reduce LV afterload to between normal and 10% above normal. Nitroprusside is generally the drug of choice because it is a potent arterial, and to a lesser extent venous, dilator with a short half-life (see Appendix 5A ). The drug appears to be as safe in very young patients as it is in adults. Calcium channel antagonists (e.g., nifedipine, diltiazem) lead to similar arteriolar vasodilatation and may improve coronary perfusion in this setting. However, their longer half-life and depressive effect on ventricular contractility make nitroprusside the preferred drug.
Rarely in patients with severe long-standing mitral valve disease or congenital heart disease with pulmonary vascular obstructive changes, RV dysfunction associated with elevated pulmonary artery pressure may limit cardiac performance. Reduction of RV afterload with vasodilating agents is occasionally dramatic in its increase of RV, and thus cardiac, stroke volume. Nitroprusside (0.5 to 3 µg · kg −1 · min −1 ), nitroglycerin (0.5 to 3 µg · kg −1 · min −1 ), or phentolamine (1.5 to 2 µg · kg −1 · min −1 ) may be effective in this setting. In infants, maintaining near-anesthesia for 24 to 48 hours with fentanyl or another intravenously administered agent may minimize paroxysms of pulmonary artery hypertension and the consequent increased RV afterload (see “ Pulmonary Hypertensive Crises ” later in this section).
Alternatively, management of neonates and infants may be based on use of the long-acting α-receptor blocking agent phenoxybenzamine (see Appendix 5A ). It is administered first at the commencement of CPB (see “Other Additives” under Perfusate in Section II of Chapter 2 ). An additional dose is usually given about 12 hours after returning to the ICU.
Heart rate is adjusted to optimal levels when necessary by atrial pacing, by ventricular pacing when atrial fibrillation is present, or by AV sequential pacing when AV dyssynchrony or dissociation is present. When tachyarrhythmias are present, pharmacologic means of control may be used (see text that follows).
If these relatively simple measures do not quickly bring cardiac performance to an adequate level, inotropic agents are begun (see Appendix 5B for details), although their disadvantages are recognized. There is no ideal inotropic agent, nor are there specific indications for specific agents. In their review, Doyle and colleagues classified inotropic drugs based on their effect on intracellular cyclic adenosine monophosphate (cAMP): cAMP-independent drugs include calcium, digoxin, and α-adrenergic agonists; cAMP-dependent agents include epinephrine (and levarterenol), dobutamine, and isoproterenol. These are β-adrenergic agonists that, coupled with dopaminergic drugs (dopamine), have variable effects on peripheral resistance. Phosphodiesterase inhibitors (amrinone, milrinone, enoximone) may enhance contractility while producing myocardial relaxation (lusitropism) and relaxation of vascular smooth muscle. They are not susceptible to receptor down-regulation.
Initially, dopamine may be infused at 2.5 µg · kg −1 · min −1 . This dose can be increased to 15 or 20 µg · kg −1 · min −1 if needed, but if a favorable response is not obtained at 10 µg · kg −1 · min −1 , it is not likely to be obtained at higher doses. Dopamine has the advantage of augmenting renal blood flow in addition to increasing cardiac contractility. Dopamine increases ventricular automaticity (hence the probability of ventricular arrhythmias), but to a lesser extent than isoproterenol. At low doses (2 to 4 µg · kg −1 · min −1 ), systemic peripheral vascular resistance is decreased or unchanged by dopamine, whereas higher doses (>6 µg · kg −1 · min −1 ) increase peripheral resistance. Tachycardia may limit the rate at which dopamine can be administered. When dopamine is ineffective, dobutamine is gradually added in similar doses. Dobutamine, although more expensive than dopamine, appears to augment myocardial blood flow more ; in general, its effectiveness is similar to dopamine. Isoproterenol may be preferred initially and is probably superior in the presence of predominantly RV dysfunction and decreased or normal heart rate because of its favorable effect on pulmonary vascular resistance.
Occasionally, hypotension exists in the presence of normal and adequate cardiac output. Under that special circumstance, norepinephrine administered through a central venous catheter is rational treatment; a very low dose (0.01 µg · kg −1 · min −1 ) is often sufficient under these circumstances. Under more dire circumstances, larger doses can be used.
Epinephrine is the catecholamine of choice of some, but its powerful vasoconstricting effects make it less desirable than dopamine or dobutamine. When an insufficient response is obtained from other drugs, or excessive tachycardia develops, epinephrine is added or substituted. The drug is initially infused at a dose of 0.01 to 0.05 µg · kg −1 · min −1 , which may be increased as needed.
Milrinone also is useful in patients with low cardiac output after cardiac surgery, because it combines a peripheral vasodilatory action with its inotropic effect. This drug is different in structure and mode of action from catecholamines in that it is a phosphodiesterase enzyme inhibitor in cardiac and vascular tissue, not a β-adrenergic receptor agonist. Administration is usually initiated with a loading dose of 5 µg · kg −1 over 10 minutes, followed by a maintenance dose of 0.3 to 0.75 µg · kg −1 · min −1 . The drug is effective in neonates and infants as well as adults, but in small patients, particular care is necessary to maintain an adequate blood volume because of the vasodilatory effect of the drug. The indications for this drug vs. catecholamines remain arguable. However, it appears to cause less tachycardia and fewer atrial arrhythmias than catecholamines.
Additionally, 10% calcium chloride is administered in a dose of 0.1 mmol · kg −1 , with supplemental doses if the ionized serum calcium level is below 1.2 mmol · L −1 .
Once the acute problems have subsided, some patients in sinus rhythm appear to require chronic augmentation of ventricular contractile function. Use of digitalis in this setting has long been argued, but there is evidence that it does increase contractility. Digitalization appears to be useful, particularly in children (see “ Atrial Arrhythmias ” under Cardiac Arrhythmias later in this section), but it is not recommended in neonates because it may impair diastolic function.
The concept of intraaortic balloon pumping (IABP) to produce diastolic augmentation of coronary and systemic blood flow was elucidated by Moulopoulos, Topaz, and Kolff in 1962. The procedure was first performed clinically by Kantrowitz and colleagues in 1968. IABP uses the principle of diastolic counterpulsation, which augments diastolic coronary perfusion pressure, reduces systolic afterload, favorably affects the myocardial oxygen supply/demand ratio, and augments cardiac output.
IABP is used in adult patients with inadequate cardiac performance not responsive to optimized preload, afterload, and heart rate or to moderate doses (up to 10 µg · kg −1 · min −1 ) of dopamine or equivalent doses of dobutamine or epinephrine (see Appendix 5B ). Whenever possible, the decision to insert an IABP is made in the OR rather than postoperatively. It is used in preference to catecholamines postoperatively for patients with severe LV dysfunction, with or without evidence of myocardial necrosis, and for patients with evidence of myocardial necrosis and inadequate cardiac output or severe ventricular arrhythmias. This technique has led to survival of some patients who would otherwise have died. Survival is less than 50% if renal failure develops from low cardiac output postoperatively. IABP has been effective in patients with ischemic heart disease and valvar and congenital heart disease.
Preoperative prophylactic insertion of the IABP is sometimes advisable. In addition to its use in myocardial infarction with low cardiac output or shock, preoperative insertion is often helpful in unstable angina, left main disease with ongoing ischemia, and ischemia leading to ventricular arrhythmias. In the era of more complex arterial revascularization for ischemic heart disease (see Chapter 7 ), IABP support is helpful intraoperatively for pre-bypass support of patients with low ejection fraction. For patients with acute mitral regurgitation or ventricular septal rupture, insertion upon diagnosis is often lifesaving. Perhaps the most frequent indication for preoperative IABP insertion is poor perfusion from either low cardiac output or peripheral arterial disease. It follows that the best survival following IABP occurs when the device is in place preoperatively.
