Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Since the late 19th century, the study of Streptococcus pneumoniae and pneumococcal infections has occupied a central position in developing a scientific basis for controlling infectious diseases. , The organism was first isolated and grown in the laboratory almost simultaneously by Sternberg and Pasteur in 1881. During the next decade, the pneumococcus was shown to be the principal cause of lobar pneumonia. To this day, pneumococcal disease remains a leading cause of morbidity and mortality among the young and the old.
In the 1890s, experiments by Georg and Felix Klemperer elucidated two important biological principles: vaccinating rabbits with killed pneumococci protected them from challenge with the same organism, and transfer of serum from immunized rabbits to unimmunized ones conferred protection. Serum, derived from whole blood, was the quintessential humoral substance, hence the term humoral immunity. This serum protected against challenge with the immunizing strains of S. pneumoniae but not others. Vaccination with these other strains provided protection against themselves but did not protect against the initial strains, demonstrating homologous protection.
Neufeld and Haendel clarified the distinction between these two groups of isolates, calling them type 1 and type 2. , A few years later, Dochez and Gillespie found a third prominent pneumococcus, which they called type 3; other pneumococci were lumped together as type 4. Because these types were identified by serologic protection, specific to each type, they were called serotypes.
In vitro experiments using sera from immune rabbits caused clumping and capsular swelling of pneumococci of the same serotype (the Quellung reaction), but not of pneumococci of the other serotypes. Most importantly, Neufeld and Rimpau showed that, whereas pneumococci were not taken up well by polymorphonuclear leukocytes, after incubation in immune serum, the bacteria were readily ingested and killed. This process of preparing pneumococci for ingestion and killing was called opsonization. Thus, by the end of the first decade of the 20th century, the medical community realized that (1) vaccination of rabbits stimulated the production of antibodies that were protective, (2) this protection was serotype-specific, (3) the protective effect could be identified by microscopic observation of clumping and capsular swelling, and (4) serotype-specific protection was related to the capacity of immune serum to enable ingestion and killing of infective organisms by phagocytic cells.
These observations were promptly tested in humans. The first large-scale clinical trial of a pneumococcal vaccine, using killed whole pneumococcal organisms, was begun in 1911 in newly assembled male mine workers in South Africa, a setting in which pneumococcal pneumonia was rampant and lethality was high. Vaccination with pneumococci, killed by boiling or by exposure to formalin, showed protection and also reduced mortality in patients with influenza pneumonia.
Maynard, Wright, Cecil and Austin, and Lister undertook studies to show that African miners , , and U.S. soldiers were protected against pneumococcal pneumonia by repeated vaccination with killed pneumococci. In these studies, whole-cell pneumococcal vaccine appeared to reduce the occurrence of pneumococcal pneumonia by 50%–80%, although interpretation of some results is limited by methodologic issues, such as small sample size or lack of an appropriate comparison group. , , Of interest, a recent reanalysis of data from trials of whole-cell killed bacterial vaccines given during the 1918 influenza pandemic suggests that whole-cell bacterial vaccines containing pneumococci may have reduced the risk of secondary pneumonia and mortality among patients with pandemic influenza virus infection. 8 At the Rockefeller Institute, Dochez and Avery isolated a “soluble specific substance” from urine and sera of infected patients; this substance precipitated when exposed to serotype-specific antiserum. , In the ensuing decade, Heidelberger and Avery concluded that this was the capsular substance that characterized serotypes and against which antibody was directed. Felton isolated this capsular polysaccharide, confirmed the role of antibody in its precipitation, and concluded that antibody to capsule was responsible for immunity to pneumococcal infection. , Felton provided Smillie with his purified capsular polysaccharide and during an outbreak of pneumococcal pneumonia in a “state hospital” (a residential institution characterized by crowding and unsanitary conditions). Smillie et al. demonstrated that vaccination with this substance aborted the outbreak, documenting that pneumococcal capsular polysaccharide was the immunizing substance. Felton et al. then showed in studies of 18,000 members of the U.S. Civilian Conservation Corps that vaccination with capsular polysaccharides reduced serotype-specific pneumonia by 59%–89%. In controlled studies of 10,900 older adults, Kaufman found a 73% reduction in pneumonia after vaccination with polysaccharides from S. pneumoniae serotypes 1, 2, and 3. In U.S. military recruits during World War II, MacLeod and colleagues undertook a randomized prospective, controlled study using a vaccine that contained capsular polysaccharide from pneumococcal serotypes 1, 2, 5, and 7 but not serotypes 4 and 12. They then observed the incidence of pneumonia due to specific serotypes. The results provided evidence for serotype-specific protection against pneumonia due to S. pneumoniae serotypes 2, 5, and 7 with reductions of 93%, 75%, and 100%, respectively. There were not enough cases to show any decrease in disease caused by serotype 1. The incidence of pneumonia due to serotypes 4 and 12 was similar in the vaccinated group and controls.
With the beginning of the antibiotic era, interest in pneumococcal infection waned, under the general assumption that this disease could be readily treated. However, a few clinician-scientists retained interest in pneumococcal vaccination, as we shall see when the historical review resumes below under Active Immunization.
