Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Challenged by complex clinical problems, the pace of innovation in plastic surgery has accelerated steadily during the past 30 years. The specialty benefits from the absence of anatomic or organ system boundaries and from the collaboration with other surgical specialists. Plastic surgeons discover new reconstructive and aesthetic challenges and continuously make medical progress. With growing sophistication, plastic surgery has matured into areas of specialization, including surgery for congenital abnormalities, maxillofacial surgery, breast surgery, hand surgery, head and neck surgery, skin and soft tissue surgery, aesthetic surgery, body contouring, wound care, microsurgery, and burn care.
Plastic surgeons, in a relatively small specialty, stay aware of innovations in each of these areas and are quick to adopt and spread these ideas into all realms of surgery. Through the combination of research and clinical experience, it is not surprising that unique solutions for perplexing clinical problems sustain the momentum of innovation in the field.
The concept of a reconstructive ladder is used to guide surgical reconstruction, ascending from simple to complex reconstructive techniques in a systemized way that considers the requirements of the defect to be repaired. Direct closure is the simplest and most straightforward technique. This may be precluded by the size of the wound or distortion of the surrounding tissue. Thus a more complex closure technique such as skin graft, local flap, or distant flap that brings in additional tissue, is required, increasing in level of complexity. Microvascular free tissue transfer represents the most complex flap option, allowing for transfer of various tissue type including skin, fascia, muscle, bone, nerve, and lymph.
When the concept of the reconstructive ladder is used, the triad of form, function, and safety is the basis for setting the reconstructive goals for any given defect. For example, in reconstructing the face, awareness of form would suggest a more complex technique such as tissue expansion instead of the simpler technique of skin grafting because it is optimal to restore with skin and soft tissue of the same thickness, texture, and color. Improvement in surgical techniques has also gradually transitioned the concept to a “reconstructive elevator,” where it emphasizes the importance of selecting the most appropriate level of reconstruction as opposed to defaulting to the least complex.
Good closure technique starts with an incision with the scalpel at right angles to the skin and continues with careful handling of tissue to avoid devitalizing the skin margins, debridement of skin edges if needed, eversion of the wound margin, and precise approximation without tension. The skin edges need to be lined up at the same level, and wound edges should just touch each other.
Minimizing tension is essential to reduce scarring. This can be done by using buried deep dermal and subdermal sutures to lessen tension on the skin sutures. Minimizing tension can be accomplished also by aligning skin incisions along relaxed skin tension lines. These lines of minimal tension, also called natural skin lines, wrinkle lines, or lines of facial expression, run at right angles to the long axis of the underlying muscles. Closure placed in one of these furrows will be under minimal tension and will heal with minimal scarring.
The development of negative pressure wound therapy in the early 1990s has expanded the option for managing wounds that cannot be closed immediately by direct closure or flap. Its application has been shown to increase the rate of granulation formation, decrease edema, decrease frequency of dressing changes, increase time to closure, and control bacterial colonization and proliferation. It has been used commonly as a bridge to creating a viable wound bed for skin graft.
A skin graft is a segment of dermis and epidermis that is separated from its blood supply and donor site and transplanted to another recipient site on the body. Survival of the transplanted skin graft requires a vascularized wound recipient bed. Graftable beds with adequate blood supply include healthy soft tissues, periosteum, perichondrium, paratenon, and bone surface that is perforated to encourage granulation tissue growth. Poor wound surfaces with inadequate blood supply include exposed bone, cartilage, tendon, implant, and fibrotic chronic granulation tissue. The wound must be free of infection and debris and interposed as a barrier between the graft and bed.
Skin grafts are classified in the following manner: autograft, self; allograft, other person; homograft, same species; and xenograft, different species. Partial-thickness skin grafts consist of the epidermis and a portion of the dermis and are called split-thickness skin grafts (STSGs). Full-thickness skin grafts (FTSGs) include the epidermis and entire dermis and portions of the sweat glands, sebaceous glands, and hair follicles. The STSG is harvested with a dermatome that can be adjusted for width and depth, usually in strips of 0.006- to 0.024-inch in thickness. The STSG can be meshed by cutting slits into the sheet of graft and expanding it. Meshed grafts are useful when there is a paucity of available donor skin, the recipient bed is bumpy or convoluted, or the recipient bed is suboptimal as with exudate. STSG can be taken from anywhere on the body; donor site considerations include color, texture, thickness, amount of skin required, and scar visibility. The STSG takes readily on the recipient site, and the donor site reepithelializes quickly from the residual dermis. Its disadvantages are contracture over time, abnormal pigmentation, and poor durability if subject to trauma. The FTSG is removed with a scalpel and is necessarily small because the donor site must be sutured closed. Containing skin appendages, the FTSG can grow hair and secrete sebum to lubricate the skin, has the color and texture of normal skin, and has the potential for growth. In general, FTSGs are taken from areas at which the skin is thin and can be spared without deformity, such as the upper eyelids, postauricular crease, supraclavicular area, hairless groin, or elbow crease. The greater thickness makes the FTSG more durable than the STSG, but this thickness also means that the graft take is not as predictable because more tissue must be revascularized from the recipient bed.
The take of either type of skin graft occurs in three phases:
Plasmatic circulation, also called serum imbibition, during the first 48 hours nourishes the graft with plasma exudate from host bed capillaries.
Revascularization starts after 48 hours with two processes. The primary is neovascularization in which blood vessels grow from the recipient bed into the graft, and the secondary is inosculation in which graft and host vessels form anastomoses.
Organization begins immediately after grafting with a fibrin layer at the graft-bed interface, holding the graft in place. This is replaced on postgraft day 7 with fibroblasts; in general, grafts are securely adherent to the bed by days 10 to 14.
Sensibility returns to the graft over time, with reinnervation beginning at approximately 4 to 5 weeks and being completed by 12 to 24 months. Pain returns first, with light touch and temperature returning later.
The most common cause of skin graft failure is hematoma under the graft, where the blood clot is a barrier to contact of the graft and bed for revascularization. Similarly, shearing or movement of the graft on the bed will preclude revascularization and cause graft loss. Additional causes are infection, poor quality of the recipient bed, and characteristics of the graft itself, such as thickness or vascularity of the donor site. Dressings can prevent some impediments to graft take. A light pressure dressing minimizes the risk of fluid accumulation. A bolster or tie-over dressing left in place for 4 or 5 days improves survival by maintaining adherence of the graft to the bed, minimizing shearing, hematoma, and seroma. A negative pressure wound therapy device can be placed on the grafted surface to stabilize the graft in place; this is especially useful for larger wounds with an irregular three-dimensional (3D) surface.
Skin grafts composed of tissue-cultured skin cells are used for the treatment of burns or other extensive skin wounds. Human epidermal cells in a single-cell suspension are grown in monolayers in vitro during a period of 3 to 6 weeks. Concerns with tissue-cultured skin are fragility, sensitivity to infection, length of time for cultivation, and potential risk of malignancy caused by mitogens present during culturing.
Tissue expansion is a technique that uses a mechanical stimulus to induce tissue growth so as to generate soft tissue for reconstructive use. It involves placing a prosthesis that is gradually enlarged by the addition of saline, which causes an increase in the surface area of the overlying soft tissue. Initially, the expanded skin is the result of stretching as interstitial fluid is forced out of the tissue, elastic fibers are fragmented, viscoelastic changes (termed creep) occur in the collagen, and adjacent mobile soft tissue is recruited. Over time, it is not just stretching but actual growth of the skin flap that creates an increase in the surface area with accompanying increases in collagen and ground substance. Histologic changes in the skin include dermal thinning, epidermal thickening, subcutaneous fat atrophy, and no effect on the skin appendages.
Tissue undergoing expansion must have the capacity for growth. Prior irradiation or scar formation may slow the rate of expansion or make it impossible. Expanders perform poorly under skin grafts, under very tight tissue, and in the hands and feet. Contraindications include expansion near a malignant neoplasm, a hemangioma, or an open leg wound.
Expanders come in various styles, and sizes range from a few cubic centimeters to 1 L or more. They can be round, square, rectangular, or horseshoe shaped. The injection ports can be remote or integrated into the wall of the expander so that no dissection of a pocket for the remote port is required. The envelope can be smooth or textured for better stabilization at one location in the tissue pocket.