Insertion by arterial puncture is generally used, despite its somewhat higher prevalence of vascular complications, because of the technical ease of balloon insertion and removal. When the patient is still on CPB, or the femoral pulse cannot be palpated because of hypotension, an incision only in the skin is made over the femoral artery. Through this incision, the femoral artery can nearly always be palpated and the arterial puncture technique used.
Important aortoiliac occlusive disease and abdominal aortic aneurysm greatly increase the risk of vascular complications or failure of insertion when the femoral route is used. In their presence, the balloon can be inserted into the ascending aorta through a purse-string suture. This is usually performed before discontinuing CPB. A pledgeted mattress suture of 2-0 or 3-0 polypropylene is placed in the midportion of the ascending aorta. A tie is placed on the shaft of the intraaortic balloon to indicate the point that should be level with the aortic wall when the balloon is in proper position within the descending aorta. An aortic stab wound is made and controlled digitally, and the balloon is introduced through the wound and passed into the descending aorta. Proper position is verified by appearance of the characteristic aortic pressure pulse when pumping is begun. The balloon shaft is usually brought out through the lower end of the sternotomy incision. When pumping is no longer needed, the patient is returned to the OR, the median sternotomy reopened, and another pledgeted mattress suture placed outside the original one. As the balloon is removed, the stab wound is controlled digitally, and the pledgeted mattress suture is made snug and tied. In severe atheromatous aortic wall disease, use of other assist devices is probably indicated (see “ Temporary Ventricular Assistance ” in text that follows).
IABP is begun in a 1 : 1 ratio with ventricular diastole, as judged by electrocardiographic (ECG) and arterial pressure pulse signals. Often the patient's hemodynamic state improves promptly; consideration is then given to weaning the patient from IABP as early as 6 to 12 hours after insertion. If catecholamines have also been required, they are reduced as rapidly as possible to 5 µg · kg −1 · min −1 of dopamine or less, or the equivalent doses of dobutamine or epinephrine (see Appendix 5B ). Once the hemodynamic state is improved, the IABP ratio is progressively reduced to 1 : 8 (or 1 : 3 with some systems). In most postoperative patients, the final reduction can be reached within 12 to 48 hours. The balloon can then be removed using a closed method. Although objections have been raised to use of this method, most of the problems have occurred when the balloon inadvertently was inserted through the superficial rather than the common femoral artery. After removing the balloon percutaneously, firm pressure is applied to the groin and held for half an hour. If circulation to the leg becomes impaired or a hematoma becomes apparent, prompt exploration in the OR is indicated.
Circulation in the leg distal to the site of balloon insertion is observed systematically. If signs of ischemia appear, generally the balloon is removed. Complications that importantly impede convalescence occur in about 3% of patients.
Ventricular assist devices (VADs), probably first used in a planned fashion by Cooley and colleagues in 1969, are now used (1) for support after cardiac operations, (2) as a bridge to transplantation, and (3) as a bridge to recovery. Without VADs, nearly all patients assisted would likely have died; however, uncertainty about this makes interpreting outcomes and indications for them difficult. It also hampers efforts to determine their direct risks. The following discussion relates to use of various temporary VADs for cardiopulmonary failure after cardiac operations. (A discussion of use of these devices for other indications can be found in Chapter 22 .)
Low cardiac output accompanying reduced ventricular function following cardiac operations occasionally prohibits separating the patient from CPB. A thorough investigation for correctable causes of low cardiac output should be made and may include ensuring aortocoronary bypass graft patency and adequacy of flow using Doppler ultrasound flow velocity or electromagnetic flow probes, and evaluating accuracy of repairs and segmental ventricular wall motion using intraoperative TEE. Preload and afterload should be optimized, appropriate pharmacologic agents administered, and IABP instituted. When these measures are insufficient, temporary ventricular assistance should be considered.
Use of a temporary VAD often follows a prolonged operation and a long period on CPB. Insertion of an implantable VAD under these circumstances is inadvisable (see Chapter 22 ). Temporary support of the failing circulation allows postponing further major operative intervention until the patient's condition improves. The temporary assist system can be used as a bridge to ventricular recovery or as a bridge to more durable mechanical circulatory support if weaning is not possible (see Chapter 22 ).
Temporary ventricular assist is set up as right or left heart bypass or as a combined biventricular bypass circuit. Cannulae for left and right heart bypasses are introduced through the midline sternotomy used during the cardiac operation. (Using cannulae previously placed makes the transition from CPB to right and left heart bypass a more controlled process.) With those cannulae in place, an additional thin-walled metal-tip right-angle cannula (28F) is introduced into the left atrium via the right superior pulmonary vein or via the roof of the left atrium through a purse-string stitch with a short tourniquet. The purse-string stitch may be felt-reinforced if tissues are thin or friable, because bleeding around the perfusion cannulae is frequently troublesome and may require reexploration. An arterial cannula (24F wire thin-wall) is placed in the pulmonary trunk.
Left heart bypass is established by connecting the left atrial cannula to the aortic cannula via a centrifugal pump. This ventricular bypass circuit provides continuous blood flow.
Right heart bypass is established by connecting the right atrial cannula to the pulmonary trunk cannula via a centrifugal pump. Pump flow is increased gradually to 4.0 to 5.0 L · min −1 for adult patients as CPB flow is reduced and discontinued. The cannulae are brought through the fascia, muscle, and skin into the left and right upper quadrants of the abdomen at the time of insertion or connection to the extracorporeal circuit. Polytetrafluoroethylene (PTFE) felt strips are placed tightly around the cannulae in the subcutaneous tissues to seal the exit tract. In some cases, the cannulae are simply brought through the wound. The midline incision may be closed primarily in some cases. More often, cardiac compression results from wound closure. The skin only may then be closed, leaving the sternal edges apart; alternatively, a silicone membrane is sewn to the skin edges to seal the mediastinum. The edges of the membrane are sealed with iodine ointment to eliminate ingress of air and present a barrier to bacteria. Bulky dressings are applied and sealed to the skin with a large iodine-impregnated plastic adhesive.
Heparin-coated tubing and centrifugal pumps are desirable to reduce trauma to blood elements by providing a more blood-compatible extracorporeal circuit. Roller pumps can also be used but seldom are, because of the perception that more blood elements are injured. Short tubing reduces foreign blood contact surface and heat loss in the extracorporeal circuit.
An alternative, pulsatile extracorporeal blood pump system is also available (ABIOMED Inc., Danvers, Mass.). The pump consists of a compliant inflow chamber to which blood is drained by gravity, separated from a rigid pumping chamber by a polyurethane inflow valve. The pumping chamber is fitted with a polyurethane outflow valve. A sac within the pumping chamber is expanded pneumatically to propel the blood. Components of the system are arranged vertically and placed at the bedside. A console provides pneumatic power, senses filling of the pumping device, and synchronizes pumping so that little attention to the system is required. Anticoagulation with heparin is necessary, and the patient must remain immobile, as with other temporary systems. It may be used in left, right, or biventricular assist configuration. Although the system provides the possible advantages of pulsatile flow and less operator attention, it costs substantially more than centrifugal pump systems.
Bypass support of a single failing ventricle is used when possible. It is frequently possible to bypass a failing RV using an extracorporeal ventricular assist system combined with IABP to support the LV. Bypass support of the failing LV seems more complicated. In theory, the LV can be sustained by left atrial–to-aortic bypass while the unassisted RV continues to provide adequate pulmonary flow. Experience has shown, however, that RV function also declines. Biventricular support is frequently advisable even when there is apparent isolated LV failure.
Each of these devices has specific methods and equipment for insertion and late management. In general, anticoagulation with heparin followed by warfarin is required for all devices with mechanical valves, and low-dose anticoagulation with heparin, warfarin, or aspirin is used for those with biological valves. However, management details vary among institutions and devices.