As in prior editions of this book, pneumococcal conjugate vaccines (PCVs) are discussed in Chapter 47. This chapter focuses on the use of unconjugated pneumococcal polysaccharide vaccines in adults, but the epidemiologic impact of PCV in children informs the use of PPSV23 and is briefly reviewed in this chapter.
S. pneumoniae can be cultured from the nasopharynx of between 15-20% of children in temperate zones in developed countries (with a higher percentage in those in day care) and 5-10% of adults. The organism causes infection either by direct extension from the nasopharynx (e.g., pneumonia, otitis media, sinusitis) or by invasion of the blood stream with spread to normally sterile sites (e.g., osteomyelitis, septic arthritis). Meningitis or spontaneous bacterial peritonitis may occur by direct or hematogenous spread.
The microorganism initially observed by Sternberg and Pasteur was called Pneumococcus by Fränkel in 1886. It was renamed Diplococcus pneumoniae in 1920 because of its distinctive morphology. Its name was changed again to Streptococcus pneumoniae in 1974 because of its many similarities to other streptococci.
Avery, MacLeod, and McCarty used the pneumococcus in 1944 to demonstrate that DNA is the active substance responsible for the genetic transformation of pneumococci from rough (nonencapsulated) to smooth (encapsulated) forms. This is one of the cornerstones of modern molecular biology. Other biologic markers, such as serotype specificity and antibiotic resistance, are genetically transferable.
Pneumococci are relatively fastidious, facultative anaerobic organisms that grow in short chains in broth culture. Microscopically, they appear as lancet-shaped gram-positive diplococci or chains of cocci ( Fig. 48.1 ). Growth requires a source of catalase, obtained by adding red blood cells to the growth medium, to neutralize the hydrogen peroxide produced by the bacteria.
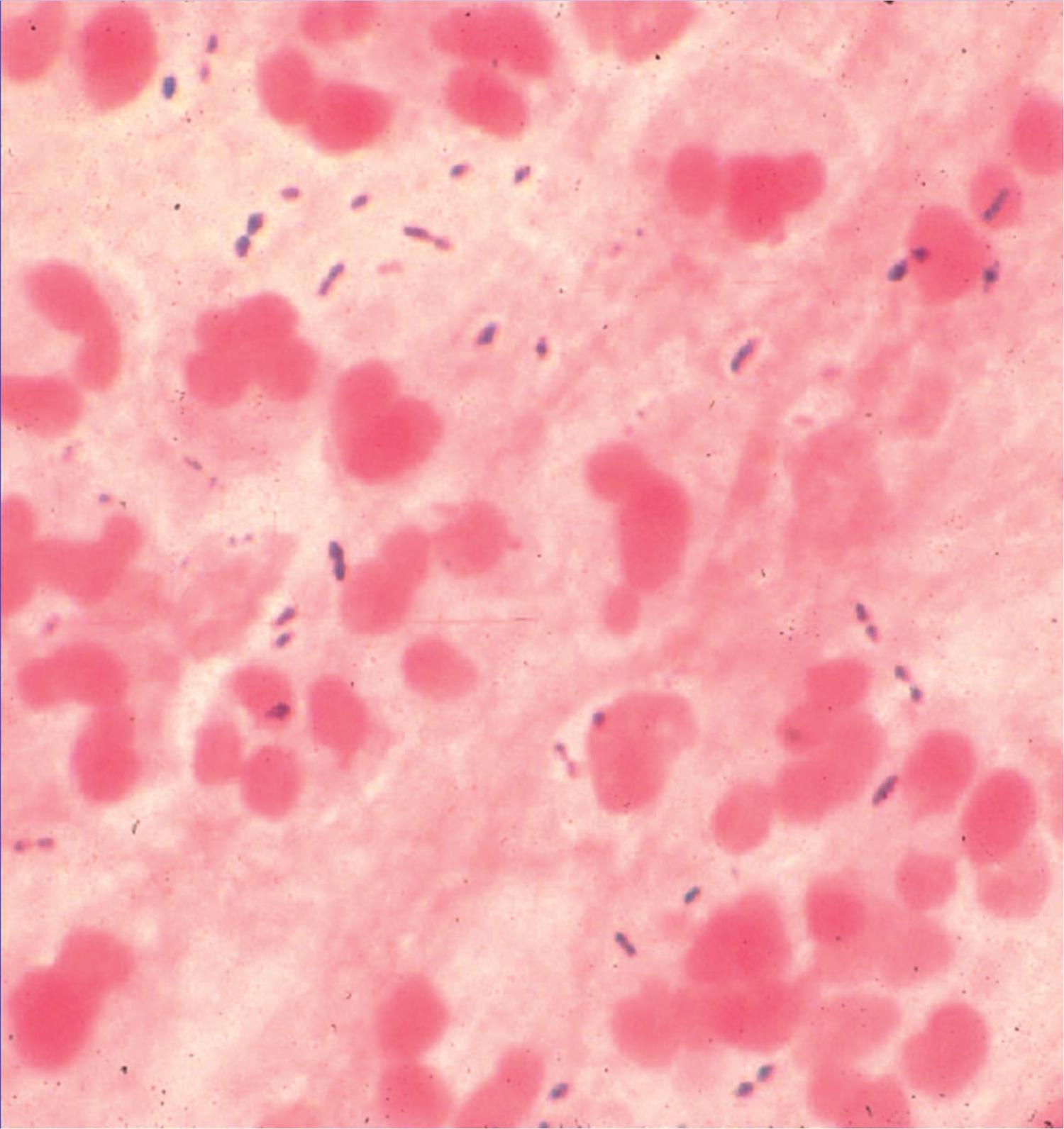
The capsular polysaccharide on the cell surface is the major virulence factor of S. pneumoniae . It interferes with phagocytosis by preventing complement C3b–mediated opsonization of bacterial cells. Antibodies to the capsular polysaccharides are protective. At least 100 pneumococcal serotypes have been identified based on antigenic differences in the capsular polysaccharide. Current polysaccharide vaccines are based on formulations of various capsular polysaccharide antigens derived from corresponding serotypes; selection is based on the frequency with which specific capsular polysaccharides cause disease in the target population.