Expanders should be placed under tissue that best matches the lost tissue ( Fig. 69.1 ). Normal landmarks, such as the eyebrow or hairline, should not be distorted. The incision to insert the expander can be placed at the edge of the defect that later will be excised because a scar in this position will be removed at the time of the next surgery. The most common reason for expander failure is construction of a pocket that is too small for the device. An expander with a curled edge may later protrude through the incision or erode through the overlying tissue. Filling of the expander is initiated approximately 2 weeks after surgery and is continued at weekly or biweekly intervals. The rate of expansion is limited by the relaxation and growth of the tissue overlying the expander. Pain and palpable tightness over the expander are clinical indicators that guide the rate of expansion. The patient is ready for the second surgical procedure when the expanded tissue is adequate to produce the desired effect. If the flap is to be advanced, it must be measured to ensure that it is large enough and has the correct geometry to cover the defect. At the second surgery, the skin is incised through the old scar, the capsule around the expander is opened, the expander is removed, and the expanded flap is advanced over the defect. It is important to confirm that the expanded tissue will replace the defect before the defect is excised. If it is not sufficient, this is handled by subtotal resection of the defect and leaving the expander in place for a second round of expansion.
Tissue expansion can be combined with other reconstructive techniques. Expander placement in the subcutaneous or submuscular plane can facilitate later repair of abdominal wall hernias. Preexpansion of transposition or rotation flaps increases the amount of tissue, enhances the flap’s blood supply, and lessens donor site morbidity. Preexpansion of free flaps increases the surface area and augments the blood supply of the future flap, may make primary closure of the free flap donor site possible, and thins the flap, which may be desirable for reconstructions calling for thinner and more pliable coverage. A disadvantage of the preexpansion of free flaps is the time needed for the expansion process because delay may not be acceptable for oncologic defects and complex wounds. In addition, the preexpanded free flap procedure is technically more difficult because of distortion of the vascular pedicle.
The advantages of expansion are the provision of matching tissue for reconstruction, normal sensibility of the transferred tissue, negligible donor defect, and enhanced success of preexpanded traditional flaps because of enhanced vascularity.
An alloplastic material is a synthetic substance implanted in living tissue. Its advantages are availability when autologous tissue is not available and the absence of donor site morbidity or scarring. Nonbiodegradable alloplastic materials do not undergo resorption as do bone or cartilage grafts. In addition, the implant can be manufactured to meet special needs, such as for controlled-release drug delivery systems.
The tissue response to different implants varies with the chemical composition and the microstructure and macrostructure of the synthetic material; these differences are used clinically. For example, the vigorous tissue ingrowth with polypropylene mesh in a hernia repair provides strong and lasting support, whereas the fibrous encapsulation around a silicone tendon prosthesis ensures free gliding of a tendon graft. However, certain properties (noncarcinogenic, nontoxic, nonallergenic, nonimmunogenic) and concerns (mechanical reliability, biocompatibility) are common to all implants.
Categorization by chemical composition is the most useful framework for the description and comparison of surgical implants. This materials scientific approach recognizes that the commonality of different groups of materials arises more from their composition than from the organ systems in which they are used. Chemically, there are three major classes of biomaterials: metallic, ceramic, and polymeric. Although they are polymers, biologic materials such as collagen need to be classified separately because they introduce new considerations of protein antigenicity.
Metals in clinical use are stainless steel, Vitallium (cobalt-chromium-molybdenum alloy), and titanium. The general requirements for a metal device are mechanical strength, suitable elastic modulus, comparable density and weight to those of the surrounding tissue, and resistance to corrosion. Very few metals have sufficient corrosion resistance to be used in the hostile environment of the living organism. Corrosion results from the electrochemical activity of unstable metal ions and electrons in physiologic salt solutions; corrosion products can be cytotoxic, leading to pain, inflammation, allergic reactions, and loosening of the device.
Ceramic materials have high stability and resistance to chemical alteration and include carbon compounds such as hydroxyapatite, which is capable of bonding strongly to adjacent bone. Used to augment the facial skeleton or as a bone graft substitute, it is a permanent microporous implant that undergoes osseointegration by providing a matrix for the deposition of new bone from adjacent living bone.
Polymers are large, long-chain, high–molecular-weight macromolecules made up of repeated units, or mers. There are a vast number of these synthetic implants in surgical use. To a large extent, this is because of the ease and low cost of fabrication and because they can be processed easily into tubes, fibers, fabrics, meshes, films, and foams. Polymers vary across an enormous range of chemical compositions, the degree of polymerization, cross-linking between chains, and the presence of chemical additives such as plasticizers to increase flexibility or resins to catalyze polymerization. With the exception of resorbable polymers, most surgical polymers are relatively inert and stimulate fibrous encapsulation. The physical form of the implant, solid versus mesh or smooth versus rough, will determine whether the entire structure is encapsulated as a whole or whether fibrous tissue will penetrate the interstices. Tissue reaction to the implant is influenced also by the chemical composition, factors such as hydrophilicity and ionic charge, and the chemical durability of the polymer. Silicone rubber, polytetrafluoroethylene, and polyethylene terephthalate polyester (Dacron) are among the most stable of polymers, whereas polyamide (nylon) is vulnerable to hydrolytic reaction and undergoes substantial degradation.
A flap consists of tissue that is moved from one part of the body to another with a vascular pedicle to maintain blood supply. The vascular pedicle may be kept intact, or it can be transected for microvascular anastomosis of the flap vessels to vessels at another site.
Skin-bearing flaps are classified according to three basic characteristics—composition, method of movement, and blood supply. Composition refers to the tissue contained within the flap, such as cutaneous, musculocutaneous, fasciocutaneous, osseocutaneous, adipofascial, and sensory flaps. The method of movement is local transfer as with advancement or rotation flaps, distant transfer as with pedicle flaps from the abdomen to the perineum, or microvascular free flaps.
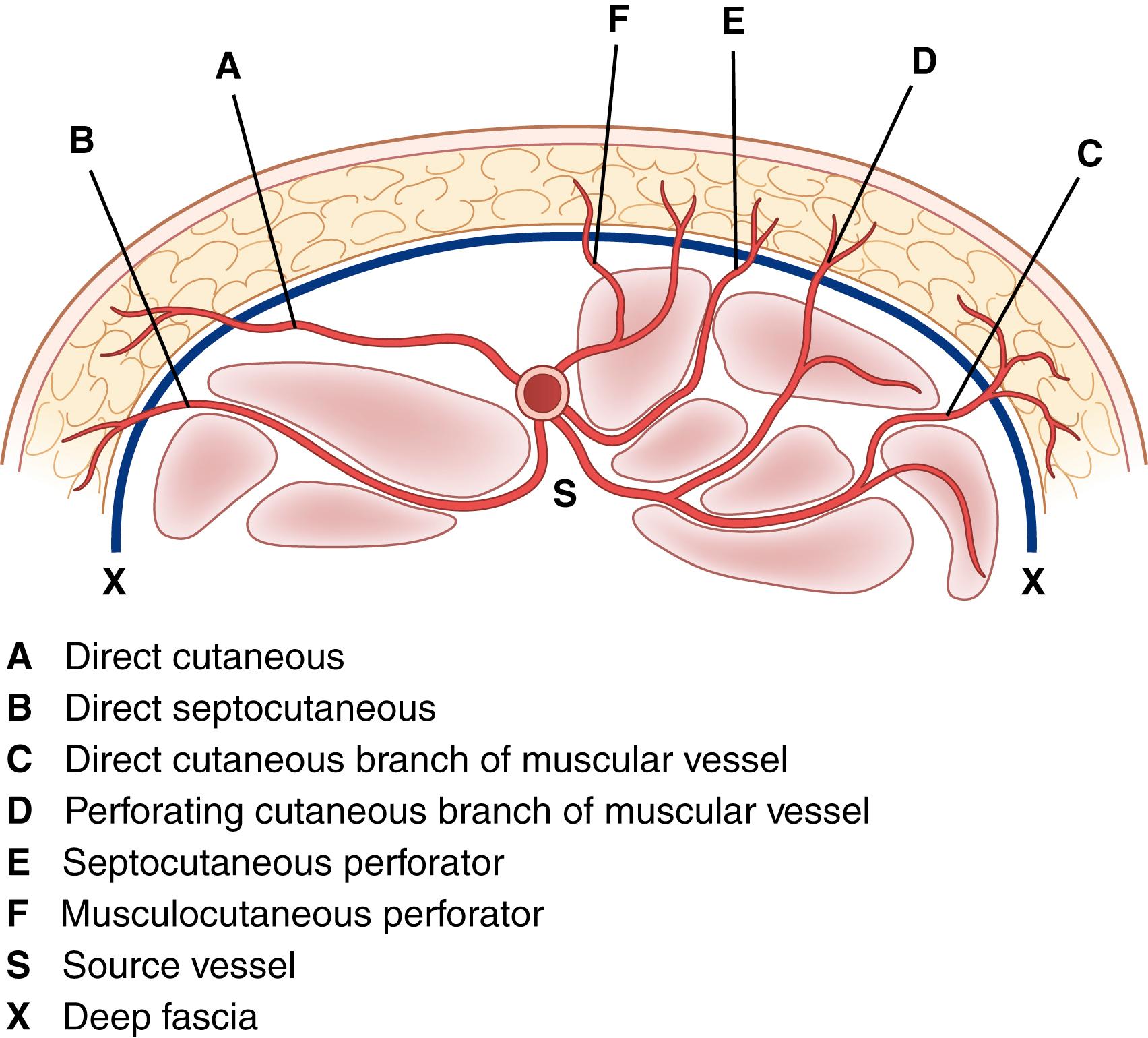
With regard to blood supply, arteries perfusing the surgical flap reach the skin component in two basic ways. Musculocutaneous arteries travel perpendicularly through muscle to the overlying skin. Septocutaneous arteries arising from segmental or musculocutaneous vessels travel with intermuscular fascial septa to supply the overlying skin. With either of these patterns, the flap can have a random pattern, which means that it derives its blood supply from the dermal and subdermal vascular plexus of vessels supplied by perforating arteries. Alternatively, it can be an axial flap designed to include a named vessel running longitudinally along the axis of the flap to penetrate the overlying cutaneous circulation at multiple points along the course of the flap’s length to provide greater length and reliability.
