Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The pleural, pericardial, and peritoneal cavities are lined by a single layer of flat mesothelial cells called the serosa . Normally, these cavities are collapsed and contain only a small amount of fluid (50 mL in each pleural cavity in adults ), enough to lubricate the adjacent surfaces as they move over each other with respiration, heartbeats, and intestinal peristalsis. In disease states, a greater amount of fluid—an effusion—accumulates. Effusions are classified clinically as transudative or exudative. Transudates result from an imbalance of hydrostatic and oncotic pressures. Common causes are congestive heart failure, cirrhosis, and the nephrotic syndrome. Exudates result from injury to the mesothelium, as occurs with pneumonia, malignancy, lupus, rheumatoid pleuritis, pulmonary infarction, or trauma. Compared with transudates, exudates have a relatively high lactate dehydrogenase and total protein concentration (Light’s criteria). The distinction is important because pleural involvement by a malignancy causes an exudate; cytologic examination is not needed for a transudate. Malignant tumors are a common cause of exudates because the serosal surfaces are a frequent site of metastasis for many tumors, as well as the site of origin for the asbestos-related tumor malignant mesothelioma.
Specimens are obtained by inserting a needle into the pleural space (thoracentesis), pericardial space (pericardiocentesis), or peritoneal cavity (paracentesis). When ultrasonography is used to guide thoracentesis, a higher rate of successful aspiration is achieved. Although peritoneal fluid is usually obtained through the abdominal wall, in women it can also be aspirated from the cul-de-sac through the vagina (culdocentesis). Fluid is also obtained by suction during thoracic, abdominal, or cardiac surgery.
Fluid is sent unfixed to the laboratory. To prevent clotting, which widely disperses cells, thus hindering their evaluation, fluid can be collected in heparinized bottles containing 3 to 5 IU of heparin per milliliter of capacity. If heparinized bottles are not available, the heparin can be poured into the bottle before the fluid is collected: contact with glass results in rapid clotting.
Fluid should be refrigerated at 4°C until the time of slide preparation. An effusion specimen is remarkably hardy—it can be refrigerated for 2 weeks or longer without compromising cellular morphology or antigenicity for immunostains. A variety of slide preparation methods are available. Slide preparation begins by shaking the container to disperse the cells evenly, then spinning a 50-mL aliquot (or the entire specimen if less than 50 mL) in a centrifuge. The supernatant is discarded, and the sediment is used to prepare smears, cytocentrifuge preparations, or thinlayer (“liquid-based”) preparations like ThinPrep and SurePath. The slides are usually alcohol-fixed and Papanicolaou-stained. If a hematologic malignancy is suspected, air-dried cytocentrifuge preparations are helpful; one slide is stained with a Romanowsky-type stain and the rest reserved, if needed, for immunocytochemical studies for lymphocyte surface markers. So-called “cell blocks” are especially useful as adjuncts to the “cytologic” preparations listed above. To prepare a cell block, the sediment is wrapped in filter paper, placed in a cassette, embedded in paraffin, and cut and stained in the manner of histologic sections. Before placing it in a cassette, however, it is helpful to coagulate the sediment. One common way is to add a few drops of plasma and several drops of a thrombin solution, but other methods are available, including an automated system called Cellient (Hologic Inc., Marlborough, MA). Clotting the specimen with drops of plasma and thrombin does not disperse the diagnostic cells as does a spontaneously formed clot, but rather congeals the sediment into a compact mass. If the fluid was not heparinized and clots are present, they should be removed and placed in cassettes for processing as cell block material.
Using more than one preparation method for effusions improves sensitivity for the detection of malignancy. A common preparation combination is one thinlayer slide and a cell block. Cell block sections are especially useful for special stains and immunohistochemistry because of the ease with which multiple duplicate slides can be prepared, the relative absence of obscuring background staining, and the standardization of the preparation for control slides. Cell blocks also make for excellent morphologic comparison with histopathologic sections (when, for example, the patient has had a prior breast biopsy) because they are fixed and stained in an identical manner.