Pneumococci readily exchange genetic material by transformation and can “trade” genetic material for capsules or acquire new ones. Vaccines that contain polysaccharides conjugated to carrier proteins (i.e., pneumococcal conjugate vaccines, PCVs) nearly eliminate colonization in children. , In doing so, they create an ecologic niche that might be occupied by other commensal bacteria but appear to be preferentially occupied by other pneumococcal serotypes. This observation exposes a basic problem with any vaccine that relies solely on antibody to capsular polysaccharide and supports the search for conserved proteins as vaccine candidates. 31,33 More information on pneumococcal proteins and pneumococcal protein based vaccine development may be found in Chapter 46.
The diagnosis of invasive pneumococcal disease (IPD) is established by isolation of the organism from blood or another normally sterile site (e.g., cerebrospinal, pleural, or synovial fluid). The diagnosis of noninvasive pneumococcal infections, such as nonbacteremic pneumococcal pneumonia, is more complicated. About 75%–80% of cases of pneumococcal pneumonia are not associated with detectable bacteremia. The finding of S. pneumoniae in sputum by Gram stain and culture in a patient who presents with a pneumonia syndrome enables the diagnosis of presumptive pneumococcal pneumonia. However, there are significant limitations to diagnostic tests of sputum. At least 40% of patients are unable to produce an adequate expectorated sputum specimen at time of hospitalization for pneumonia, and, once antibiotics have been administered for longer than 18 hours, the diagnostic yield from sputum Gram stain and culture is less than 25%. ,
A rapid immunochromatographic assay reliably detects cell-wall polysaccharide from S. pneumoniae in urine from 60% to 80% of patients who have bacteremic pneumococcal pneumonia and 40% to 60% of those with pneumococcal pneumonia but with negative blood cultures. In adults, there are few false-positive results. The assay is not used in children, who have a greater nasopharyngeal colonization burden that results in false-positive results. This immunochromatographic test is also approved for the rapid diagnosis of pneumococcal meningitis through testing of CSF samples, with a sensitivity of at least 95% and a specificity of 100%. The test has also been used to detect S. pneumoniae antigen in pleural fluid specimens, bronchoalveolar lavage fluid samples (with utility limited by contamination via nasal colonization), and blood cultures. Multivalent serotype-specific immunochromatographic assays are being used in disease surveillance studies.
An approach under investigation aims to diagnose pneumococcal infection through nucleic acid–amplification techniques. Although polymerase chain reaction (PCR)-based assays are highly sensitive, their use to detect S. pneumoniae in respiratory samples can be limited by the non-specificity of the gene targets, some of which may also be present in other organisms, such as Streptococcus pseudopneumoniae and nonpneumococcal streptococcal viridans group that are oropharyngeal colonizers, leading to false-positive results. More importantly, the test may also detect asymptomatic pneumococcal colonization; consequently, a positive test may not indicate pneumococcal infection. The first concern may be addressed by the use of gene targets found only in S. pneumoniae . The second concern may potentially be addressed by the use of quantitative real-time PCR methods to prevent false-positive results resulting from colonization with small numbers of organisms. , In patients with meningitis, PCR is highly sensitive and specific for detection of S. pneumoniae in CSF samples. ,
Although in the 1940s pneumococci were readily susceptible to penicillin, diminished antibiotic susceptibility is now relatively common. This degree of resistance only rarely impacts patient care because pneumococcal penicillin susceptibility depends on the site of infection, since the concentration of antibiotic achievable varies with the site of infection, specifically whether central nervous system infection is thought to be present.