Local flaps contain tissue lying adjacent to the defect that usually matches the skin at the recipient site in color, texture, hair, and thickness. Flaps should be the same size and thickness as the defect and be designed to avoid distortion of local anatomic landmarks, such as the eyebrow or hairline. They can be planned so that the donor site can be closed directly. Local flaps rely on the inherent elasticity of skin and are most useful in the older patient whose skin is looser. In some cases, the site from which the flap is raised is closed with a skin graft. Commonly used local skin flaps include rotation flaps, transposition flaps, and advancement flaps. By definition, these flaps are random flaps because they are raised without regard to any known blood supply other than the subdermal plexus.
Failure of a skin flap usually involves necrosis of the most distal portion of the transferred tissue. This could be caused by a flap design in which the size of the flap exceeds its inherent vascular supply, or it could be a result of extrinsic mechanical compromise of the flap pedicle by pressure from a hematoma, compressive dressings, or twisting or kinking of the flap. Measures to optimize viability include proper flap design and avoidance of extrinsic pedicle compression, undue tension with wound closure, and venous congestion caused by excessive flap dependency.
Consideration of a muscle as a potential flap is possible because muscles have an independent, intrinsic blood supply. This vascular pedicle may be a dominant one, capable of sustaining the entire muscle independently. A minor pedicle, regardless of the size of the vessel, is defined as one that maintains only a lesser portion of the muscle. Many muscles have multiple unrelated sources of blood supply so that each nourishes only a segment of the muscle, thus called segmental pedicles. Some muscles have both a dominant pedicle and a segmental blood supply. One example is the latissimus dorsi muscle with a dominant pedicle, the thoracodorsal artery in the axilla, and additional segmental perforating branches from the intercostal and lumbar vessels posteriorly. Muscle flaps are classified according to their principal means of blood supply and the patterns of vascular anatomy ( Fig. 69.2 ):
Type I: Single pedicle (e.g., gastrocnemius, tensor fascia lata)
Type II: Dominant pedicle with minor pedicles (e.g., gracilis, trapezius)
Type III: Dual dominant pedicles (e.g., gluteus maximus, serratus anterior)
Type IV: Segmental pedicles (e.g., sartorius, tibialis anterior)
Type V: Dominant pedicle, with secondary segmental pedicles (e.g., latissimus dorsi)
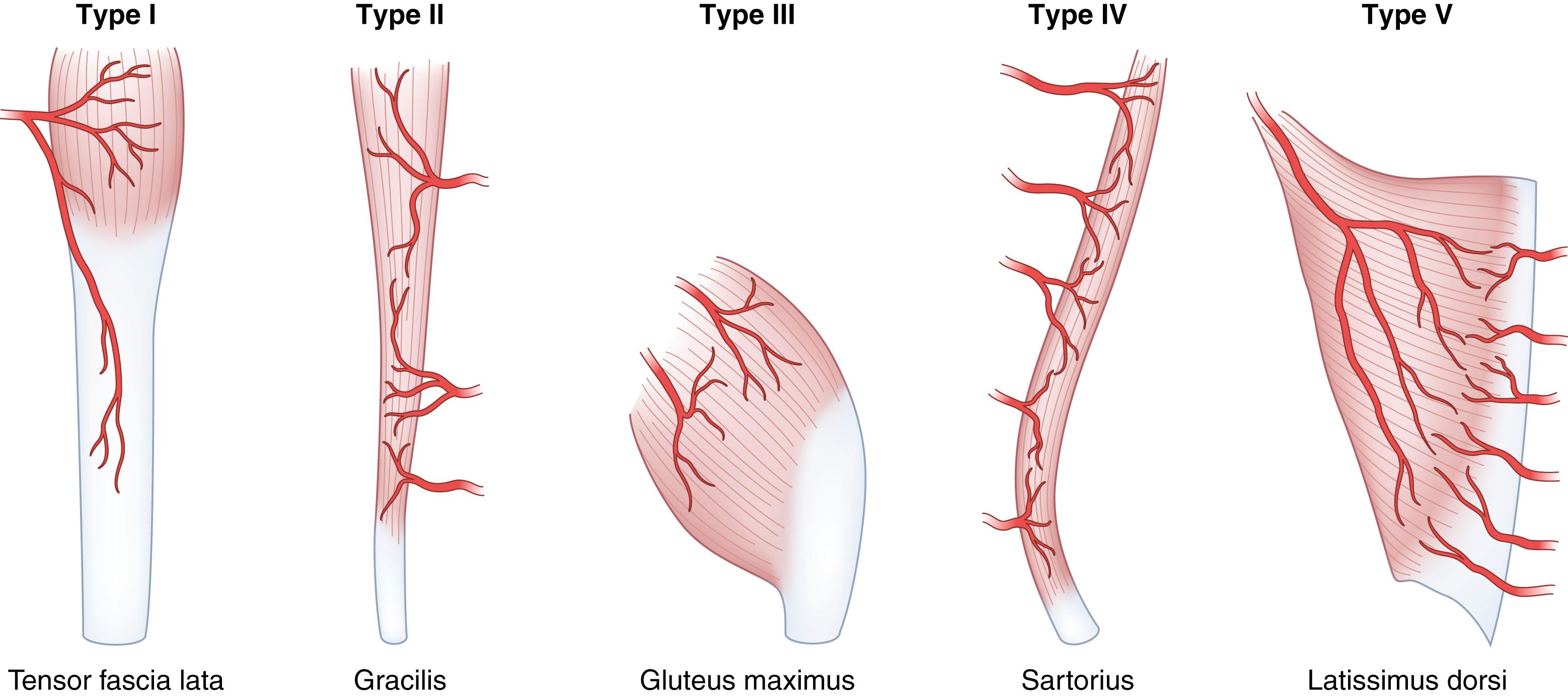
In terms of reliability of the vascular anatomy and usefulness as a flap, large muscles with a recognized dominant pedicle supplying most of a flap (types I, III, and V) are most useful. The territory of the pedicles in type II muscles may vary, and type IV muscles are useful only when smaller flaps are needed. Connections between regions within a given muscle supplied by more than one pedicle are through small-caliber choke vessels with bidirectional flow. An example of a flap depending on these choke vessels is the transverse rectus abdominis musculocutaneous (TRAM) flap in which the superior epigastric pedicle alone can support the lower half of the muscle normally supplied by the inferior epigastric vessels below the watershed level at the umbilicus. In muscle, venous territories are parallel with arterial vessels (i.e., venous outflow is adjacent to and in a direction opposite from flow in the major arterial pedicles). In a pattern analogous to that of the bidirectional choke vessels, venous flow from one territory to another occurs through oscillating veins that are devoid of valves.
Compared with skin flaps, muscle flaps have more robust blood supply and demonstrate superiority in wounds compromised by irradiation or infection. The vascular anatomy is predictable and easily identifiable, and the muscle can be put into use as a functional unit for a dynamic tissue transfer. A major consideration with muscle flaps is whether the loss of function is acceptable. In an effort to limit the functional loss associated with use of an entire muscle, methods of functional preservation have been devised. If some portion of the muscle chosen as the flap is left innervated and attached at its insertion and origin, function is preserved after transfer of the remainder of the muscle. This can be done by splitting the muscle into segments, provided each is supplied by a different dominant pedicle.
A musculocutaneous flap, also called a myocutaneous flap, is a muscle flap designed with an attached skin paddle. Each superficial skeletal muscle carries blood supply to the skin lying directly over it through musculocutaneous or septocutaneous perforators. The number and pattern of these musculocutaneous perforators vary with each specific muscle; this means that the extent of the skin territory is different for each muscle unit. Through dissection of injected cadaver specimens, the number, size, and location of perforators have been described; this information, combined with clinical experience, is used to predict the cutaneous territories on the superficial muscles.