Bleeding from the primary operative site is controlled by reversal of heparin with protamine. Platelet infusion, transfusion of fresh frozen plasma (FFP) or other blood products, and pharmacologic agents to promote normalization of the blood clotting subsystem are administered as indicated. A heparin-bonded extracorporeal circuit is adequate to prevent clotting in the short term. When bleeding from the operative site has ceased or slowed, heparin therapy is restarted. Usually this occurs within 12 hours after completing the operation. Activated clotting time (ACT) is used to monitor heparin effect; desired ACT is approximately 160 to 200 seconds (assuming a control level of 100 to 120 seconds).
Postoperative care of patients on left, right, or biventricular bypass is labor intensive. Continuous bedside care, often by more than one nurse, is required, as is ready availability of a cardiopulmonary perfusionist or other personnel capable of managing the extracorporeal assist system. Bleeding is frequently a nuisance or even a major complication requiring frequent monitoring of blood clotting by ACT. Smooth operation of the system requires frequent infusion of blood products to maintain adequate atrial pressures. Body temperature is monitored continuously, and measures are taken to heat or cool the blood in the extracorporeal circuit or the patient's body with a heating/cooling blanket. The VAD system may be fitted with ports for hemofiltration to remove excess extracellular fluid if there is marked edema. Occasionally, hemodialysis is necessary. Ventilatory assist is required.
Separating the patient from temporary ventricular assistance requires judgment and patience. There is a tendency to remove systems too early to avoid complications directly related to prolonged extracorporeal circulation. It is advisable to wait a day or so longer rather than rush the process of separation. Ability of the heart to support the circulation is tested by reducing flow into the extracorporeal circuit, thereby raising atrial pressures and allowing flow through the supported ventricle. There is a limit to which flow in an extracorporeal circuit can be reduced without introducing danger of clotting. Flow of less than about 1 L · min −1 should be avoided. Heparin levels should be maintained, and duration of testing interval should be short. Cardiac function is monitored by TEE and continuous measurement of arterial and pulmonary artery pressures, atrial pressures, cardiac output, and ![]() . If the heart cannot sustain adequate cardiac output under conditions of low-flow bypass, the separation process is abandoned, full flow is resumed, and plans are made for later attempts at separation or conversion to an implantable device. Simply removing the temporary device in anticipation that the heart will sustain adequate function is usually unsuccessful.
. If the heart cannot sustain adequate cardiac output under conditions of low-flow bypass, the separation process is abandoned, full flow is resumed, and plans are made for later attempts at separation or conversion to an implantable device. Simply removing the temporary device in anticipation that the heart will sustain adequate function is usually unsuccessful.
Results are judged either by recovery and long-term survival after removal of the device or by success in maintaining the patient until cardiac transplantation is accomplished. Clearly, some patients not only survive but also have an excellent long-term functional result. Of some importance in this regard is the finding that myocardial cellular atrophy is not a complication of prolonged ventricular assistance. Despite excellent intensive care, patients with VADs are at risk of thromboembolism, hemorrhage, and infection.
The report of the combined registry for clinical use of mechanical VADs summarized experience with 965 patients. For about half of them, successful weaning from the device was accomplished, and about half of those weaned were discharged from the hospital alive. Two-year survival among patients discharged from the hospital was 82%, with most being in NYHA functional class I or II. A higher proportion of patients receiving LV assist devices had satisfactory outcomes than was the case in those receiving biventricular assist devices. The probability of survival was similar in patients receiving nonpulsatile centrifugal pump devices and those receiving pulsatile pneumatic devices. Survival to discharge for the multi-institutional experience was about 30% for postcardiotomy support. Individual institutions have recorded discharge of up to 60%, and this seems to be related to greater experience with the devices and earlier establishment of support.
The complex scientific, surgical, ethical, moral, political, philosophical, and financial considerations necessarily involved in the decision for mechanical circulatory support do not permit a simple listing of indications for this therapy.
There is general agreement that a low cardiac output (cardiac index ≤ 1.5 L · min −1 · m −2 ) and elevated ventricular diastolic pressure (reflected in high left or right atrial pressure) after continuing CPB an hour beyond the usual time of discontinuance, with IABP support and administration of catecholamines in moderate doses, indicate a low probability of survival. Trial separation from CPB has failed (usually reflected in a progressive drop of systemic arterial blood pressure, elevation of atrial pressures, reduction of venous oxygen saturation, and metabolic acidosis). Under these conditions, in institutions prepared for it and, if necessary, cardiac transplantation (even if this means transportation to another institution), temporary ventricular assistance is usually indicated. Patients with continuing low cardiac output in the ICU despite IABP and catecholamine support also should be considered for temporary ventricular assistance. Use of temporary ventricular assistance is appropriate for patients of any age whose myocardial problem is expected to resolve within a few days, and for patients younger than 65 to 70 years of age whose myocardial problem is not expected to resolve but who are acceptable candidates for cardiac transplantation. In the latter group, the temporary device is used for support while it is determined whether there is good function of major organ systems and the patient is neurologically intact.
Further experience with VADs may lead to discarding current criteria in favor of more liberal use; in part, this is because LV assistance is more effective than IABP in preserving myocardial structure and function, at least in experimental studies. In general, the younger the patient, the better the prognosis from the now-repaired cardiac disease, and the fewer the other subsystem diseases and failures, the stronger the indication for temporary ventricular assistance.
Right and left heart bypass (biventricular assist) works only if pulmonary function is adequate to support gas exchange. In situations of severe compromise of the respiratory system, support of that system is also required. Portable CPB systems (cardiopulmonary support [CPS]) include both a centrifugal blood pump and a membrane oxygenator, with heparin-coated tubing. These systems are usually referred to as extracorporeal membrane oxygenation (ECMO). Successful use has been reported, particularly in infants and children, and there is little doubt that in some patients, it is an effective means of biventricular support. (It may be used occasionally in venovenous configuration to support respiration in the face of severe postoperative arterial desaturation from impaired pulmonary function. ) This methodology has also been used in patients undergoing myocardial infarction or sustaining a catastrophic event in the cardiac catheterization laboratory, and as a support technique during angioplasty.
Only two cannulae have to be inserted, one in the right atrium and the other in a systemic artery. These cannulae are present during surgery and may simply be attached to the system. Oxygenation is achieved in a closed system without an air-to-blood interface. Alternatively, separate venous and arterial cannulae may be inserted through the femoral artery and femoral or jugular vein by percutaneous technique (see “Cardiopulmonary Bypass Established by Peripheral Cannulation” under Special Situations and Controversies in Section III of Chapter 2 ). This places the cannulae in a position for ready control of hemorrhage. Centrally placed cannulae are removed so that the wound may be closed. Percutaneous placement of cannulae and use of the CPS system can be used for emergency support of the failing circulation at the bedside. The CPS system does not ordinarily provide decompression of the LV. LV unloading depends on total diversion of blood from the right atrium to the extracorporeal circuit and the ability of the LV to empty pulmonary venous return to the aorta. A beating heart and absence of aortic valve regurgitation are necessary.
Heparin anticoagulation is required to prevent clotting or excess fibrin formation in the oxygenator membrane. ACT is maintained at approximately 160 to 220 seconds by continuous infusion of heparin.
In the pediatric population, overall survival after ECMO support following a cardiac operation is 30% to 40%. It may be more effective in situations complicated by pulmonary hypertension or in the presence of a two-ventricle repair, compared with repairs in which there is a cavopulmonary connection or aortopulmonary shunt. In adults, ECMO seems more cumbersome than temporary LV or biventricular assistance, and hospital survival may be lower than in children. However, Smedira and colleagues reported 30-day survival of 38% in 202 adults supported by ECMO with intent to wean or bridge to transplant. Patients who survived 30 days had a 63% 5-year survival. Risk factors for death included older age, reoperation, subsystem failure while on ECMO, and occurrence of a neurologic event while on ECMO.