As with many other infectious diseases, rates of invasive pneumococcal disease (IPD) are highest at the extremes of age. Estimated IPD rates (per 100,000 population) in the United States for 2018 from the Centers for Disease Control and Prevention (CDC) Active Bacterial Core surveillance (ABCs) program were 13.3 for children younger than 1 year; 9.5 for 1-year-olds; 4.3 for 2- to 4-year-olds; 2.8 for 18- to 34-year-olds, 16.6 for 50- to 64-year-olds; 20.2 for 65- to 74-year-olds, 25.5 for 75- to 84-year olds; and 38.7 for persons 85 years of age and older ( Fig. 48.2 ). These rates have fallen greatly in young children and to a lesser degree in older age groups since widespread use of 13-valent protein conjugate vaccine in infants. Combining all ages, the overall IPD rate was 13.2 cases per 100,000 population for Blacks and 9.1 for Whites.
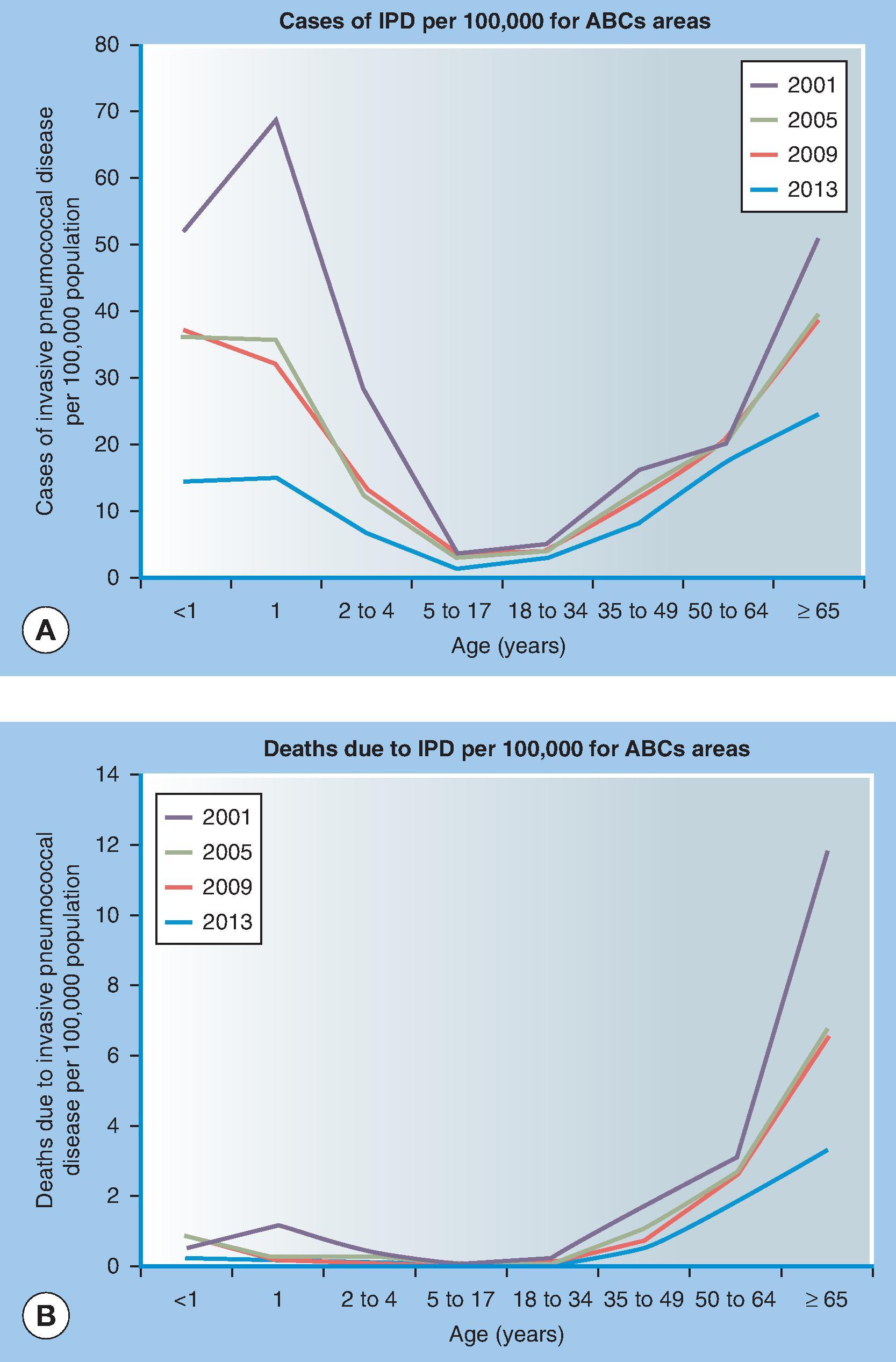
IPD is also more lethal in older adults with the 2018 attributed IPD death rate in persons 85 years of age and older of 8.4 per 100,000 population, compared with 1 per 100,000 in children younger than 1 year of age. A total of 31,400 cases and 3480 deaths resulting from IPD are estimated to occur in the United States annually. Pneumonia with bacteremia accounts for the majority (71%) of cases of IPD. Bacteremia without a focus and meningitis account for 14% and 7% of cases, respectively.