In addition to the musculocutaneous branches supplying the overlying skin, source vessels branch within the muscle into channels that perforate the deep fascia to anastomose within the subdermal plexus and nourish the skin. The source vessel and its perforating muscular branches can be dissected out of the muscle without jeopardizing skin perfusion. This requires intramuscular dissection to separate the perforators from the muscle and is the basis for the development of perforator flaps. This makes the retention of muscle unnecessary for the survival of the skin paddle; thus, its inclusion serves a passive role, primarily to avoid tedious intramuscular dissection of the vascular tree. In effort to preserve the muscle unit, a growing number of perforator flaps have been described, including the deep inferior epigastric perforator flap, which carries the same skin and subcutaneous tissue as the TRAM flap for breast reconstruction. By sparing of the rectus muscle, abdominal wall bulging and other complications are less. The superior gluteal artery perforator flap carries the skin territory of the gluteus maximus musculocutaneous flap and preserves the muscle.
Growing knowledge about musculocutaneous skin circulation has led to the identification of vascular pedicles emerging between muscles, traveling in the intermuscular septum, and entering the deep fascia. Termed septocutaneous perforators , these vessels supply the fascial plexus, which gives off branches to an overlying cutaneous territory.
The anatomic features of a fasciocutaneous flap are the fascial feeder vessels, also called the fascial perforators , which are branches of source vessels to a given angiosome. An angiosome is the 3D block of tissue supplied by a source artery; the entire surface of the body is composed of a multitude of angiosome units. The fascial feeder vessels do not perforate the deep fascia but terminate within the fascial plexus. The fascial plexus is not a structure but a confluence of multiple adjacent vascular intercommunications that exist at the subfascial, fascial, suprafascial, subcutaneous, and subdermal levels ( Fig. 69.3 ).
The concept of fasciocutaneous flaps arose from the observation that the size of a skin flap could be increased if it were oriented along a longitudinal axis on the extremity and if the deep fascia were included. Subsequent anatomic studies have confirmed the presence of septocutaneous pedicles supplying a regional fascial vascular system. The larger septocutaneous pedicles tend to be fairly constant in location, and a number of specific fasciocutaneous flaps have come into wide use (e.g., anterior lateral thigh flap, radial forearm flap, and lateral arm flap).
The design of fasciocutaneous flaps has been learned by experience, and the limits of these flaps still remain to be discovered. There are no set rules because deep fascial perforators are frequently anomalous in caliber and location, not only among individuals but also on opposite sides of the same person. The expected range of flap size is learned through the experience of other surgeons.
One of the most useful features of a fasciocutaneous flap is that it can be distally based. Unlike in a muscle flap in which the dominant pedicle is closest to the heart, blood flow in the fascial plexus is multidirectional. The flow to the corresponding angiosome is equivalent for a distal fascial perforator and proximal fascial perforator. This means that a flap pedicle can be distally based with a reliable skin territory and transposed to cover a defect located at the end of an extremity. For example, the distal-based sural flap uses the skin of the calf, based on a distal perforator of the peroneal artery, for transfer to cover the foot and ankle, thus obviating the need for a free microvascular transfer.
In addition to the advantages provided by a distally based flap design, a fasciocutaneous flap can confer sensibility if a sensory nerve is included. Compared with musculocutaneous flaps, they are accessible on the surface of the body and have the great advantage that no functioning muscle is expended. The comparative disadvantages are the anatomic anomalies in the fascial vascular system and the unanswered question as to whether they are as effective as muscle in the irradiated or infected wound.
Perforator flaps evolved as an improvement over musculocutaneous and fasciocutaneous flaps. They rely on evidence that neither a passive muscle carrier nor the underlying fascial plexus of vessels is necessary for flap survival, provided the musculocutaneous or fasciocutaneous vessel is carefully dissected out and preserved. Advantages of perforator flaps include preservation of functional muscle and fascia at the donor site and versatility of flap design with regard to including as little or as much bulk tissue as required. Disadvantages are the difficult dissection needed to isolate the perforator vessels, longer operating time associated with this dissection, anatomic variability of position and size of perforator vessels, short pedicle length available, and fragile nature of these small blood vessels.
A perforator is a blood vessel passing through the deep fascia and contributing blood supply to the fascial plexus. Perforators arise from a source or mother vessel to a given angiosome. There are direct and indirect perforators. Direct perforators are those that travel directly from the mother vessel to the plexus; these include septocutaneous and direct cutaneous branches. Indirect perforators supply other deep structures on their route from the mother vessel to the plexus (e.g., the musculocutaneous perforator passing through muscle).
Because of the small size of the vessels and their anatomic variability, Doppler ultrasound is used routinely to locate the perforators before perforator flap elevation. This is not highly accurate, thus clinical experience remains crucial in this developing area. Technical recommendations for harvesting a perforator flap include identification of at least one vessel with a diameter of 0.5 mm or more, inclusion of at least two or more perforators, sufficient pedicle length for the procedure, and preservation of a subcutaneous vein to use for venous outflow in situations in which the deep system of perforator veins proves anomalous.
The use of perforator flaps continues to evolve. Current work includes flap thinning, a technique for removing excess adipose tissue from the perforator flap as it is raised. This would provide a large delicate segment of vascularized skin for reconstruction in areas such as the ear, in which contour is important. Another innovation is the discovery of new flaps based on perforators smaller than 0.8 mm in diameter found superficial to the fascial plane. By eliminating the dissection needed to trace a perforator through the muscle, operating time is shortened and there is potential for developing a much larger number of suitable flaps. The challenge with these suprafascial free flaps is the supermicrosurgery needed for anastomoses in such small vessels.
Microvascular free tissue transfer, commonly known as free flap, transplants distant tissue with its arterial and venous supply from another part of the body to be anastomosed to vessels at the recipient site. The transferred tissue may be skin, fat, muscle, cartilage, fascia, bone, nerves, bowel, or omentum as needed to reconstruct a given defect. Selection of tissue for transfer depends on the size, composition, and functional capabilities of the tissue needed; technical considerations, such as vessel size and pedicle length; and donor site deformity that will be created with regard to function and aesthetic appearance.
Preoperative planning starts with selection of the patient and analysis of the defect. Environmental factors, such as previous surgery and prior irradiation, which impair the quality of tissue and vessels, may be an indication for angiography to assess the available vasculature. Muscle does not tolerate warm ischemia for longer than 2 hours; skin and fasciocutaneous flaps can tolerate ischemia times of 4 to 6 hours. Planning is the most important factor to minimize the effects of ischemia, and all structures at the recipient site should be ready for the tissue transfer when the donor pedicle is divided. Sound technique requires healthy vessels of reasonable size with good outflow for the anastomosis, which must be made without tension. This may require mobilization of the vessels to gain more length. Vein grafts have been shown to reduce the success rate and are not a primary choice but may be needed if the pedicle is short or the vessels in the field are damaged. Both end-to-end and end-to-side arterial anastomoses have similar patency rates, although end-to-side anastomosis is preferred if there is vessel size or wall thickness discrepancy or the continuity of the recipient vessel must be preserved. Dissection and manipulation of the microvessels frequently cause vasospasm. This can be relieved with topical lidocaine or papaverine, stripping the adventitia to remove sympathetic nerve fibers, or mechanical dilation of the vessels. Failure of reperfusion in an ischemic organ after reestablishment of blood supply is termed the no-reflow phenomenon . The severity of this effect correlates with ischemia time.
Postoperative anticoagulation is not a uniform practice for elective microvascular transfers, and studies have not shown improved survival rate with anticoagulation regimen. Postoperative monitoring of free tissue transfers is critical because rapid identification of postoperative free flap ischemia permits intervention and flap salvage. Most free flap thromboses occur in the first 48 hours after surgery, and salvage rates are high. Clinical evaluation includes observation of skin color, capillary refill, fullness, and color of capillary bleeding. If a flap is buried, a temporary skin island can be added for monitoring purposes or an implantable monitoring device can be used. Many devices are available for flap monitoring, including temperature probes, pulse oximetry, photoplethysmography, hand-held pencil Doppler probes, and implantable Doppler probes.
Tissue survival rates for free tissue transfers exceed 98%. Reexploration rates range from 6% to 25%, and thrombosis of the arterial anastomosis is the most common finding at reoperation. This is termed primary thrombosis when technical faults lead to anastomotic failure. These faults include narrowing of the lumen; sutures tied too loosely so that media of the vessel is exposed in the gap and clot forms; sutures tied too tightly that they tear through the vessel; too many sutures with subendothelial exposure and clot formation; and sutures that inadvertently take a bite of the back wall of the vessel, which obstructs the lumen. Secondary thrombosis refers to kinking or compression of vessels by hematoma or edema, which leads to decreased inflow. With reexploration, salvage rates have been seen to vary from 54% to 100% in different series.
The principles and techniques of microvascular surgery are under continual refinement. An area of current emphasis is the identification of tissue transfers that better suit the needs of the recipient site and minimize donor site sequelae, which has led to minimally invasive and endoscopic techniques for harvesting of flap tissue through smaller incisions. It has also led to the development of tissue transfers such as perforator flaps, which preserve functional muscle and fascia at the donor site, and suprafascial free flaps, which require supermicrosurgery techniques.