The imponderables, and therefore the uncertainties, as to indications for this type of support after cardiac surgery are similar to those for temporary VADs; in addition, the need for nearly full heparinization and the presence of an oxygenator in the circuit are disadvantages of ECMO.
Postoperative morbidity and mortality can result from cardiac arrhythmias, which may occur either when the cardiac subsystem has been otherwise functioning normally or as a complication of low cardiac output. Atrial and ventricular pacing wires, routinely placed at operation (see Chapter 5) and left for 3 to 10 days postoperatively, are of clear benefit in diagnosing and treating postoperative arrhythmias.
Ventricular electrical instability includes PVCs, ventricular tachycardia, and ventricular fibrillation. Controversy exists concerning the proportion of postoperative patients with frequent PVCs and some types of ventricular tachycardia who are at risk of developing ventricular fibrillation. Despite this, it is prudent to assume that such arrhythmias early postoperatively place the patient at an increased risk of sudden death. Therefore, to detect such arrhythmias, the ECG is monitored continuously for at least the first 48 hours after operation.
Unifocal isolated PVCs occurring outside the T waves fewer than six times per minute are not known to increase the risk of cardiac death and are compatible with normal convalescence. Other arrhythmias indicate an abnormal convalescence and may require special management. Rarely, atrial fibrillation or isolated premature ventricular beats occurring early (within 2-4 hours) after operation in the presence of hypothermia or low potassium level lead to profound ventricular instability, including ventricular fibrillation.
Management of ventricular electrical instability occurring in cardiac surgery patients after postoperative day 1 or 2 has been importantly affected by the results of the Cardiac Arrhythmia Suppression Trial (CAST). This trial demonstrated that drugs used to suppress ventricular electrical instability can themselves be lethal and are often ineffective. Application of these findings to the postoperative patient and implications for treatment are not well understood. For example, CAST patients for the most part had persisting ischemia; most postoperative patients (coronary disease or not) are probably well revascularized. In the postoperative period, however, many antiarrhythmic agents are proarrhythmic; therefore, treating ventricular arrhythmias other than ventricular tachycardia is probably not necessary.
In patients with an ejection fraction under 40% with postoperative ventricular tachycardia, early electrophysiologic study is indicated. Paradoxically, patients in whom such studies demonstrate acute suppression of PVCs or ventricular tachycardia with the administration of drugs have a good prognosis without drug treatment; such patients probably should be discharged from the hospital on no drug therapy. By contrast, patients whose ventricular arrhythmias cannot be suppressed with drugs have a relatively poor prognosis, and consideration should be given to implanting an implantable cardioverter-defibrillator (ICD; see “Ventricular Tachycardia and Ventricular Fibrillation in Ischemic Heart Disease” in Section V of Chapter 16 ).
When during the first day or two of convalescence, ventricular electrical instability develops in the form of PVCs occurring more than six times per minute or ventricular tachycardia persisting more than 20 to 30 seconds, treatment is prudent even if cardiac performance is good ( Appendix 5C ). Often, PVCs or bursts of ventricular tachycardia can be suppressed by atrial or ventricular pacing using temporary electrodes, without need for drug therapy. When the hemodynamic state is impaired by ventricular tachycardia, immediate direct current cardioversion (100 J initially in adults and, if ineffective, 200 J) is indicated. When these measures are ineffective, advice of an experienced electrophysiologically oriented cardiologist is indicated.
Reported prevalence of atrial fibrillation after adult cardiac operations is 20% or higher. New-onset atrial fibrillation usually occurs within 5 days of cardiac surgery, with peak occurrence on the second day. This arrhythmia uncommonly results in major morbidity or death but can complicate convalescence considerably. It is most likely to develop in patients in whom chronic atrial fibrillation has been present before operation and who have left the OR in sinus rhythm.
The largest group of patients in whom atrial fibrillation occurs but who did not have it preoperatively are those undergoing CABG. In this group, discontinuance postoperatively of a β-adrenergic receptor blocking agent that has been taken regularly before operation increases the risk of developing atrial fibrillation during the postoperative period; this complication can be expected in about 40% of such situations. Older age at operation is also a risk factor for developing atrial fibrillation postoperatively. This arrhythmia develops in about 4% of patients younger than 40 years of age undergoing CABG, compared with 30% of those aged 70 or older. Chronic obstructive pulmonary disease, chronic renal disease, valvar heart disease, atrial enlargement, increased sympathetic tone, obesity, and prior pericarditis also appear to increase its prevalence. Other preoperative and intraoperative variables do not appear to be correlated with development of atrial fibrillation after cardiac operation.
New-onset atrial fibrillation after cardiac surgery increases morbidity, length of stay, and cost. Morbidity includes hemodynamic compromise and occasionally embolic events. New postoperative atrial fibrillation is statistically correlated with advanced age but is also seen across a wide spectrum of adult cardiac operations. The peak incidence is 2 to 3 days postoperatively, but it may occur earlier or following discharge. Postoperative atrial fibrillation is usually transient; with treatment, most patients return to sinus rhythm within 2 to 3 days. Patients with preoperative atrial fibrillation are unlikely to return to sinus rhythm, especially if atrial fibrillation has been present for more than 6 months.
Prevalence of new atrial fibrillation after cardiac surgery can be reduced by prophylaxis with a β-adrenergic receptor blocking agent. For this purpose, propranolol may be given in doses of 10 to 20 mg three or four times a day, beginning the morning after operation. Alternatively, atenolol may be given in doses of 25 mg three times per day, which is often better tolerated than propranolol. The benefit from β-blockers as prophylaxis against postoperative atrial fibrillation is greatest when the drug is initiated either prior to or immediately after surgery. Amiodarone (a class III antiarrhythmic agent) is also an effective drug for preventing atrial fibrillation, with approximately a 4% to 50% reduction in its prevalence after cardiac surgery. A meta-analysis suggested that amiodarone provided similar protection against postoperative atrial fibrillation as β-blockers and sotalol (another class III antiarrhythmic agent). Pretreatment with digoxin or verapamil does not reliably prevent atrial fibrillation.
When atrial fibrillation develops postoperatively, several protocols are appropriate, depending on need for rate control versus conversion to sinus rhythm. In postoperative patients with chronic atrial fibrillation with stable and effective hemodynamics who are not in the ICU, digoxin is usually effective for rate control, and is indicated for patients with heart failure and LV dysfunction. In the ICU, rate control of atrial fibrillation with preserved ventricular function is most effectively accomplished with intravenous administration of β-blockers (esmolol, metoprolol, or propranolol) or non-dihydropyridine calcium channel antagonists (verapamil, diltiazem), exercising caution in the presence of hypotension. Intravenous amiodarone is also useful for rate control ( Table 5-3 ). It should be noted that intravenous digoxin or calcium channel antagonists should not be used in patients with a preexcitation syndrome; these agents may paradoxically accelerate the ventricular response.
| Drug | Loading Dose | Onset | Maintenance Dose | Major Side Effects |
|---|---|---|---|---|
| Acute Setting | ||||
| Esmolol | 0.5 mg · kg −1 IV over 1 min | 5 min | 60-200 µg · kg −1 · min −1 IV | ↓ BP, HB ↓ HR, asthma, HF |
| Metoprolol | 2.5-5 mg IV bolus over 2 min; up to 3 doses | 5 min | NA | ↓ BP, HB ↓ HR, asthma, HF |
| Propranolol | 0.15 mg · kg −1 IV | 5 min | NA | ↓ BP, HB ↓ HR, asthma, HF |
| Diltiazem | 0.25 mg · kg −1 IV over 2 min | 2-7 min | 5-15 mg · h −1 IV | ↓ BP, HB, HF |
| Verapamil | 0.075-0.15 mg · kg −1 IV over 2 min | 3-5 min | NA | ↓ BP, HB, HF |
| Amiodarone | 150 mg over 10 min | Days | 0.5-1 mg · min −1 IV | ↓ BP, HB, pulmonary toxicity, skin discoloration, hypothyroidism, hyperthyroidism, corneal deposits, optic neuropathy, warfarin interaction, sinus bradycardia |
When digoxin is used for rate control, a recommended protocol is found in Appendix 5D . When approximately two thirds of the estimated digitalizing dose has been given and has not accomplished control of the ventricular rate, oral administration of propranolol should be started unless the patient has poor ventricular function or pulmonary disease. If the situation is urgent, propranolol may be given intravenously in doses of 0.5 mg every 2 minutes, to a total intravenous dose of 4 mg in adults. Alternatively, treatment with verapamil may be initiated in doses of 40 mg orally two to three times daily. This drug may be infused intravenously in a dose of 0.075 mg · kg −1 to a maximum dose of 5 mg, but its action is very short when given in this manner, and oral administration should be started promptly.