In some laboratories, an unfixed wet smear stained with toluidine blue is prepared first to identify any fluid that contains large numbers of malignant cells. It is helpful to separate such fluids from the routine staining cycle to prevent cross-contamination.
Leftover fluid is stored in the refrigerator in case additional slides are needed. Fresh fluid is sometimes useful for other studies like flow cytometry and cytogenetic or molecular genetic analysis.
General categories such as “negative for malignant cells” and “positive for malignant cells” are commonly used to report results because they succinctly and unambiguously communicate an interpretation. Inconclusive findings—when abnormal cells are too poorly preserved or too few to support a definite diagnosis of malignancy—are commonly reported as “atypical cells present” (connoting a low degree of suspicion) or “suspicious for malignancy” (connoting a high degree of suspicion). Suspicious diagnoses occur in about 5% of specimens. In this situation, if the patient does have a malignancy involving the serosal cavity, fluid is likely to reaccumulate and subsequent specimens may be diagnostic of malignancy. Criteria for the adequacy of an effusion specimen have not been established.
Cytology is more sensitive than blind biopsy for detecting serosal malignancy (71% vs 45%), presumably because fluid provides a more representative sample. Estimates of the sensitivity of cytology for diagnosing serosal malignancy range from 58% to 71%. The cancer detection rate by cytology is increased by 2% to 38% when multiple sequential specimens are examined. This still leaves a substantial false-negative rate. Thoracoscopy is the procedure of choice for patients with a strong clinical suspicion of pleural disease but a negative cytology result.
The specificity of a cytologic diagnosis is very high: false-positive diagnoses occur in less than 1% of cases. When they occur, false-positive and false-suspicious diagnoses are caused by mesothelial cell atypia in the setting of pulmonary infarction, tuberculosis, chemotherapy, acute pancreatitis, ovarian fibroma, and cirrhosis. In children, false-positive results are from misinterpreting benign lymphoid cells as lymphoma or neuroblastoma. Immunocytochemistry is an essential adjunct to cytomorphology in selected cases and substantially improves diagnostic accuracy.
Confirming malignancy when morphology alone is equivocal
Distinguishing adenocarcinoma from mesothelioma
Screening an effusion for lobular breast cancer
Establishing the primary site of a malignant effusion, e.g., a patient with:
An occult primary
Multiple primaries
Establishing vulnerability of advanced lung and other cancers to targeted therapy and immunotherapy (e.g., ALK, ROS1, programmed cell death ligand 1)
Assessing receptor status (e.g., HER2) for patients with breast and gastric cancers
The circumstances outlined above represent common applications for immunohistochemistry. Antibodies against claudin-4, carcinoembryonic antigen (CEA), B72.3, Ber-EP4, and a number of other markers have high sensitivity and specificity for malignancy and are extremely useful in a variety of settings, particularly for resolving cases that are cytologically equivocal. Detailed discussion of specific applications is found in the sections that follow.
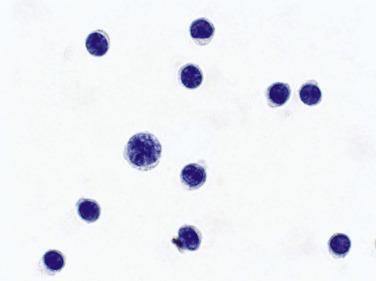
Benign effusions contain mesothelial cells, histiocytes, and lymphocytes in varying proportions. Because some bleeding is common during specimen collection, red and white blood cells are common contaminants.