Although IPD occurs all 12 months of the year, the incidence of IPD in adults is much greater in winter than in summer months. This seasonal variation is closely associated with viral respiratory infections that are also more common in winter. Exposure of children to winter respiratory viruses and asymptomatic colonization by bacteria is probably a major factor, but a mechanistic explanation remains to be elucidated. This increase is not clearly attributable to lower ambient temperature or air pollution. Factors such as photoperiod-dependent variation in host susceptibility may also be involved. , Although most IPD is community acquired, IPD can be nosocomially acquired. A population-based study in Finland reported that nosocomial pneumococcal bacteremia accounted for 10% of cases.
In the preantibiotic era, S. pneumoniae caused 95% of cases of pneumonia. This number has steadily declined, such that recent large, prospective studies have identified pneumococcus as a cause in approximately 5% to 10% of patients who are hospitalized for community-acquired pneumonia (CAP). However, these studies failed to identify an etiologic agent in 50% or more of patients with CAP. Two recent studies that included only patients who were able to provide expectorated sputum found etiologic agents in 85%–95% of cases, with pneumococcus causing about 25% of cases. , The incidence of pneumococcal pneumonia remains nearly twice as high in the UK and Europe when compared with the United States, possibly related to the continued prevalence of cigarette smoking and the failure to give pneumococcal vaccine to adults in those regions.
Those at the extremes of age are particularly susceptible to pneumococcal infection, as shown in Fig. 48.2 . Numerous pneumococcal outbreaks have been reported from nursing homes, and other settings of crowded living or working conditions, including military bases, prisons, shelters, mines, or shipyards.
Certain racial and ethnic groups are at increased risk with studies in the United States showing substantially higher rates of IPD in Blacks than in Whites. Higher rates of IPD have also been reported among the White Mountain Apaches in Arizona, , the Alaskan Native population, and aboriginal populations in Australia. The biologic explanation for these ethnic predispositions remains to be elucidated. Crowding, mixing of people from disparate locations, physical exhaustion, and stress contribute to well-documented outbreaks of pneumococcal pneumonia in adults. These conditions, together with poor nutrition, general poor health, and age might explain some of these outbreaks.
Cigarette smoking is a strong and independent risk factor for IPD in adults. A case-control study of immunocompetent adults 18 to 64 years of age found a 4-fold higher risk of IPD in current smokers and a 2.5-fold higher risk of IPD in those with passive exposure to smoke compared with adults who had never smoked and had no passive exposure. Persons with chronic pulmonary diseases (such as chronic obstructive pulmonary disease, emphysema, and asthma) are also at increased risk of pneumococcal disease. By damaging pulmonary clearance (and perhaps by increasing bacterial adherence to epithelial cells), respiratory viruses, but especially influenza virus infection strongly predisposes to pneumococcal pneumonia; other upper and lower respiratory viruses may also do so, albeit to a lesser degree.
Diseases that compromise the ability to generate antibody responses render individuals especially susceptible to IPD. Best studied are patients with multiple myeloma, lymphoma, Hodgkin disease, and HIV infection, even before the onset of AIDS. In persons with HIV/AIDS in the pre–HIV-treatment era, the age-adjusted incidence of IPD was several hundred-fold greater than in the non-HIV-infected population, approaching 500 per 100,000 per year. , Transplant recipients are also at high risk of IPD, with an overall incidence of 146 cases of IPD per 100,000 per year. , The risk is highest in liver (354 cases per 100,000) and lung (239 cases per 100,000) transplant recipients. The question of generating lasting antibody levels after receiving a pneumococcal vaccine will be discussed below and in Chapter 69. Conditions that interfere with function of polymorphonuclear phagocytes are also associated with increased risk of IPD. Diabetes mellitus, chronic renal failure, and cirrhosis of the liver are good examples.
Additional circumstances are also worth noting. Because the spleen plays a central role in removing nonopsonized bacteria from the bloodstream, persons who do not have a functioning spleen are at a high risk of rapidly progressing, overwhelming infection caused by S. pneumoniae . Persons with chronic CSF leaks or cochlear implants also have a greatly increased risk of pneumococcal meningitis. In addition, ascites predisposes to pneumococcal peritonitis, and the risk for pneumococcal endocarditis appears to be greatly increased by prior damage to heart valves. The increase in risk for pneumococcal disease is also markedly greater for patients with immunocompromising conditions than for other chronic diseases. For example, in 2000 the rates of IPD cases per 100,000 person-years were nine among healthy adults compared with 51 among adults with diabetes, 63 among adults with chronic lung disease, 94 among adults with chronic heart disease, and 100 among adults with alcohol abuse syndrome. Among immunocompromised adults, disease rates per 100,000 person-years are even higher, with rates of 300 among adults with solid cancer, 423 among adults with HIV/AIDS, and 503 among adults with hematologic malignancy. ,
The human nasopharynx is the only natural reservoir for S. pneumoniae. Colonization of the nasopharyngeal epithelium represents the first step in the pathogenesis of pneumococcal infections. , Colonization is an active immunizing process leading to antibody production both in children and in adults. , If the host is relatively healthy and capable of producing antibody (or already has antibody because of prior infection, colonization or vaccination), then disease is less likely to occur. In contrast, if the host is debilitated, has poor pulmonary clearance or is unable to produce antibody, disease is far more likely to occur.