In patients with new-onset or recurrent postoperative atrial fibrillation, successful conversion to sinus rhythm is usually possible and is advantageous for postoperative hemodynamics. Digoxin is not effective in converting postoperative atrial fibrillation. Amiodarone is currently the first-line drug for pharmacologic conversion of atrial fibrillation. A bolus dose of 5 mg · kg −1 in saline is given over a 30- to 40-minute period, followed by a continuous infusion of 0.25 to 0.5 mg · min −1 for 4 to 8 hours. Following conversion, oral amiodarone is generally recommended for 4 to 6 weeks.
Ibutilide (a class III antiarrhythmic agent) is also highly effective in converting postoperative atrial fibrillation and is at least as effective as amiodarone at converting new-onset atrial fibrillation (greater than 75% success). Adult patients are pretreated with intravenous magnesium (1-2 g) to reduce risk of torsades de pointes, and the serum potassium level is normalized. Higher doses of magnesium (up to 4 g divided into pre- and post-ibutilide infusion) have been recommended by some. Under continuous ECG monitoring, 1 mg of ibutilide is administered intravenously over 10 minutes. The dose can be repeated once. The major risk is a 1% to 4% occurrence of torsades. Ibutilide should be avoided in patients with very low ejection fraction, because of the high risk of ventricular arrhythmias. When ventricular response rate is rapid, pretreatment with esmolol may increase the efficacy of conversion with ibutilide.
Theoretical concerns have been raised about using ibutilide (which can be proarrhythmic with prolongation of QT interval) after amiodarone (which also prolongs QT interval but rarely causes torsades). When intravenous amiodarone is not effective in conversion to sinus rhythm, several studies indicate that ibutilide is frequently successful and reasonably safe. In a study of ICU patients (noncardiac surgical) who were not successfully converted from atrial fibrillation to sinus rhythm with amiodarone, 80% were successfully converted with ibutilide. Nonsustained torsades de pointes occurred in 11% of patients, all of whom had depressed LV function. Torsades de pointes has been reported in about 5.5% of women and 3% of men who receive ibutilide. It usually occurs within 45 minutes of completion of ibutilide infusion and resolves without treatment in about 40% of patients. Treatment of more than transient torsades includes intravenous magnesium plus lidocaine or cardioversion if the patient is hemodynamically unstable. Deaths from torsades have rarely been reported in this setting. Similarly, amiodarone can be safely administered if initial treatment with ibutilide fails.
Alternatively, in patients in whom atrial fibrillation or flutter is importantly embarrassing circulation, electrical cardioversion is indicated. Using bipolar temporary atrial wires placed at operation (one each on the right atrium and left atrium), low-energy cardioversion techniques have been developed that result in an 80% conversion early postoperatively; anesthesia is unnecessary.
When atrial fibrillation persists for more than 48 hours, anticoagulation with heparin or warfarin is indicated for stroke prevention.
Atrial flutter can be a difficult arrhythmia to control when it occurs postoperatively. Although not always successful, the best treatment is rapid atrial pacing via the two atrial epicardial wires placed at operation (see Appendix 5E ).
Ibutilide is also effective in converting atrial flutter (>80% success) and is probably more effective than amiodarone. Ibutilide is also effective in converting atrial flutter in pediatric patients. For patients weighing less than 60 kg, the recommended dose is 0.01 mg · kg −1 intravenously over 10 minutes.
Important episodes of paroxysmal atrial tachycardia or paroxysmal atrial contractions occurring after cardiac operations may also be treated by rapid atrial pacing. If these arrhythmias persist, however, the diagnostic and therapeutic advice of an electrophysiologically expert cardiologist is needed. Specific treatment depends on the precise nature of the supraventricular tachycardia. In general, administration of catecholamines should be reduced as much as possible; if additional support is necessary, milrinone is considered (see “ Noninvasive Methods ” under Treatment of Low Cardiac Output earlier in this section). β-Adrenergic receptor blocking agents such as propranolol are useful (see earlier discussion). Multifocal atrial tachycardias are often best treated by verapamil given intravenously.
Junctional ectopic tachycardia (JET) may be a particularly difficult problem in children, with a prevalence of 5% to 8%. It is associated with operations to close VSDs, perioperative transient AV block, repair of AV septal defect, and young age. In 70 of 71 patients with postoperative JET, Walsh and colleagues obtained control of the arrhythmia and hemodynamic stability using a combination of sedation, hypothermia to 34°C, and procainamide. Amiodarone may also be effective.
Adequacy of pulmonary function early postoperatively while the patient is still intubated and receiving controlled ventilation is judged by the response of Pa o 2 and arterial carbon dioxide pressure (Pa co 2 ) to the minute volume of ventilation and ventilating gas mixture being used. The response of the arterial oxygen levels can be expressed as the alveolar-arterial oxygen difference, P(A − a)O 2 , which in intact humans is normally only a few mmHg. Nearly all patients early after cardiac operations have abnormally large alveolar-arterial oxygen differences due to intrapulmonary right-to-left shunting of 3% to 15%. This assumes that no right-to-left intracardiac shunting is present. Response of the carbon dioxide levels can be expressed as the minute volume of ventilation required to maintain Pa co 2 at 30 to 45 mmHg. Usually this is about 15 to 20 mL · kg −1 in both adults and children.
When anesthesia and heavy sedation are no longer present and the ventilator is in a mode of intermittent mandatory ventilation (IMV), the patient's ventilatory rate is a useful guide to the ease with which the respiratory center's needs are met, and thus to the adequacy of pulmonary function. In an adult, a patient-triggered respiratory rate of 8 to 12 breaths · min −1 , with the usual tidal volume, inspiratory gases, and end-expiratory pressure, indicates adequacy of lung function.
Positive end-expiratory pressure (PEEP) may be used as a routine or on indication, with a setting of 5 to 8 cm H 2 O for adults and children older than 12, and 4 cm H 2 O for younger patients. Unless the hemodynamic state is suboptimal, PEEP does not alter it, even in infants. PEEP is used because of studies that suggest it is associated with larger lung volumes, fewer perfused but nonventilated alveoli during ventilation, and smaller P(A − a)O 2 after extubation. PEEP is generally not advisable in patients with chronic obstructive lung disease (to avoid air trapping and rupturing a bulla, with consequent pneumothorax) and in infants and children who have undergone a Fontan operation or cavopulmonary anastomosis (to avoid still further elevation of jugular venous pressure; see Chapter 41 ).
Continuous positive airway pressure (CPAP) may be used in infants once their cardiovascular state is stable, obviating the need for intermittent positive-pressure breathing (IPPB) and IMV. Should IPPB be required initially, the infant is transferred to IMV and sometimes to CPAP for several hours before extubation.