The introduction of supermicrosurgery, which allows the anastomosis of smaller caliber vessels and microvascular dissection of vessels ranging from 0.3 to 0.8 mm in diameter, has led to the development of new reconstructive techniques. Free perforator-to-perforator flaps using suprafascial vessels can be transferred more quickly and the tissue can be obtained from better concealed parts of the body. If a discrete perforator can be identified anywhere on the body, a flap can be designed around it. This has been called a freestyle flap . The constraints of using only described territories can be disregarded and the donor site selected solely on the basis of the best possible match for color, contour, and texture at the recipient site. Disadvantages are the anatomic variation of the perforators and the need for supermicrosurgical technique. The supermicrosurgical technique includes the use of 12-0 nylon sutures with 50-μm to 30-μm needles, which requires a high magnification microscope and a technically adept surgeon.
Craniosynostosis refers to the premature fusion of one or more of the cranial sutures, leading to characteristic deformities of the skull and face. It occurs at an overall frequency of approximately 1 in 2500 live births and is usually sporadic. Any suture may be involved in craniosynostosis, and skull growth is restricted perpendicular to the affected suture. Treatment of craniosynostosis is indicated to correct the deformity and to normalize the shape of the head, to protect the eyes by restoring brow projection, and to minimize the risk for development of increased intracranial pressure and associated developmental and visual sequelae. The timing of treatment is based on which suture is fused and on the protocol at a given center, but correction during the first 6 months of life appears to be associated with better neurodevelopmental outcomes.
Surgical treatment of craniosynostosis is generally done with a coronal approach; techniques differ, but all involve release or excision of the fused suture. The cranium then expands and remodels. Residual bone defects reossify secondarily, a process that is robust in the infant up to 2 years of age ( Fig. 69.4 ). The use and implementation of resorbable plates have allowed for improved outcomes and decreased morbidity in these patients.
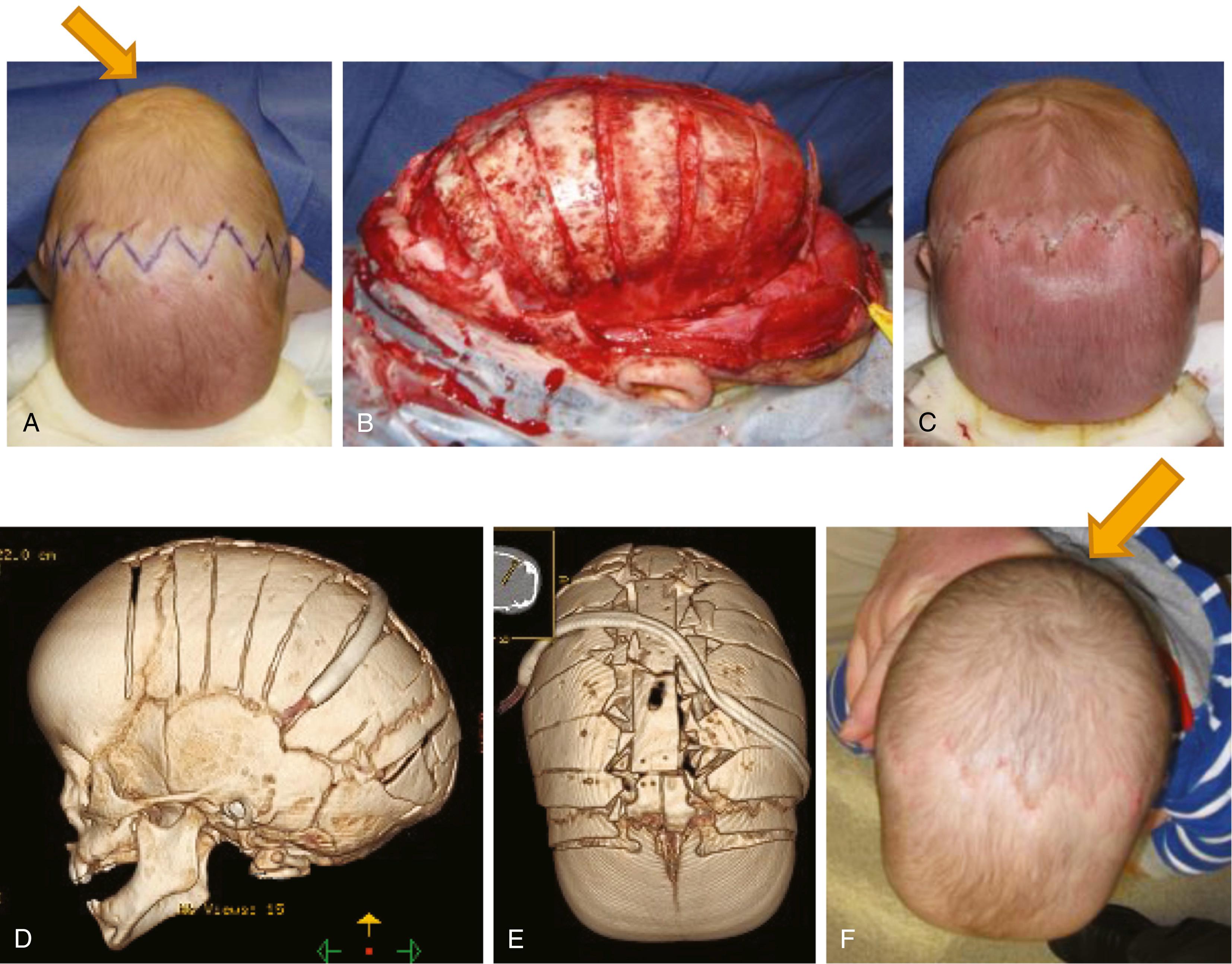
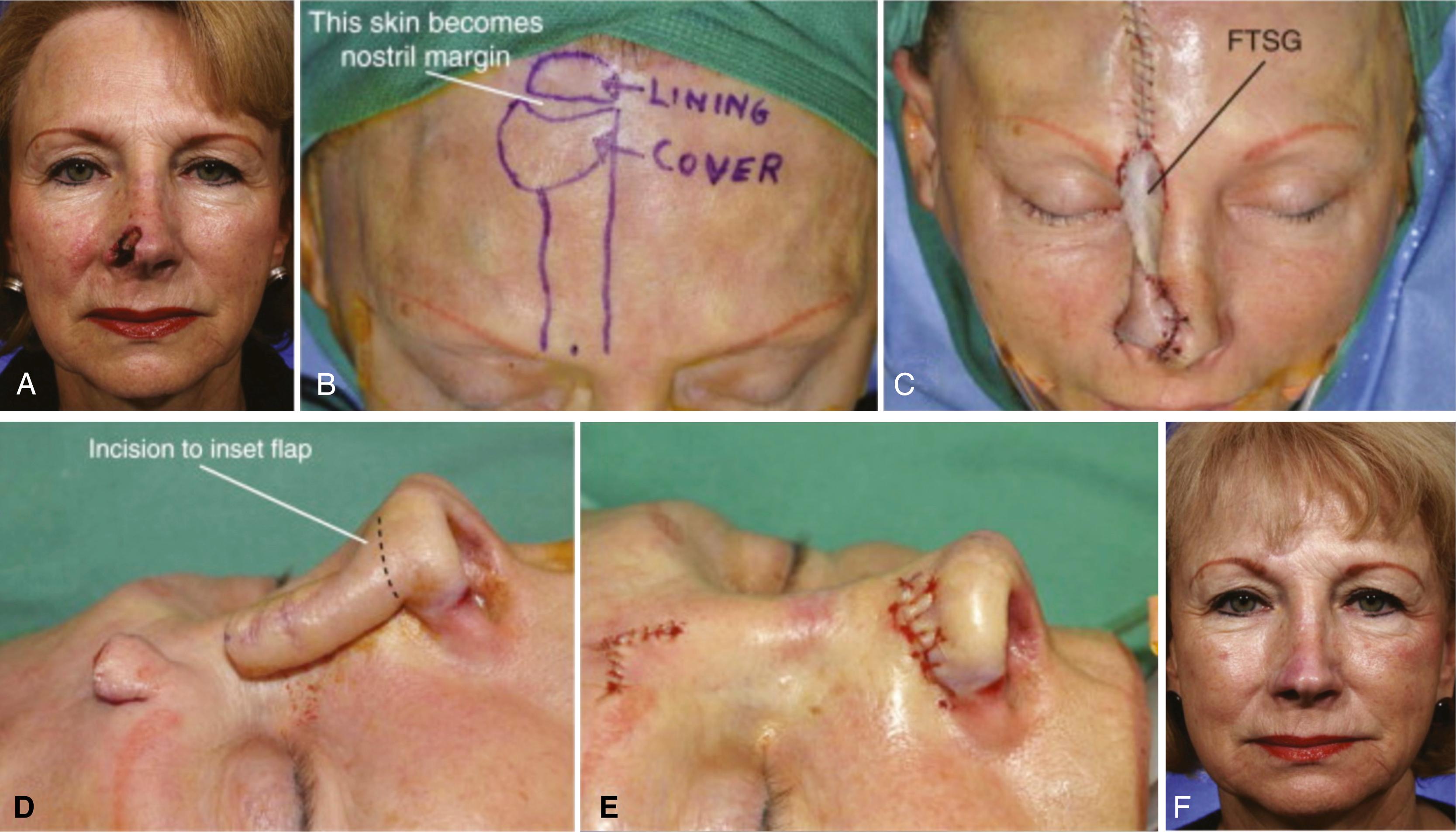
Other less common congenital abnormalities of the head include agenesis of one or a number of layers of scalp or cranium. Aplasia cutis congenita usually refers to a focal defect of skin on the vertex. The defect may include any proportion of skin, bone, or dura. Treatment depends on the size of the defect and layers involved and may encompass local wound care or surgical reconstruction with flaps or grafts in infancy. The cause of this rare condition is unknown and likely varies from case to case. A classification system for aplasia cutis congenita has been developed and is related to the presence of other associated anomalies.