Often numerous
Dispersed as isolated cells
Occasional small clusters with “windows”
Round cells
Round nucleus
Single nucleolus
Dense cytoplasm with clear outer rim (“lacy skirt”)
Mesothelial cells can be sparse or numerous in benign effusions ( Fig. 4.1 ). They are mainly dispersed as isolated cells or occasional small clusters. Large clusters with more than 12 cells are highly unusual in benign effusions. Binucleation and multinucleation are common, and a few mesothelial cells in mitosis can be seen in benign effusions. The dense cytoplasm reflects the abundance of tonofilaments, and the clear outer rim (“lacy skirt” or “halo”) corresponds to long, slender microvilli, better visualized with electron microscopy. Two or more mesothelial cells in groups are often separated by a narrow space or “window.” Less commonly, mesothelial cells have one or more cytoplasmic vacuoles.
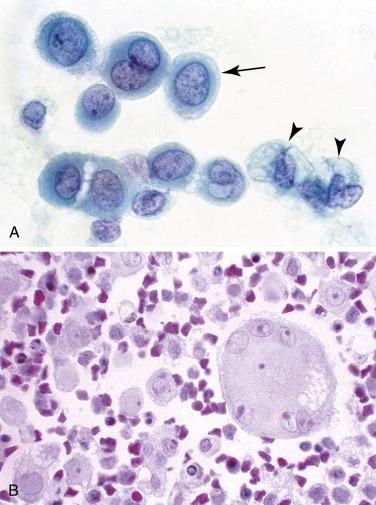
With acute or chronic injury, mesothelial cells undergo hyperplasia and hypertrophy and can have significant nuclear atypia, but they remain predominantly dispersed as isolated cells. Such “reactive” mesothelial cells generally comprise a spectrum of cells that range from normal to “atypical,” with variation in nuclear size, a coarse chromatin texture, irregular nuclear contours, or prominent nucleoli ( Fig. 4.2 ).
Mesothelioma
Metastatic malignancy
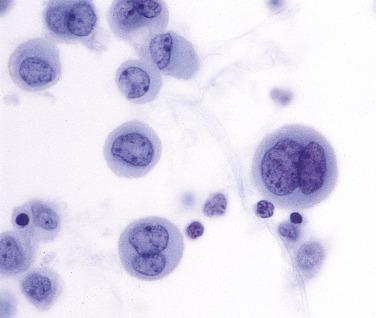
Malignant mesothelioma should be considered in any adult with unexplained ascites or a unilateral pleural effusion, particularly if imaging studies show findings characteristic of mesothelioma. Morphologic clues include cells that are much larger than normal mesothelial cells or fluid that contains numerous clusters of 12 or more cells, even if the cells themselves are not particularly atypical. Such large groups are uncommon in benign effusions. Clinical correlation is important: some medical conditions, including anemia, cirrhosis, lupus, pulmonary infarction, renal failure, and acquired immunodeficiency syndrome, are notorious causes of benign mesothelial atypia. On the other hand, if the patient has a large unexplained unilateral pleural effusion, particularly with radiographic evidence of pleural thickening, additional evaluation (eg, pleural biopsy), should be considered to exclude mesothelioma.
Metastatic malignancy should be considered when a population of cells is identified that is morphologically distinct from the mesothelial cells, histiocytes, and lymphocytes. In a minority of malignant effusions, a “second population” of cells is not evident. This is particularly true with lobular carcinoma of the breast and melanoma, the cells of which mimic normal histiocytes or mesothelial cells. Special stains are then needed to resolve the case.