Traditionally, continued intubation after cardiac operations has been recommended to maintain more precise control of cardiopulmonary physiology. However, early extubation may shorten ICU and hospital stay. Therefore, a protocol beginning with anesthesia induction may be designed to expedite awakening and spontaneous breathing.
Once the patient has been extubated, Pa co 2 and Pa o 2 levels, as well as the visually estimated work of breathing, are useful indices of pulmonary function. Pa co 2 of less than 45 mmHg in adults, less than 50 mmHg in young children, and less than 55 mmHg in small infants indicates an adequate minute volume of ventilation. Higher values indicate inadequate alveolar ventilation. Pa o 2 is often mildly depressed at this time and usually remains so for the first few days after cardiac surgery performed with CPB ( Fig. 5-9 ). This results from the somewhat widened alveolar-arterial oxygen difference. These measurements are valuable, but when a patient is comfortable, breathing easily and slowly, and the chest radiograph is essentially normal, it is highly probable pulmonary function is adequate and convalescence will be satisfactory.
![Figure 5-9, A, Arterial oxygen tension (Pa o 2 ) , venous admixture ( ) , and arteriovenous oxygen content difference [C(a − v)O 2 ] measured preoperatively and at intervals early postoperatively in 10 adults undergoing operation with cardiopulmonary bypass. B, Pre- and postoperative values for minute ventilation ( ) , frequency (f), and tidal volume (V t ) in the same patients. Air- and O 2 -breathing results have been combined, and mean values for the group at each time are shown. Figure 5-9, A, Arterial oxygen tension (Pa o 2 ) , venous admixture ( ) , and arteriovenous oxygen content difference [C(a − v)O 2 ] measured preoperatively and at intervals early postoperatively in 10 adults undergoing operation with cardiopulmonary bypass. B, Pre- and postoperative values for minute ventilation ( ) , frequency (f), and tidal volume (V t ) in the same patients. Air- and O 2 -breathing results have been combined, and mean values for the group at each time are shown.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/PostoperativeCare/13_3s20B978141606391900005X.jpg)
After cardiac surgery with CPB, the lungs are more likely to have dysfunction, albeit mild and transient, than any organ other than the heart itself. This dysfunction has multiple causes. In part it is caused by absence of pulmonary blood flow during total CPB and by its near absence during partial CPB. Among other things, this results in very low sheer stresses in the pulmonary capillaries. This appears to accentuate neutrophil activation, because neutrophils appear to be exquisitely sensitive to sheer stress. Leukocytes are also activated by the general damaging effects of CPB and incite an inflammatory response in the pulmonary vasculature. During total CPB and lung ischemia, plasma thromboxane B 2 increases, and this may contribute to a pulmonary vascular inflammatory response. Cytokines interleukin (IL)-6 and IL-8 increase with CPB and may also contribute to membrane damage and neutrophil activation in the lung. The alveolar-capillary barrier becomes more permeable than normal, and after cardiac surgery using CPB, macromolecules enter the pulmonary interstitium and ultimately the alveoli, promoting development of pulmonary edema. During the early hours after CPB, radioactive albumin injected intravenously can be detected in considerable quantity in fluid aspirated from the tracheobronchial tree (Digerness SB, Kirklin JW: personal communication; 1972). Postoperative disturbances of lung function and increases of alveolar polymorphonuclear leukocytes have been linked to important reduction of pulmonary surfactant activity. In addition, multiple and not yet totally defined factors encourage development of large areas of atelectasis, either segmental or occasionally lobar; in particular, the left lower lobe has a strong tendency to atelectasis, even in patients who are otherwise convalescing normally.
In some patients, direct trauma to the lungs occurs and contributes to pulmonary dysfunction. Should secretions be retained during or early after the operation, these also contribute to dysfunction. Left (and occasionally, right) phrenic nerve injury may occur after carefully performed cardiac operations, increasing the tendency to pulmonary dysfunction early postoperatively. However, left lower lobe atelectasis is considerably more common than left phrenic nerve paralysis; Markand and colleagues found the left phrenic nerve to be paralyzed in only 11% of patients experiencing left lower lobe atelectasis early after open heart operations. In most patients, the left phrenic nerve recovers within 6 months from its paralysis. Mechanical obstruction of the lower trachea or the bronchi can produce pulmonary dysfunction that may go unrecognized unless care is taken to identify and treat it. Localized or more extensive pulmonary edema may develop in the presence of low or normal left atrial pressure, no doubt related to changes in pulmonary venular and capillary permeability, the causes of which are only partially understood. This phenomenon seems to be more marked in elderly patients. Less commonly, frank pulmonary hemorrhage developing as CPB is discontinued or early thereafter can cause serious bronchial obstruction and contribute in a major way to pulmonary dysfunction. This is probably a more severe result of the same factors that lead to pulmonary edema in patients with normal or low left atrial pressures.
Patient-specific risk factors for pulmonary dysfunction after cardiac surgery have long been recognized, but formal identification and quantification are rare. In a formal observational study, young age at operation was identified as a risk factor, particularly when the patient was younger than about 2 years old ( Fig. 5-10 ). Similar observations were made by Lell and colleagues. Increased risk with young age is associated with the increased tendency of the very young to develop whole body edema after CPB. In the experience of most institutions, and in a study by Gallagher and colleagues, older age , particularly older than 60 years, has also been associated with increased prevalence of pulmonary dysfunction after cardiac surgery. Chronic obstructive lung disease is an important risk factor for pulmonary dysfunction postoperatively and increases the overall risk of operation because it predisposes patients to increased work of breathing and air trapping. Preoperative pulmonary arterial hypertension , even when associated with low pulmonary arteriolar resistance, predisposes infants to pulmonary dysfunction and also to paroxysms of pulmonary arteriolar constriction and pulmonary hypertension early postoperatively (see “ Pulmonary Hypertensive Crises ” later in this section). Congenital morphometric pulmonary abnormalities like the alveolar hypoplasia frequently present in patients with congenital heart disease and Down syndrome increase the prevalence of postoperative pulmonary dysfunction. Some drugs given during the period before operation increase the prevalence of pulmonary complications postoperatively; amiodarone , used to control cardiac arrhythmias, has been linked to severe pulmonary dysfunction after cardiac surgery.
The type of oxygenator used is probably related to the amount of pulmonary dysfunction generated by the operation, with oxygenators other than membrane-type being risk factors for pulmonary damage. Proper filters in the arterial tubing may reduce pulmonary dysfunction postoperatively. Longer duration of CPB is a risk factor. This is probably related in part to the direct correlation between duration of CPB and increase in the patient's extracellular water ( Fig. 5-11 ). The amount of C3a generated by complement activation during CPB is a risk factor for pulmonary dysfunction; this may relate to a greater amount of neutrophil activation with higher levels of C3a. Use of external cardiac cooling , particularly by ice slush rather than cold saline, increases the prevalence of left phrenic nerve paralysis, hence the tendency to postoperative pulmonary dysfunction.
Postoperative events can increase the probability of pulmonary dysfunction. Elevated left atrial pressure , with consequent elevation of pulmonary capillary and venular pressures, aggravates the tendency toward increased lung water and pulmonary dysfunction. The longer the patient is on a ventilator, the greater the chances of continuing pulmonary dysfunction. Paralysis of the phrenic nerve and the consequent elevation of the left hemidiaphragm predispose patients to continuing pulmonary dysfunction, particularly small patients.