Congenital anomalies of the external ear may occur in isolation or as part of craniofacial microsomia. Common external ear deformities include prominent ears, constricted ears, cryptotia (failure of the upper pole of the ear to stand out from the head), and microtia (a small or abnormally formed outer ear). The most common type of microtia is a malformed vestigial cartilaginous structure associated with a soft tissue component of lobule. In cases of isolated microtia, there is often conductive hearing loss associated with absence of the external auditory canal. This is most important in bilateral cases in which a bone-anchored hearing aid is required.
Reconstruction of typical microtia can take two general approaches: autologous or nonautologous. Nonautologous reconstruction involves placement of a high-density polyethylene implant under the skin. This approach results in excellent results but requires harvest of a flap (commonly temporoparietal fascia flap based off the superficial temporal artery +/- skin grafting in certain cases). Disadvantages include the presence of a foreign body that may become exposed through the temporoparietal fascia flap or graft, susceptibility to infection, and difficulty with salvage in case of complications. The second approach more commonly used involves the use of autologous tissue (rib cartilage) to shape an ear framework, which is then buried in a subcutaneous pocket. The meticulous shaping of the framework, creation of a thin skin pocket, and use of drains allow the skin to contour around the intricate framework. The procedure requires multiple stages but results in a reconstructed ear that has good form and is capable of responding to trauma and infection like other parts of the body. The disadvantage is the need to harvest cartilage from the multiple ribs.
Craniofacial microsomia, also known as hemifacial microsomia, is a constellation of abnormalities involving deficient development of parts of the face related to the first and second branchial arches. Deformity can be unilateral or bilateral and can involve the orbit, mandible, external ear, facial nerve, and facial soft tissue. Each or all of the structures may be involved and to varying degrees. The cause is unknown but is thought to be related to in utero vascular compromise of the stapedial artery. Treatment of craniofacial microsomia is complex, and the approach has to be tailored for individual patients. Functional problems, such as airway compromise or eye exposure, are treated in childhood; reconstruction of other structural defects is delayed until the patient is almost full-grown. Novel approaches using distraction osteogenesis of the mandible, pioneered by long bone distraction techniques by Ilizarov, have been modified by surgeons such as McCarthy and used to treat and avoid long-term complications in these patients.
For patients with craniofacial anomalies such as those described as well as for those with cleft lip and palate, the current standard is team care at an established craniofacial center. With referral to a craniofacial center at birth, the craniofacial team can make a diagnosis, carry out genetic testing, educate the family, and outline short-term and long-term plans in a coordinated manner, bringing in multiple specialists (e.g., plastic surgeons, neurosurgeons, oral surgeons, orthodontists, speech pathologists, otolaryngologists, ophthalmologists, social workers, nurse practitioners, developmental psychologists, and pediatricians).
Cleft lip and palate are relatively common congenital anomalies. They may be unilateral or bilateral. Most are isolated anomalies, but many syndromes have clefts as one of the features. The genetics of cleft lip and palate is complex, and the condition is multifactorial. The pathophysiologic mechanism of cleft lip and palate is incompletely understood, but the deformity and its variations are well described. A minimum of three operations, and usually four, will be required to correct the deformity. These are performed at specific times corresponding to the developmental stage of the patient. Commonly the sequence is as follows: cleft lip repair at 3 to 6 months, cleft palate repair before 1 year (or before speech development), and alveolar bone graft when permanent dentition begins and after orthodontic preparation. Future surgeries are reserved for the end of skeletal maturity and include possible septorhinoplasty in the late teenage years, possible lip and nose revisions, and LeFort I maxillary advancement, if indicated. At times during these two stages, secondary procedures for speech improvement are done in almost 5% to 20% of the cases.
A cleft lip is characterized by a partial or complete lack of circumferential continuity of the lip. Most cleft lips occur in the upper lip where one of the philtral columns normally lies, and they extend into the nose. The deformity involves the mucosa, orbicularis oris muscle, and skin. The nasal deformity is characterized by a slumped and widened ala (nostril) that is posteriorly misplaced at its base. The nasal floor is nonexistent in complete clefts, and the nasal septum is deviated.
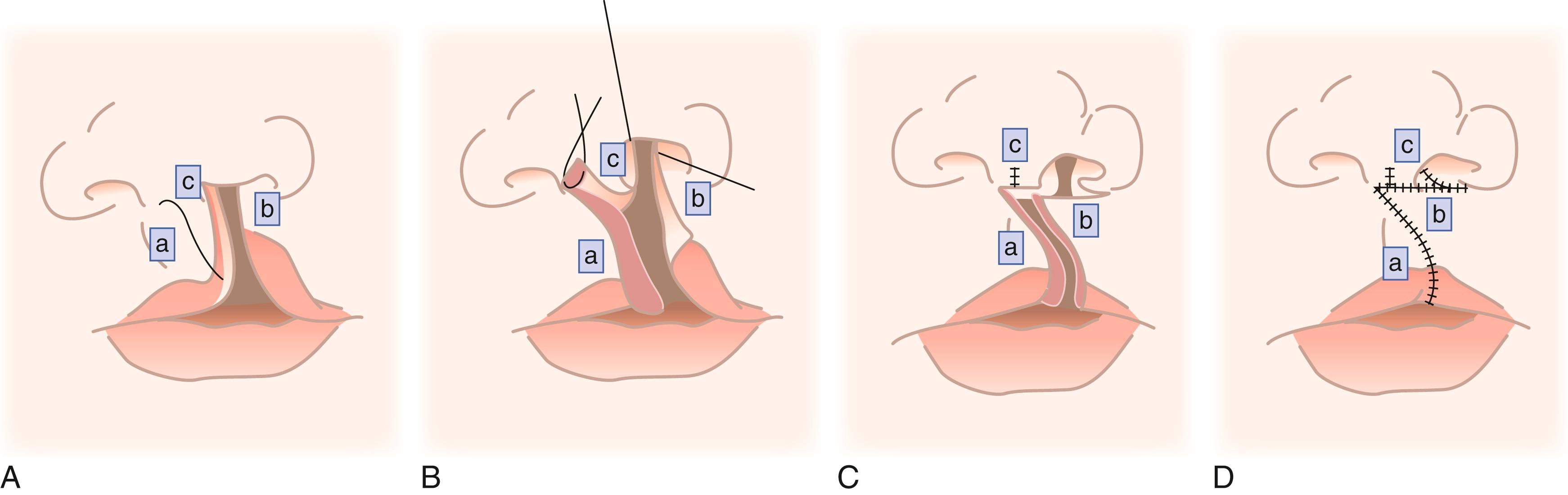
There are many techniques for repair of a cleft lip, but most are a variation of the rotation-advancement repair. Millard introduced this technique of downward rotation of the medial portion of the lip and advancement of the lateral portion into the defect created by the rotation. The repair is based on the principle that existing elements need to be returned to their normal position to restore the normal anatomy while remaining cognizant of future growth and the effects of surgery on growth ( Fig. 69.5 ). , Many leaders have modified their techniques and have adapted variations, which leads to the creativity and innovation of the field.