Smaller nucleus than that of mesothelial cells
Nucleus often folded
Cytoplasm granular or vacuolated
No “windows” between adjacent cells
Dense aggregates (cell block sections)
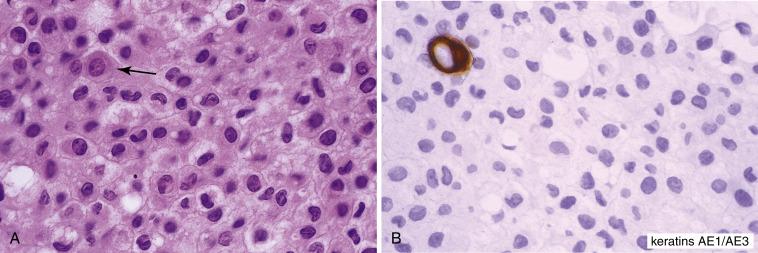
Some effusions contain abundant histiocytes ( Fig. 4.3A ). A particularly marked histiocytic reaction to irritation of the serosal surfaces has been termed nodular histiocytic/mesothelial hyperplasia . This is a nonspecific chronic inflammatory reaction that should not be misconstrued as a malignancy in cytologic or histologic specimens. When abundant, histiocytes can form aggregates on smears and liquid-based preparations, and they tend to sediment together in cell block preparations, forming mass-like aggregates that mimic malignancy. Immunohistochemistry can be useful to distinguish histiocytes from mesothelial cells and metastatic carcinoma: histiocytes are immunoreactive for CD68, CD163, and PU.1 and negative for keratin proteins ( Fig. 4.3B ); the reverse is true for mesothelial cells and metastatic carcinoma.
In many benign disorders, effusions give a nonspecific cytologic picture. Thus pleural fluid in congestive heart failure or pulmonary infarction is morphologically indistinguishable from pericardial fluid caused by renal failure and peritoneal fluid due to cirrhosis. Fortunately, the features of some benign conditions are sufficiently characteristic to narrow the differential diagnoses or even indicate the specific cause. To give a very unusual example, finding undigested meat and vegetable matter in pleural fluid strongly suggests esophageal rupture (Boerhaave’s syndrome).
Acute pleuritis, pericarditis, and peritonitis are usually the result of bacterial infection. Bacterial infection of the pleura occurs in the setting of pneumonia, which secondarily involves the overlying pleura and results in an empyema. The classic symptoms are cough, fever, sputum production, and chest pain, but elderly patients often present with nonclassic symptoms (anemia, fatigue, failure to thrive) and thus may not be diagnosed in a timely fashion. An empyema requires drainage.
Acute infection of the peritoneal cavity is often secondary to inflammation of or injury to the bowel, as in spontaneous bacterial peritonitis. It is important to screen such cases carefully for malignant cells because acute infection can be a complication of metastatic malignancy.
A pleural effusion is considered “eosinophilic” when eosinophils account for 10% or more of the nucleated cells present. Between 5% to 16% of exudative effusions are eosinophilic effusions. The most common causes are pneumothorax, hemothorax, and thoracic surgery. The introduction of air or blood into the pleural space, a common cause of an eosinophilic effusion, can occur simply with repeated thoracenteses. Less common causes include drug reactions, parasitic infections, pulmonary infarction, and the Churg-Strauss syndrome. In about one-third of cases the origin remains obscure. Most cases resolve spontaneously. Eosinophilic pericardial and peritoneal effusions are less common than eosinophilic pleural effusions.
Cytologic preparations are usually cellular and remarkable for a high concentration of eosinophils. With alcohol-fixed Papanicolaou-stained slides, the defining “eosinophilic” cytoplasmic granules are either orangeophilic or pale-green and inconspicuous, and the cells are identified more on the basis of their bilobed nuclei ( Fig. 4.4 ). The granules are brightly eosinophilic on cell block preparations stained with hematoxylin and eosin and on air-dried Romanowsky-stained slides. Charcot–Leyden crystals are present in some cases and, curiously, are more common in fluids that have been refrigerated for more than 24 hours.
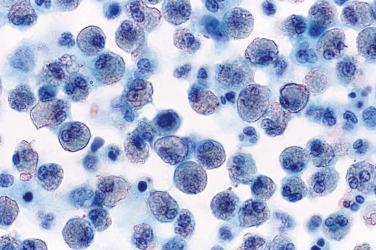
A pleural effusion consisting mostly of small lymphocytes is a relatively common but nonspecific finding ( Fig. 4.5 ). Cytologic preparations are often highly cellular and composed almost exclusively of dispersed small lymphocytes. Mesothelial cells and histiocytes are either conspicuously absent or present in only small numbers.