Colonization studies consistently demonstrate much higher rates of carriage among children than adults. , After acquisition, the duration of carriage is also longer in children compared with adults, and S. pneumoniae is thought be introduced into households by children and then spread to other family members. Among adults, carriage rates may be elevated among certain populations, including adults with AIDS, military trainees, cigarette smokers, and native Alaskans, but is generally lower in elderly persons.
Although PPSVs are not widely regarded as effective in preventing colonization, rates of serotype-specific nasopharyngeal carriage among vaccine recipients in crowded adult settings significantly decreased in PPSV recipients. A study at a U.S. Army technical school found that PPSV4 reduced serotype-specific carriage by 55% (from 3.3% to 1.8%, P < .01). Klugman and colleagues calculated carriage reductions among gold miners in South Africa of 70% for PPSV6 (from 23% to 7%, P < .001) and 65% for PPSV13 (from 51% to 18%, P = .005).
After PCV7 was universally recommended for infants in the United States in 2000, IPD caused by those serotypes nearly vanished, not only in vaccine recipients, but also in children and adults who had not received PCV7. , This effect is called the indirect or herd effect of the vaccine. The reduction in pneumococcal disease in unvaccinated adults lagged behind that in vaccinated children by a few years but ended up with similar declines. Reductions have also been reported from other countries where PCVs are routinely used in infants, including Canada, England, Israel, Germany, and France. , By 2013, the routine adoption of PCV13 for American children in 2010 resulted in a 58% to 72% decline in IPD as a result of the inclusion of six new serotypes not previously included in PCV7. The net effect was the vaccine serotypes to fall in incidence and proportion among adults, leaving the non-PCV13 serotypes to predominate in circulation.
Fränkel demonstrated protective humoral immunity in 1886 by noting that rabbits that recovered from pneumococcal infection were immune to reinfection. The 1891 contributions of Georg and Felix Klemperer to the origin of serum therapy were discussed in Background. , , These early investigations also led to the discovery of different “types” of S. pneumoniae based on the absence of cross-protection. On this basis, they are considered the discoverers of serum therapy.
In the 1920s and 1930s, before the discovery of antibiotics, Cecil, Finland, and other investigators documented modest benefit from using serotype-specific serum therapy to treat pneumococcal infections. , ,
Current commercially available human intravenous immunoglobulin preparations contain varying amounts of antibody to pneumococcal capsular polysaccharides. , Chronic prophylaxis with intravenous immunoglobulin is used in persons who have deficient antibody production and chronically low serum levels of IgG.
From the 1920s through 1940s, as discussed in Background, the work of teams led by Dochez, Avery, Heidelberger, Felton, Kaufman, Smillie, and MacLeod showed that humoral protection resulted specifically from antibody to the pneumococcal polysaccharide capsule. , In 1946, two hexavalent pneumococcal polysaccharide vaccines were marketed in the United States, but were voluntarily withdrawn within a few years, because physicians had little interest in a vaccine to prevent a disease they believed they could treat reliably with penicillin.
In 1964, Austrian and Gold reported that pneumococcal infections were still lethal, despite appropriate antimicrobial therapy, and Austrian renewed efforts to develop a pneumococcal capsular polysaccharide vaccine. Within a decade, the serotype-specific efficacy of PPSV was tested by Smit and colleagues in two vaccine trials in young adult gold miners in South Africa, one with a six-valent and one with a 12-valent PPSV ( Table 48.1 ) , Vaccination was associated with a significant reduction in the risk of vaccine-type pneumococcal pneumonia confirmed by radiography and positive blood culture. Vaccine efficacy was estimated to be 76% with the six-valent vaccine and 92% with the 12-valent vaccine. In an analysis of both trials combined, PPSV was reported to reduce the risk of vaccine-type pneumococcal bacteremia by 82%. ,
| Reference, Vaccine Valency | Study Population | Outcome | VE a (%) | 95% CI | No. Events/No. Vaccinated | No. Events/No. Unvaccinated |
|---|---|---|---|---|---|---|
| Smit (1977), 6-valent | Gold miners in South Africa | Vaccine-type pneumococcal pneumonia | 76 | 53–88 | 9/983 | 78/2036 |
| All-cause pneumonia | 37 | 9–56 | 37/83 | 121/2036 | ||
| Bronchitis | 8 | −17 to 28 | 84/983 | 190/2036 | ||