Cleft palate can also be complete or incomplete. The goals of palatal repair are the development of normal speech and prevention of regurgitation of food into the nose. Normal speech requires velopharyngeal competence to close the oral cavity off from the nasal cavity to produce pressure consonants. This requires static physical separation of the two cavities in the region of the hard palate and dynamic closure of the soft palate against the posterior pharyngeal wall with a functioning levator veli palatini muscle. In a cleft palate, the levator veli palatini muscle fibers are oriented abnormally along the cleft. Thus, all modern techniques of cleft palate repair involve repair of the nasal lining and oral mucosa and reorientation and repair of the levator veli palatini muscle. The primary measure of outcome of cleft palate repair is normal speech. The third procedure necessary in most cases is alveolar bone grafting. Cancellous bone, usually from the ilium, is used to restore bone continuity along the dental arch, allowing the teeth in the cleft edge a better chance of survival, and as a foundation for dental implants for missing teeth associated with the cleft, to close a nasolabial fistula (if present), and to produce support for the nasal base.
Other procedures are indicated for some patients, but this generally cannot be predicted in infancy. Approximately 15% of patients will continue to demonstrate velopharyngeal insufficiency after initial palate repair, and secondary palatal lengthening or other approaches to promote velopharyngeal closure are indicated typically after 3 to 5 years of age. Septorhinoplasty is usually necessary to correct residual nasal deformity after the cessation of skeletal growth and after final dental restoration and orthodontics. A subset of patients with unilateral cleft lip and palate will develop maxillary hypoplasia that is iatrogenic and related to scarring and growth retardation from lip and palate surgery. Depending on the degree of maxillary hypoplasia, LeFort I maxillary advancement after achieving skeletal maturity may be indicated. In sum, treatment of a child born with a cleft lip and palate does not end after palate repair but rather requires observation by a craniofacial team throughout development into adulthood and must be tailored for each individual.
Vascular anomalies are divided into two major groups: tumors and malformations. Vascular tumors are characterized by increased abnormal proliferation of endothelium. Hemangioma is the most common vascular tumor; others include hemangioendotheliomas, tufted angiomas, hemangiopericytomas, and malignant tumors such as angiosarcoma. Vascular malformations are the result of abnormal development of arterial, capillary, venous, or lymphatic components of the vascular system. They may involve only one component or may be mixed and are named for the component vessels. They can be high flow, low flow, or mixed. Correct diagnosis depends on the history (e.g., hemangiomas develop in infancy and are usually not visible at birth), physical examination (e.g., malformations with an arterial component may have a palpable pulse or thrill), and imaging to determine the extent of disease and to assist with making the diagnosis.
The natural histories of the different anomalies are diverse. Hemangiomas typically involute spontaneously; 50% involute completely by the age of 5 years. More recently, the quick treatment of infantile hemangiomas during the involution stage with beta blockers has resulted in a reduction in cases needing surgical intervention. Early treatment and natural history reduces the indications for surgery to those lesions that are affecting vision or the airway or are large enough that, even after involution, the abnormal remaining skin will require surgical modification. In contrast, capillary malformations start as patches, but over time, they typically enlarge and become thick and verrucous; for these lesions, early treatment is indicated. Some vascular malformations or tumors have systemic effects, depending on their mass, status as high or low flow, and thrombosis and consumption of coagulation factors. Treatment of these lesions involves complete resection, when feasible, or debulking if complete resection is not possible. Sclerotherapy is the mainstay of treatment of venous malformations. For arteriovenous malformations, sclerotherapy is useful as an adjunct to surgery but insufficient alone because of the development of collaterals. For these malformations, sclerotherapy and embolization are followed immediately by surgical resection.
Neck masses in the pediatric patient are most likely infectious or congenital noncancerous lesions. In addition to vascular malformations, other common pediatric neck masses include dermoid cysts, teratomas, branchial cleft anomalies, thyroglossal duct cysts, thymic cysts, ranulas, cartilaginous rests, heterotopic neuroectodermal tissue, neurofibromas, ectopic salivary tissue, lymphadenopathy, and malignant tumors. Branchial cleft anomalies may be cysts, sinuses, or fistulas. Cysts and sinuses are located in the anterior cervical triangle and are derived from the first cleft (near the external auditory meatus) and second cleft (below the hyoid) 98% of the time. The treatment of these lesions is surgical excision, and care must be taken due to the intimate association with neurovascular structures. Thyroglossal duct cysts may arise anywhere along the course of the thyroglossal duct from the foramen cecum at the base of the tongue to the thyroid gland. Thyroglossal duct cysts usually present in the first or second decade of life as painless anterior neck masses, and there may be an associated sinus track. Indications for surgery include recurrent infection, tissue diagnosis, and improved cosmesis. Thyroid scan is indicated before excision to rule out a functioning ectopic thyroid gland.
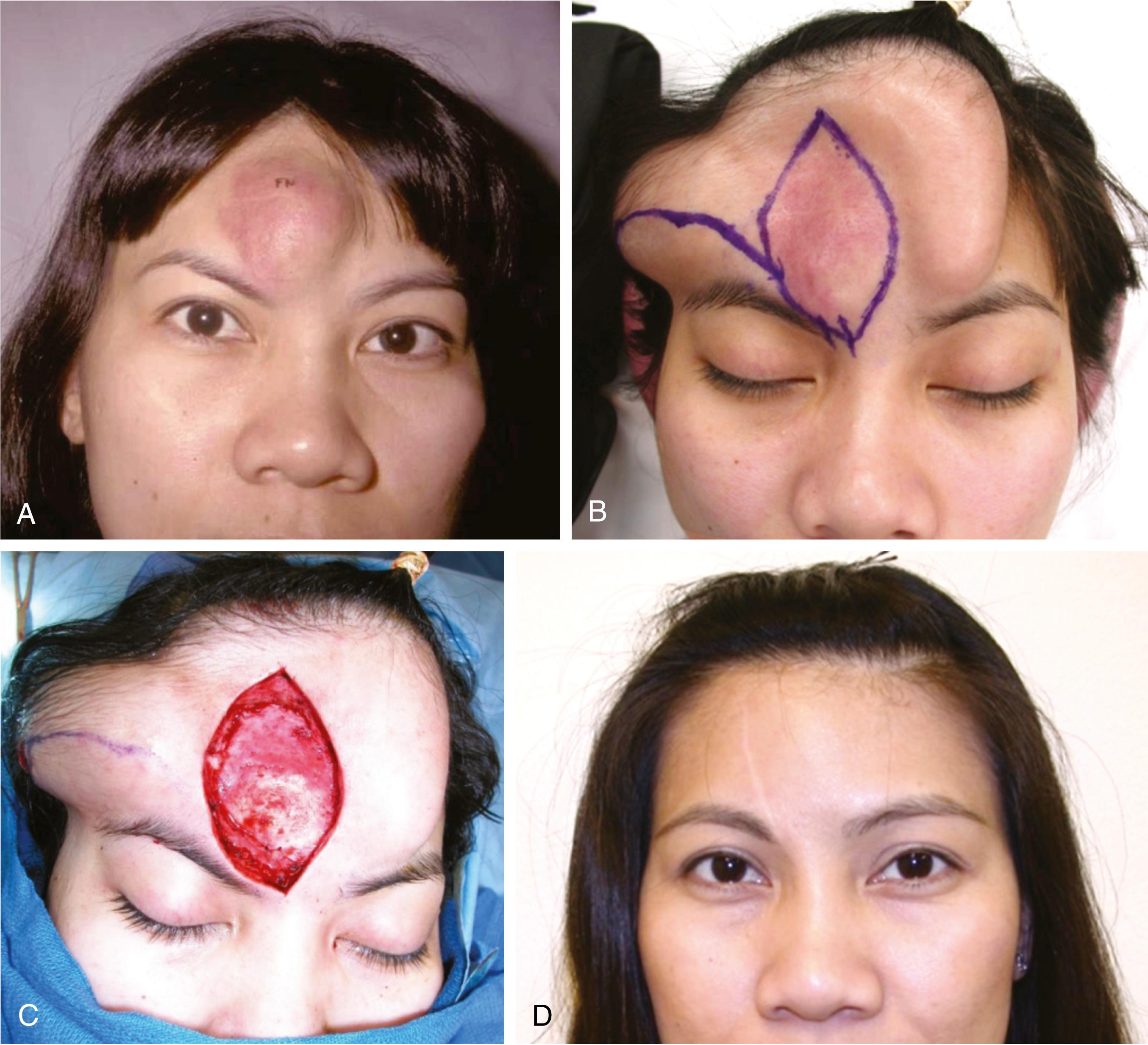
Congenital melanocytic nevi are hamartomas consisting of nevus cells. Nevi are classified by size as small (<1.5 cm), medium (1.5–19.9 cm), large (20–49.9 cm), and giant (>50 cm). The classification dictates the prognosis and reconstructive approach. Risk of melanoma occurring in a melanocytic nevus varies by report but is estimated to be less than 5% in small or medium lesions and typically presents after puberty. In large and giant nevi, the reported risk of melanoma development is up to 10%. Unlike the case for small or medium nevi, malignancy in large and giant nevi typically occurs in the first three years of life. Large and giant nevi also have an increased incidence of leptomeningeal involvement that can be diagnosed by magnetic resonance imaging (MRI). In addition, psychosocial and developmental issues associated with larger nevi are significant, so early excision and reconstruction are recommended for large and giant nevi.