Mild pulmonary dysfunction in normally convalescing patients slowly improves without specific therapy except for ambulation and breathing exercises; however, residues of dysfunction may still be present 10 days after operation. Occasionally in the patient who appears to be convalescing normally and is out of the ICU for 3 to 6 days, orthopnea and paroxysmal nocturnal dyspnea may develop insidiously. This is particularly likely to occur in patients who have marked LV hypertrophy or poor LV function preoperatively, and it may occur even though their left atrial pressure was less than 15 mmHg when last monitored in the ICU. The chest radiograph may have been normal or may have shown evidence of a mild increase in interstitial fluid. Response to diuresis in such a patient is dramatic and, as a rule, terminates the problem. The hypothesis is that fluid that has accumulated in the interstitial spaces throughout the body during and early after CPB returns to the vascular space 24 to 72 hours after operation. Blood volume is thereby increased at a time when the renal response is subnormal, and diuresis and control of blood volume do not follow. In this setting, LV end-diastolic, left atrial, and pulmonary venous pressures rise and symptoms develop. Weight gain and other gross evidence of fluid retention may be absent.
Occasionally, patients who have convalesced without difficulty begin to cough up thick tracheobronchial secretions 48 to 72 hours after operation. Often, seemingly paradoxically, whatever dyspnea and tachypnea may have been present begins to lessen with these events. Presumably at this time, protein-rich fluid that has been in the alveoli and interstitium of the lung since CPB (or soon thereafter) begins to be moved by ciliary action out of the terminal bronchioles and into the larger airways, from which it can be cleared by coughing.
Lung volumes are usually reversibly decreased early after cardiac surgery, particularly vital capacity and total lung volume. This is probably the result of the summed effects of multiple small areas of atelectasis, occasionally left lower lobe collapse, occult pulmonary edema and pleural fluid, and reduced inspiratory efforts. These usually revert to normal within 3 to 6 months.
The goal of treatment of the pulmonary subsystem is the earliest possible return of the patient to extubated spontaneous breathing and ambulation. In an era characterized by the ability to intensively treat both large and small patients after cardiac surgery, the distinctly beneficial effects of early extubation are often forgotten. These include a decline in intrapleural pressure and an increase in LV end-diastolic diameter (or volume), improved ventricular systolic function (due to increased preload associated with shifting of some of the blood volume toward the chest), and improved cardiac output.
Patients who are convalescing normally are extubated either in the OR or during the early hours after operation. In most ICUs, it is advisable to extubate patients in the daytime rather than at night; this consideration is a reasonable cause of delay of extubation for a few hours. Otherwise, when pulmonary function is good, extubation should be delayed only under special circumstances, including the presence of cardiac assist devices and the possibility of early reoperation. In general, criteria for extubation include stable and satisfactory cardiac performance, lack of important cardiac arrhythmia, and appropriate awakening with satisfactory neurologic status. There should be no anticipation of return to the OR (e.g., for bleeding) and satisfactory mechanics and ventilatory lung function, as assessed by arterial blood gas analysis and a clinical estimate of inspiratory force and volume. Increasing emphasis has been placed on the advisability of routine extubation of the normally convalescing patient within 6 hours of arrival in the ICU.
A major cause of postoperative pulmonary dysfunction is atelectasis and the associated loss of functional alveolar units. Standard prophylactic measures include chest physiotherapy and use of devices to encourage deep inspirations to recruit atelectatic areas. Zarbock and colleagues demonstrated a beneficial effect of prophylactic nasal positive airway pressure at 10 cm H 2 O for at least 6 hours.
Treatment of patients with pulmonary dysfunction who are still intubated during the early hours after operation is in large part an intensification of the usual management, as effective specific therapy is not available. Fractional inspired oxygen (F io 2 ) is appropriately adjusted upward if P(A − a)O 2 is large. Minute volume of respiration (not only measured, but judged visually by excursion of the chest wall) and respiratory rate are adjusted to maintain Pa co 2 at about 30 to 35 mmHg. The airway is kept clear by appropriate tracheal suctioning (see “ Pulmonary Hypertensive Crises ” later in this section for special precautions in neonates and infants). When hemoconcentration develops from leakage of plasma from the intravascular space into the interstitial space of the lungs, and at times into all other organs and the pleural and peritoneal spaces, administration of concentrated serum albumin is helpful in counteracting this trend. Diuretics are useful because they reduce extracellular fluid volume and, in turn, extravascular lung water. During all this, efforts continue to reduce ventilatory support and work toward extubation of the patient (see Appendix 5F ). Spontaneous breathing trials and decreasing levels of pressure support appear equally effective in patients who are difficult to wean.
A prolonged period of endotracheal intubation is necessary when:
Criteria for extubation are not met.
Neurologic complications are present.
Severe dysfunction of the cardiac subsystem is present.
Persistent chest drainage or a residual cardiac defect make early return to the OR likely.
During periods of prolonged ventilation, active decisions are required regarding use of continuous vs. intermittent sedation and neuromuscular blockade, depending on hemodynamic stability, effectiveness of ventilation, and timing of ventilator weaning. Commonly used sedation and paralytic agents are described in Tables 5-4 and 5-5 .
| Drug | Action | Bolus Dose | Onset | Duration | Continuous Infusion | Comments |
|---|---|---|---|---|---|---|
| Cisatracurium | Non-depolarizing | 0.15-2 mg · kg −1 | 1-2 min | 25-90 min | 3 µg · kg −1 · min −1 | Generally given as continuous infusion. Preferred for renal or hepatic failure |
| Vecuronium | Non-depolarizing | Initial: 0.05-0.1 mg · kg −1 | 2-3 min | 45-90 min | 0.8-1.7 µg · kg −1 · min −1 | Preferred agent for patients without hepatic or renal failure. Given as intermittent bolus or continuous infusion |
| Maintenance: 0.1-0.2 mg · kg −1 every hour | ||||||
| Pancuronium | Non-depolarizing | Initial: 0.1 mg · kg −1 | 2-3 min | 60-100 min | Not recommended | Higher risk of tachycardia. Rarely used because of long duration and potential for accumulation |
| Maintenance: 0.01 mg · kg −1 every hour |
| Drug | Class | Initial Bolus Dose | Maintenance Bolus | Continuous Infusion | Comments |
|---|---|---|---|---|---|
| Midazolam | Benzodiazepine | 0.02-0.08 mg · kg −1 up to max of 5 mg | Same as initial | 0.04-0.2 mg · kg −1 · h −1 titrate to sedation | Adverse effects: hypotension, nausea, vomiting |
| May accumulate; use with caution in patients with renal dysfunction | |||||
| Fentanyl | Opioid analgesic | 0.35-1.5 µg · kg −1 | Same as initial, every 30-60 min | 0.7-10 µg · kg −1 · h | Adverse effects: bradycardia, nausea, vomiting, respiratory depression |
| Propofol | Short-acting lipophilic general anesthetic | 1-3 mg · kg −1 over 20-30 s | 50-200 µg · kg −1 · min | May cause hypotension and bradycardia | |
| Dexmedetomidine | α 2 -Adrenergic agonist; sedative | Adults: 1 µg · kg −1 infused over 10 min Pediatric: none |
0.2-0.7 µg · kg −1 · h | Not recommended for more than 4 days to avoid withdrawal symptoms. May cause bradycardia and hypotension | |
| Haloperidol | Antipsychotic agent | Adults: 2-5 mg intramuscularly or intravenously | Adults: same as initial dose every 4-8 hours as needed to stabilize psychiatric symptoms | May alter cardiac condition and prolong QT interval. May cause extrapyramidal symptoms |
When patients are agitated after cardiac surgery, particularly during ventilator weaning, the first priority is evaluating cardiac and pulmonary subsystems to ensure that agitation is not an indicator of important cardiac or pulmonary dysfunction. If these subsystems have satisfactory function, the next level of intervention targets communication with the patient by nursing or physician staff, or both, to allay patient anxiety. When these interventions are insufficient, pharmacologic agents commonly used to alleviate agitation and anxiety in the ICU include benzodiazepines (e.g., diazepam, lorazepam, midazolam), opioid analgesics (e.g., fentanyl, hydromorphone, morphine, remifentanil), propofol, dexmedetomidine, and neuroleptics (e.g., haloperidol).