Malignancy
Tuberculosis
Status post coronary artery bypass
Despite the absence of malignant cells, a malignancy is a common cause of a lymphocytic effusion. A malignancy involving the pleura might evoke a peritumoral lymphocytic response, but the tumor itself might not shed cells into the fluid. It is not uncommon for the initial pleural fluids in a patient with pleural mesothelioma to consist only of lymphocytes. Alternatively, the malignancy might be nearby (e.g., in the lung) and obstructing lymphatic outflow but not directly involving pleural surfaces.
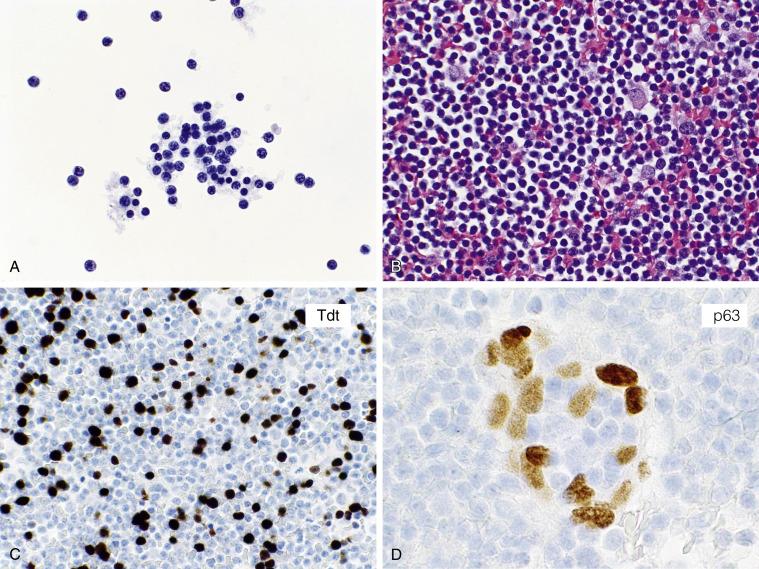
An effusion is very rarely the initial manifestation of a lymphoid malignancy. Thus it is not cost-effective to evaluate every lymphocytic effusion consisting of mostly small round lymphocytes by flow cytometry or immunocytochemistry. On the other hand, if the patient is known to have a lymphoma or thymoma, an immunophenotypic workup is justified to exclude pleural involvement ( Fig. 4.6A–D ).
Effusions caused by small lymphocytic lymphoma and chronic lymphocytic leukemia (CLL) are very uncommon. Because these are B-cell neoplasms, immunocytochemical or flow cytometric evaluation of lymphocyte surface markers is helpful in confirming the diagnosis. In a patient with CLL and a peripheral lymphocytosis, however, contamination of the effusion by peripheral blood during a traumatic tap should be excluded before diagnosing pleural involvement. Even a small amount of blood containing leukemic cells can result in a false-positive diagnosis.
The diagnosis of tuberculosis can be confirmed by microbiologic studies or pleural biopsy, which reveals caseating granulomas and acid-fast organisms. The differential diagnosis includes other benign effusions of nontuberculous origin, as in patients after coronary artery bypass surgery.
Less than 5% of patients with rheumatoid arthritis develop symptomatic pleural involvement by their disease. In almost all cases, joint disease precedes the development of pleuritis, but occasionally pleuritis precedes or is synchronous with the onset of joint disease. The pleural effusion is usually unilateral but can be bilateral, and some patients have a synchronous pericardial effusion. Radiographic studies reveal pulmonary nodules in a minority of patients; presumably, these are rheumatoid nodules. The effusion can last for days, months, or sometimes several years. Most resolve without any specific treatment (except perhaps thoracentesis), but some patients require corticosteroids.
The cytologic picture is so characteristic that it has been termed pathognomonic. Examination of pleural fluid therefore can be extremely useful to confirm the diagnosis of rheumatoid pleuritis and exclude the possibility of coincident disease, especially a malignancy.
