Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A 75-year-old man with long-standing hypertension and type 2 diabetes mellitus underwent open repair of an infrarenal aortic aneurysm. His baseline creatinine was 1.6 mg/dL, and this rose to 2.0 mg/dL on the second postoperative day. On the fifth postoperative day, he developed progressive hypoxemia, fever, and hypotension. A contrast-enhanced scan of the pulmonary vessels was not suggestive of pulmonary embolism. He was started on a β-lactam and an aminoglycoside for probable pneumonia. Over the next 12 hours, he became hypotensive and anuric. Renal replacement therapy was started, and antibiotic therapy was shifted to a quinolone. The patient’s renal function recovered after several weeks, and he was eventually discharged.
The authors wish to thank Dr. Mark Nunnally and Dr. Robert Sladen for their respective contributions to the previous edition of this chapter.
Postoperative acute kidney injury (AKI) is a term that describes a spectrum of diminished renal function that develops after surgery. The most current consensus guidelines provided by the Kidney Disease Improving Global Outcomes (KDIGO) Work Group provide a definition based on serum creatinine (S Cr ) and urine output. This definition consolidates two previously validated groups of consensus criteria known as RIFLE and AKIN into one system for clinical practice and research.
AKI is defined by any one of the following criteria and is then staged according to Table 165.1 :
Increase in S Cr greater than or equal to 0.3 mg/dL (26.5 μmol/L) within 48 hours; or
Increase in S Cr greater than or equal to 1.5 times baseline, which is known or presumed to have occurred within 7 days; or
Urine volume less than 0.5 mL/kg/h for 6 hours
| Stage | Serum Creatinine | Urine Output |
|---|---|---|
| 1 | 1.5–1.9 times baseline OR ≥0.3 mg/dL increase within 48 hours |
<0.5 mL/kg/h for 6–12 hours |
| 2 | 2.0–2.9 times baseline | <0.5 mL/kg/h for ≥12 hours |
| 3 | 3.0 times baseline OR Increase in S Cr to ≥4.0 mg/dL OR Initiation of RRT OR In patients <18 years, decrease in estimated glomerular fltration rate to <35 mL/min per 1.73 m 2 |
<0.3 mL/kg/h for ≥24 hours OR Anuria for ≥12 hours |
The cause of AKI has traditionally been classified as prerenal (decreased perfusion), renal (parenchymal injury), or postrenal (urinary obstruction). Although these distinctions remain somewhat useful for conceptual understanding, they do not represent the complexity of the pathophysiology involved, and thus the field is moving away from them. In many cases, postoperative AKI is a consequence of sustained prerenal injury that culminates in an extreme form of AKI: ischemic acute tubular necrosis (ATN).
In animal models, transient low perfusion creates a prerenal state characterized by oliguria and low urinary sodium, with salt and water retention. This is the normal renal response to hypovolemia and is mediated by the renin-angiotensin-aldosterone system (RAAS), other neurohumoral factors, and intrinsic tubuloglomerular feedback. With increased perfusion, urine flow returns to normal. This is therefore a hemodynamic event with preserved tubular function; it is reversed by restoration of normal hemodynamic function. More prolonged perfusion deficits result in oliguria (with high urine sodium) that does not reverse when perfusion increases. In this scenario an intrinsic insult occurs, characterized by backleak of ultrafiltrate across the disrupted tubular basement membrane and facilitated by tubular obstruction with cellular debris. The latter is a persistent hemodynamic event with loss of tubular function; it is not reversed by restoration of normal hemodynamics.
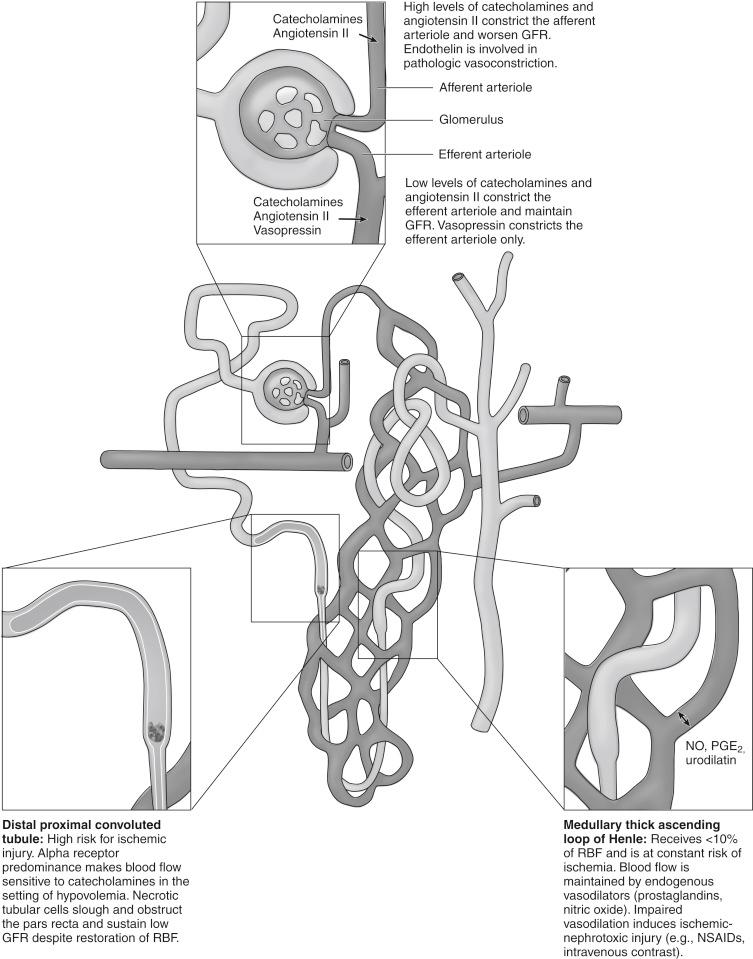
The pathogenesis of AKI reflects the kidney’s unique sensitivity to insult. Fig. 165.1 demonstrates several at-risk areas of the nephron. The medulla and inner cortex have marginal oxygenation and are therefore at risk for ischemic ATN from reductions in intrarenal oxygen delivery. This could result from intravascular volume depletion, hypotension, diminished cardiac output, and/or anemia. In nephrotoxic ATN, damage to the tubules is the result of inflammatory signaling, free radical damage, disturbances in cellular metabolism, and disruption of intrinsic renal vasodilators such as prostaglandins and nitric oxide. Tubular cell necrosis and apoptosis lead to loss of function, disruption of renal cellular architecture, and nephron obstruction by cellular debris. Once this has occurred, restoration of renal blood flow can no longer reestablish glomerular filtration rate (GFR).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here