Prolonged intubation (≥48 hours) entails problems necessitating fastidious care of the patient. A pneumothorax occasionally develops from positive-pressure breathing, usually requiring urgent aspiration and underwater drainage. Bronchospasm may become a particular problem, probably as a reflex response to proteinaceous tracheobronchial fluid often present under these circumstances. Administration of nebulized isoetharine every hour can be helpful. In extreme cases, aminophylline is given as a continuous intravenous infusion in 10% dextrose in a dose of 0.15 mg · kg −1 · min −1 . An initial loading dose of about 4 mg · kg −1 is given over 20 minutes. Neonates, infants, and young children who require intubation and ventilation for more than about 14 days often have phrenic nerve paralysis; in these patients, plication of the hemidiaphragm often permits prompt extubation. Plication usually does not interfere with recovery of phrenic nerve and diaphragmatic function.
In those unusual circumstances when intubation in older children and adults is necessary for more than about 10 days, consideration is given to tracheostomy. Both patient comfort and effectiveness of ventilation are thereby usually increased. This procedure has disadvantages but is sometimes helpful in weaning the patient from the ventilator. Tracheostomy is rarely performed in neonates, infants, and young children, because it is difficult to manage in this age group.
When the patient meets all criteria for extubation, reintubation is usually unnecessary. However, reintubation is indicated when:
Pa co 2 rises above 50 mmHg over 4 hours in the absence of appropriate respiratory compensation for metabolic alkalosis.
Signs of decreasing cardiac output are noted.
Signs of exhaustion from breathing spontaneously are observed.
Excessive pulmonary secretions with ineffective coughing are evidenced.
When the situation is borderline, proper management requires careful observation by senior members of the team. Reintubation should be performed by a competent professional.
Pulmonary hypertensive crisis is a phrase used to describe a serious syndrome of hyperacute rise in pulmonary arterial pressure usually accompanied by bronchospasm, often followed within seconds or accompanied by profound reduction in cardiac output and fall in Sa o 2 ; these sequelae may be irreversible. The syndrome occurs most commonly, but not exclusively, among neonates and infants who are intubated after an operation for repair of a congenital cardiac defect associated with pulmonary arterial hypertension. Pulmonary artery pressure is usually essentially normal or mildly elevated after repair, until an episode of pulmonary hypertensive crisis. The crisis may appear spontaneously but usually occurs during or shortly after endotracheal tube suctioning, particularly if the endotracheal tube is too far down the trachea and makes contact with the carina. Multiple crises may occur in a single patient. The prevalence of pulmonary hypertensive crises is greatest about 18 hours after operation, but they can occur before or after that time. During an acute hypertensive crisis, atypical cardiac tamponade can result from acute RV dilatation.
Although recognized for many years, its precise cause at the time it occurs is unknown. Congenital cardiac defects accompanied by large pulmonary blood flow and relatively low pulmonary vascular resistance are strong patient risk factors for their development. Chief among them are truncus arteriosus and AV septal defect. The histology of the pulmonary arteries and arterioles is normal or mildly abnormal, with all changes considered to be clearly reversible. The syndrome is not seen in patients with fixed pulmonary hypertension or severe pulmonary vascular disease. Procedural risk factors have not been clearly identified, although absence of pulmonary blood flow during CPB, accumulation of neutrophils in the lung, and damage to pulmonary endothelial cells are known to occur and provide a favorable setting for development of pulmonary hypertensive crises. Release of arachidonic metabolites from some cells in the lung (probably pulmonary endothelial cells), failure of pulmonary endothelial cells to inactivate bradykinin, and release by injured pulmonary endothelial cells of the vasoconstrictive platelet-activating factor are probably all encouraged by events during CPB, perhaps particularly if these cells were preconditioned before operation by a high pulmonary blood flow. Postoperative risk factors nearly certainly include acute hypoxia, which is known to produce acute hypoxic vasoconstriction, probably using some of the mechanisms just described. Catecholamine administration appears to predispose patients to these crises.
Because pulmonary hypertensive crisis may be fatal despite intense therapy, its prevention is critical. An important preventive measure in neonates and infants is maintenance of paralysis and sedation (with pancuronium [or a similar agent] and fentanyl) for at least the first 20 to 24 postoperative hours, and for at least another 24 hours or longer if the patient remains intubated (see Section IV, “ General Care of Neonates and Infants ,” later in this chapter). Fentanyl has been shown to be a safe agent in neonates and adults, with little adverse effect on cardiac function or systemic and pulmonary circulations. A paralytic agent (see Table 5-4 ) is given along with fentanyl to prevent rigidity. Fentanyl (in a dose of 25 µg · kg −1 ), along with pancuronium, should be given before suctioning the endotracheal tube in any neonate or infant who is not already well paralyzed and sedated, because it appears to minimize the possibility of a pulmonary hypertensive crisis. In addition to sedation, aggressive treatment of acidosis, including hyperventilation to P co 2 = 30 to 34 mmHg, decreases prevalence and severity of pulmonary hypertensive crises. When the monitored pulmonary artery pressure becomes paroxysmally elevated early postoperatively, efforts are made to control and reduce it. A high F io 2 is maintained because this is as useful an agent as exists for reducing pulmonary vascular resistance. Direct infusion of nitroprusside into the pulmonary artery has also been useful. Many agents have been advanced as being optimal on the grounds that they selectively reduce pulmonary vascular resistance, but few do so, and no clearly superior one has been identified.
There are encouraging results from use of inhaled nitric oxide to reduce pulmonary artery pressure in cardiac transplant recipients, patients on LV assist devices, and generally in infants and children prone to pulmonary hypertensive crises. Journis and colleagues found that 15 of 17 children having critical pulmonary hypertension following operation for congenital heart disease responded to inhaled nitric oxide (20 ppm) after failure of conventional medical treatment ( Table 5-6 ). Pulmonary arterial pressure decreased without a change in systemic arterial pressure but with concomitant increase of Sa o 2 and ![]() . Other investigators have also shown an increase in cardiac index and improvement of indices of RV function after nitric oxide treatment. Intrapulmonary shunt fraction may decrease as well. The response in postoperative patients with valvar heart disease may be less satisfactory.
. Other investigators have also shown an increase in cardiac index and improvement of indices of RV function after nitric oxide treatment. Intrapulmonary shunt fraction may decrease as well. The response in postoperative patients with valvar heart disease may be less satisfactory.
| T 0 (before NO) | T 1 (20 min after NO) | P | |
|---|---|---|---|
| HR (beats · min −1 ) | 153 ± 22 | 145 ± 21 | .008 |
| Systolic P pa (mmHg) | 60 ± 14 | 40 + 11 | .0003 |
| Mean P pa (mmHg) | 42 ± 14 | 27 ± 8 | .0008 |
| Diastolic P pa (mmHg) | 31 ± 14 | 18 ± 10 | .0003 |
| Systolic P ao (mmHg) | 68 +12 | 69 ± 7 | .18 |
| Mean P ao (mmHg) | 55 ± 11 | 56 ± 6 | .4 |
| Diastolic P ao (mmHg) | 44 ± 10 | 46 ± 6 | .12 |
| P la (mmHg) | 7.2 ± 1.9 | 6.8 ± 1.5 | .3 |
| P cv (mmHg) | 6.6 ± 2.5 | 5.2 ± 1.8 | .04 |
| |
50 ± 13 | 67 ± 12 | .003 |
| Sa o 2 (%) | 87 ± 8 | 96 ± 3 | .0007 |
a Nitric oxide inhaled at 20 ppm reduces pulmonary artery pressure and improves ![]() while having no effect on systemic artery pressure when given to a group of patients following operations for defects associated with pulmonary hypertension.
while having no effect on systemic artery pressure when given to a group of patients following operations for defects associated with pulmonary hypertension.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here