| Smit (1977), 12-valent | Gold miners in South Africa | Vaccine-type pneumococcal pneumonia | 92 | 38–99 | 1/540 | 25/1135 |
| All-cause pneumonia | 32 | −42 to 68 | 9/540 | 28/1135 | ||
| Bronchitis | 42 | −7 to 68 | 13/540 | 47/1135 | ||
| Austrian (1976), 6- or 13-valent | Gold miners in South Africa | Vaccine-type pneumonia and/or vaccine-type bacteremia | 79 | 65–87 | 17/1493 | 160/3002 |
| Riley (1977), 14-valent | Papua New Guinea highlanders age ≥10 yr | All-cause mortality | 21 | 1–37 | 133/5946 | 170/6012 |
| Respiratory mortality | 27 | 0–46 | 68/5946 | 94/6012 | ||
| Death from pneumonia uncomplicated by chronic lung disease | 43 | 6–66 | 23/5946 | 41/6012 | ||
| All-cause pneumonia | 26 | −13 to 52 | 36/2713 | 48/2660 | ||
| Cough illness | 19 | −3 to 36 | 114/2713 | 138/2660 | ||
| Cough illness with pneumococci isolated from blood culture or lung aspirate | 86 | 48–97 | 2/2714 | 14/2660 | ||
| Austrian (1980), b 12-valent | Inpatients at Dorothea Dix Psychiatric Hospital in Raleigh, NC | All-cause pneumonia | −22 | −49 to 0 | 154/607 | 144/693 |
| Members age ≥45 yr of the Kaiser Permanente Health Plan in San Francisco | Vaccine-type bacteremia | 100 | P = .06 | 0/6782 | 4/6818 | |
| Vaccine-type pneumonia | 15 | −47 to 51 | 24/6782 | 28/6818 | ||
| Non–vaccine-type infections | −15 | −135 to 44 | 16/6782 | 14/6818 | ||
| All-cause pneumonia | 2 | −16 to 7 | 268/6782 | 274/6818 | ||
| Gaillat (1985), c 14-valent | Residents of 48 long-term care institutions in France | All-cause pneumonia | 79 | 53–91 | 7/937 | 27/749 |
| Simberkoff (1986), 14-valent | U.S. Veterans, immunocompetent, and either age ≥55 yr or with renal, hepatic, cardiac or pulmonary disease, alcoholism, or diabetes mellitus | Pneumococcal pneumonia or bronchitis | −15 | −48 to 11 | 43/1145 | 28/1150 |
| Pneumococcal pneumonia | −0.8 | −29 to 43 | 17/1145 | 16/1150 | ||
| All-cause pneumonia | −39 | −110 to 8 | 56/1145 | 41/1150 | ||
| All-cause mortality | −28 | −57 to −5 | 211/1145 | 171/1150 | ||
| Koivula 1(1997), 4-valent | Residents in a small town in Finland age ≥60 yr | All-cause pneumonia | −17 | −66 to 17 | 69/1364 | 64/1473 |
| Pneumococcal pneumonia | 15 | −43 to 50 | 26/1364 | 33/1473 | ||
| Örtqvist (1998), 23-valent | Immunocompetent persons age 50–85 yr who had been previously discharged from a hospitalization for community-acquired pneumonia in Sweden | All-cause pneumonia | −20 | −72 to 11 | 63/339 | 57/352 |
| Pneumococcal pneumonia | −28 | −150 to 34 | 19/339 | 16/352 | ||
| All-cause mortality | −5 | −88 to 38 | 29/339 | 28/352 | ||
| Vaccine-type bacteremic pneumonia | 79 | −77 to 98 | 1/339 | 5/352 | ||
| Honkanen (1999), 23-valent | Persons age ≥65 yr in northern Finland | All-cause pneumonia | −20 | −50 to 10 | 145/13980 | 116/12945 |
| Pneumococcal pneumonia | −20 | −90 to 20 | 52/13908 | 40/12945 | ||
| Vaccine-type bacteremia | 60 | −40 to 90 | 2/13980 | 5/12945 | ||
| Alfageme (2006), 23-valent | Immunocompetent COPD patients age 61–73 yr in Seville, Spain | Community-acquired pneumonia from pneumococcal infection or of unknown etiology | 24 | −24 to 54 | 25/298 | 33/298 |
| Pneumococcal pneumonia | 100 | P = .06 | 0/298 | 5/298 | ||
| Community- acquired pneumonia from organisms other than pneumococci | −7 | −62 to −0.01 | 8/298 | 1/298 | ||
| All community-acquired pneumonia | 3 | −52 to 38 | 33/298 | 34/298 | ||
| Furumoto (2008), 23-valent | Persons age 40–80 yr with chronic lung disease in Japan | Pneumonia | 0 | −105 to 52 | 13/87 | 12/80 |
| Acute pulmonary disease exacerbation | 30 | 9–46 | 42/87 | 55/80 | ||
| Maruyama (2010), 23-valent | Nursing home residents in Japan | All-cause pneumonia | 45 | 22–61 | 63/502 | 104/04 |
| Pneumococcal pneumonia | 64 | 32–81 | 14/502 | 37/504 | ||
| Kawakami (2010), 23-valent | Persons age ≥65 yr in Japan | All-cause pneumonia | 25 | −18 to 53 | 67/391 | 81/387 |
| All-cause pneumonia hospitalization | 27 | –16 to 56 | 60/391 | 76/391 |
a VE is calculated based on the formula VE = 1 − relative risk of disease in vaccinated versus unvaccinated. A negative VE estimate indicates a higher risk in vaccinated compared with unvaccinated persons; a zero VE estimate indicates no difference in risk between vaccinated and unvaccinated persons; and a positive VE indicates a lower risk in vaccinated compared with unvaccinated persons. If the confidence intervals for the VE estimate cross zero, that is, extend from negative to positive, or include zero, the VE estimate is not statistically significant.
b Study results obtained from Broome CV. Efficacy of pneumococcal polysaccharide vaccines. Rev Infect Dis . 1981;3(Suppl):S82–S96.
c Study results obtained from Fedson DS, Liss C. Precise answers to the wrong question: prospective clinical trials and the meta-analyses of pneumococcal vaccine in elderly and high-risk adults. Vaccine . 2004;22:927–946.