Abundant clumps of granular debris
Macrophages
Cytologic preparations are sparsely or moderately cellular. An abundant granular material dominates the picture ( Fig. 4.7A ). It can stain green, pink, red, or orange with the Papanicolaou stain, and it aggregates into small and large clumps with irregular edges. Large, island-like masses can be appreciated in cell block material. The predominant cell is the macrophage, which is round or spindle-shaped; multinucleated macrophages are seen in most but not all cases ( Fig. 4.7A and B ). Lymphocytes and polymorphonuclear leukocytes may be seen. Mesothelial cells are noticeably absent.
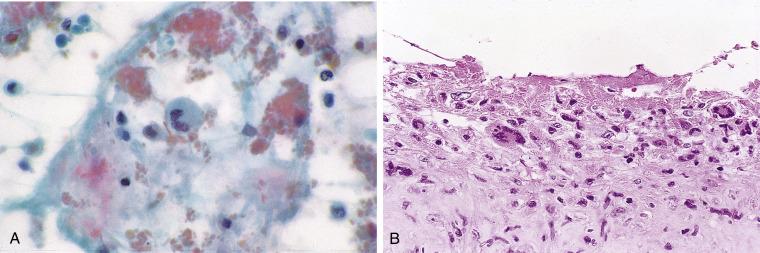
The characteristic granular debris is different from fibrin, which is usually strand-like rather than coarsely granular. Although the elongated macrophages resemble the spindle cells seen in squamous and other cancers, their nuclei are normochromatic.
About one-third of patients with systemic lupus erythematosus (SLE) develop a pleural or pericardial effusion. Peritoneal effusions are less common but do occur. Rarely, an effusion is the initial manifestation.
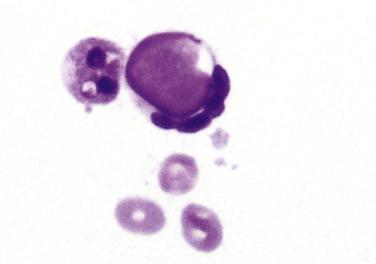
The characteristic cell is the lupus erythematosus (LE) cell, a neutrophil or macrophage that contains an ingested cytoplasmic particle called a hematoxylin body . The hematoxylin body may be green, blue, or purple with the Papanicolaou stain, and magenta with Romanowsky-type stains, and has a glassy, homogeneous appearance ( Fig. 4.8 ). Filling the cytoplasm of the neutrophil or macrophage, it often pushes the nucleus to one side, indenting it into a crescent-like shape. Hematoxylin bodies are thought to represent degenerated nuclei. Similar cells that contain ingested nuclei with a visible chromatin structure (rather than the glassy, structureless hematoxylin body) are called tart cells after the patient in whom they were first described. LE cells are present in just 27% of effusions in patients with SLE, and only in those with a known diagnosis of SLE.
Most viral pneumonias associated with a pleural effusion result in a nonspecific cytologic picture. The cytopathic changes characteristic of the herpes viruses and cytomegalovirus are rarely seen in serous effusions. Although fungal infections are common in immunocompromised patients, organisms are rarely seen in pleural, pericardial, and peritoneal fluids. Candida species, Cryptococcus neoformans, Coccidioides immitis, Blastomyces dermatitidis , and Aspergillus niger have been described in fluids in rare instances.
Pneumocystis jirovecii (carinii) has been identified in pleural and peritoneal effusions from immunocompromised patients. Papanicolaou stains show foamy exudates similar to those seen in respiratory specimens. The trophozoites measure 2.5 to 5.0 μm and have pale cytoplasm and a dot-like nucleus; they may be intracellular (within macrophages) or extracellular and are well seen on air-dried preparations stained with a Romanowsky-type stain. Cyst forms measure 4 to 7 μm and can be seen with special stains like the methenamine silver stain.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here