In the 1970s, a large randomized, double-blind, placebo-controlled clinical trial of a 14-valent polysaccharide vaccine, was conducted in Papua New Guinea. Study participants were rural highlanders who lived in poorly ventilated longhouses and had frequent respiratory infections. A total of 11,958 persons 10 years of age and older were enrolled in the trial and followed for 3 years. The risk of lower respiratory illness with vaccine-type pneumococci isolated from blood culture or lung aspirate was reduced by 86% (see Table 48.1 ).
The results of the South Africa trials provided the primary data used to support the licensure of PPSV14 in the United States in 1977. Since the mid-1970s, many randomized trials of PPSVs have been conducted among adults in the United States, Europe, and Japan. In 1983 the product was reformulated to include the most common adult serotypes, in the form of PPSV23. Efficacy results are described in detail below, with multiple meta-analyses finding PPSV23 reduces serotype-specific nonbacteremic pneumococcal pneumonia (NBPP) and IPD by 48% to 87%.
The currently available 23-valent vaccines (PPSV23) contain 25 µg of purified pneumococcal capsular polysaccharides from each of the following serotypes: 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F. Selection of the vaccine serotypes was based primarily on the distribution of serotypes causing IPD in various global case series in the late 1970s and early 1980s. , Before the introduction of PCV7 for children in the United States, these 23 serotypes accounted for more than 85% of isolates from adult IPD cases in the United States. , Since the introduction of PCV7 and then PCV13, and the resulting indirect protection, IPD caused by serotypes contained in PPSV23 have also declined in older adults. , , In 2018, pneumococcal serotypes contained in PPSV23 accounted for approximately 63% of IPD cases in adults 65 years of age and older in the United States, whereas PCV13 serotypes accounted for approximately 27% of adult IPD cases. ,
Two vaccine manufacturers currently produce PPSV23 Merck (Pneumovax 23) and Chengdu Institute of Biological Products for China. These vaccines consist of the 23 polysaccharide capsules as described above, dissolved in isotonic saline, with phenol 0.25% added as a preservative. The vaccine does not contain an adjuvant. Techniques that simultaneously measure the consistency of molecular size and the antigenicity of individual polysaccharides have helped to ensure uniformity between different lots of vaccine.
PPSV23 is administered as a single 0.5-mL dose intramuscularly or subcutaneously, however, intramuscular administration is considered less likely to cause injection-site reactions and, thus, is the preferred route. Influenza vaccine can be given simultaneously with PPSV23 at separate injection sites. , The vaccine is stored at 2°C to 8°C and is stable for 30 months.
Two types of standard assays are used to assess antibody responses to pneumococcal vaccines. Enzyme-linked immunosorbent assays (ELISAs) quantify the amount, but not the function, of serum antibody to pneumococcal capsular polysaccharide. In contrast, opsonophagocytic assays (OPAs) measure the degree to which serum antibody opsonizes pneumococci for uptake in vitro by human polymorphonuclear leukocytes and are, therefore, regarded as functional assays. Because ELISA measures total serotype-specific antipneumococcal antibody levels, including what are presumably functional and nonfunctional antibodies, the ELISA antibody concentrations may not correlate perfectly with functional antibody activity. For example, post-vaccination serum from healthy young adults and elderly adults may show similar levels of antibody by ELISA, but may provide very different levels of protection in vivo. Another complicating factor is that the capsular polysaccharide antigens used in the ELISA assays also contain contaminating cell-wall polysaccharide that is common to all pneumococci. Most individuals have natural antibodies to cell-wall polysaccharide. Consequently, absorption of the serum specimen with cell-wall polysaccharide has been used in recent years to remove non–serotype-specific cross-reacting antibodies. Even after removal of cell-wall polysaccharide antibodies, a variable amount of antibody to unrelated epitopes may still be measured by ELISA. A heterologous serotype 22F pneumococcal polysaccharide can also be used to adsorb antibodies to common epitopes. , Serotype 22F was chosen for this purpose because the capsular polysaccharide is readily available and at the time was thought not likely to be included in future conjugate vaccines. Other approaches include adsorption with serotypes 25 and 72, instead of 22F. , Such absorption has improved the correlation between immunoglobulin (Ig) G levels determined by both ELISA and OPA in most, , but not all, studies.
Further insight into functional status can be obtained by ELISA if avidity studies are performed. In these studies, a range of concentrations of sodium thiocyanate is added into the ELISA wells. If low concentrations reduce the amount of antibody measured by ELISA, the antibody is judged to be of low avidity which correlates with low OPA activity and poor protection of mice following challenge with serotype-specific S. pneumoniae .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here