Options for removal of larger nevi include serial excision, excision and grafting, excision and closure with distant flaps, and tissue expansion. Replacement with like tissue is the goal, and therefore tissue expansion is the mainstay approach.
Facial trauma has decreased in frequency in the United States, and this is attributed in part to the advent of seat belt laws and improved collision safety. However, it remains part of multisystem trauma from motor vehicle accidents, assaults, and combat injuries. Improvements in body armor have resulted in better survival of combat injuries but proportionally more facial injuries.
Surgical emergencies in the patient with facial trauma include airway compromise, life-threatening hemorrhage, and reversible structural injury to the eye or optic nerve. Other injuries, such as lacerations or extraocular muscle entrapment, are treated within the first 24 hours. Fractures are treated within the first 2 weeks. Evaluation of the patient with facial trauma follows the advanced trauma life support protocol and includes looking for intracranial trauma and cervical spine injury. Acute airway compromise usually occurs in the setting of combined mandibular-maxillary trauma with hemorrhage and soft tissue swelling. Endotracheal intubation should be attempted and need not be avoided because of concern about the facial injury. Nasotracheal intubation is contraindicated in the case of severe nasoorbitoethmoid and skull base fractures. Cricothyroidotomy is performed if oral or nasal endotracheal intubation is unsuccessful and should be converted to tracheostomy after the patient has been stabilized. Maxillomandibular fixation by itself is not an indication for tracheostomy because endotracheal intubation may be maintained through the nasal or oral route using an armored tube that can be routed behind the molars without kinking. An alternative technique is to exit the endotracheal tube through a submental incision, which alleviates some of the practical difficulties of working around an oral tube.
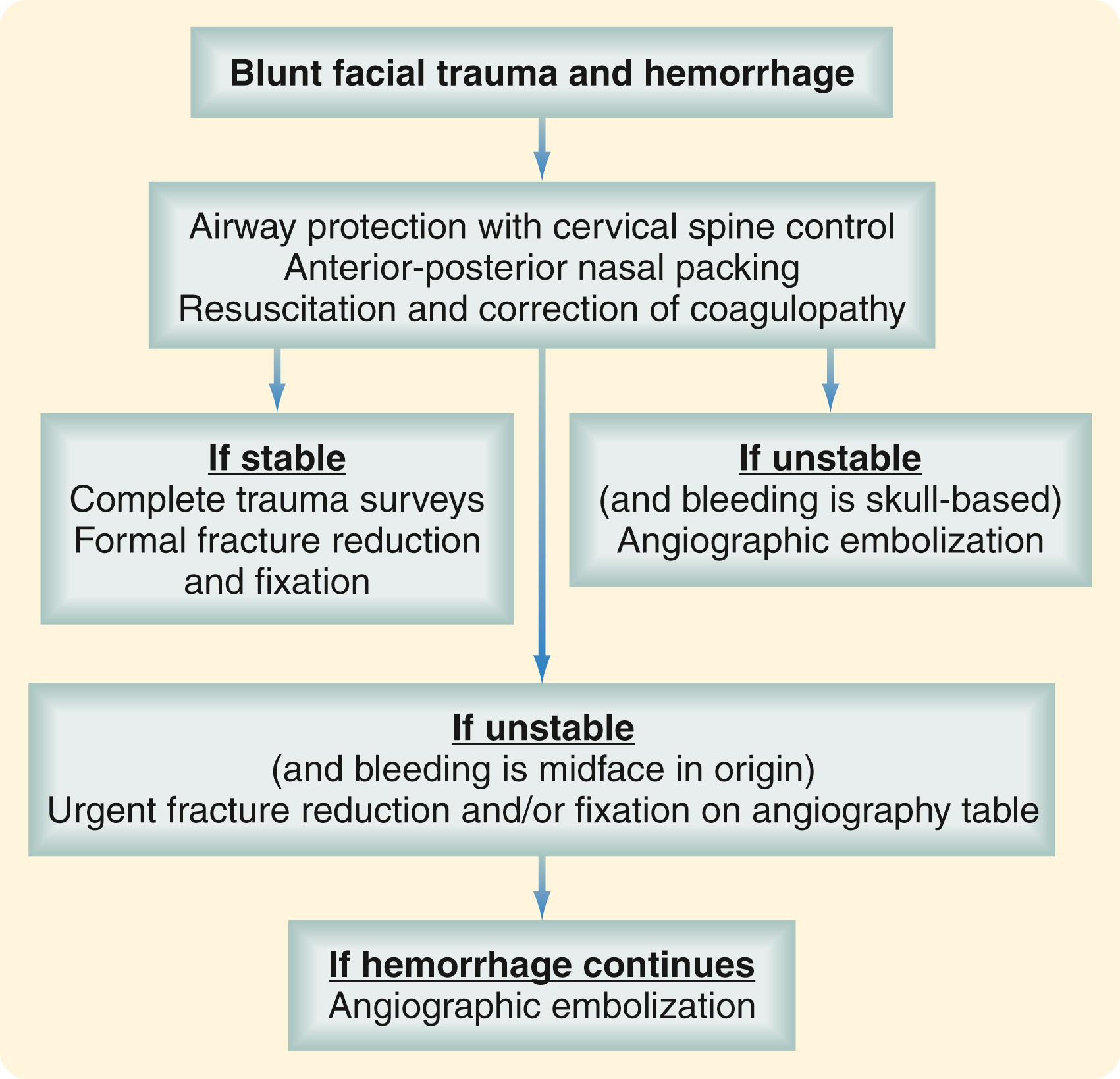
Life-threatening hemorrhage, defined as three units of blood loss or hematocrit below 29%, occurs in a small percentage of patients with facial trauma. In most cases, bleeding is effectively controlled with pressure, packing, and, in the case of significant soft tissue avulsion, rapid placement of temporary bolster sutures. Blind attempts to clamp and ligate vessels should be avoided because this is usually unnecessary and may result in injury to critical structures, such as the facial nerve. With penetrating trauma, hemorrhage is controlled in the operating room with vessel identification and ligation and, if that is unsuccessful, by angiographic selective embolization. With blunt trauma, severe hemorrhage is usually from the internal maxillary artery. The most effective way to control bleeding, especially when it is associated with midfacial fractures, is fracture reduction and stabilization. This can be accomplished quickly by temporary placement in maxillomandibular fixation using rapid techniques such as fixation screws. Severe hemorrhage from skull base and nasoethmoid fractures can often be controlled with anteroposterior nasal packing. Placement of Foley balloon catheters in each nasal airway serves to tamponade the bleeding and stabilizes the packing. Current protocols for control of hemorrhage in blunt facial trauma settings involve selective angiography if these measures fail ( Fig. 69.6 ). Angiographic embolization is effective but is associated with significant morbidity, including the possibility of stroke or necrosis of midfacial structures, such as the palate. In unstable patients, fracture reduction and nasal packing may be attempted on the angiography table to be followed immediately with embolization, if necessary.
Injuries to the orbit and contents can result in blindness; it is critical to recognize promptly and to treat reversible injuries that are vision threatening. Conditions that require emergent intervention include increased intraocular pressure, globe rupture, and optic nerve impingement. Acute increased intracranial pressure is manifested by pain and vision loss and can result from causes such as hematoma or decreased orbital volume because of fracture or a foreign body. Treatment involves rapid alleviation of intraocular hypertension by lateral canthotomy, inferior cantholysis, and administration of mannitol, acetazolamide (Diamox), and steroids. Urgent ophthalmology consultation is indicated. Vision loss may result from mechanical compression of the optic nerve. Computed tomography (CT) will diagnose the presence of a bone fragment or foreign body; such a finding should prompt emergent surgical decompression to preserve vision. Extraocular muscle entrapment presents as the inability to move the eye on the trajectory controlled by the entrapped muscle and is associated with pain on attempted motion. Especially in children, the pain may be severe and accompanied by nausea or vomiting. Muscle entrapment should be treated by surgical release of the entrapped contents. This should be done in the acute setting because delaying the treatment of entrapment for 1 week or longer after injury typically results in failure of the entrapped muscle to regain excursion.
The primary diagnostic studies for facial injury are physical examination and CT. Systematic physical examination can detect deformity, soft tissue injury, cerebrospinal fluid leak, and facial nerve injury. Palpation is used to identify bony stepoffs or midface instability. The eyes are examined for proptosis or enophthalmos, extraocular muscle function, and visual acuity. In patients who cannot cooperate with a physical examination and for whom there is reasonable suspicion of periorbital injury, a forced duction test should be performed. The occlusion is evaluated for subjective or objective malocclusion. Extraocular muscle entrapment, acute enophthalmos, and malocclusion are indications that surgical treatment of facial fractures will be required. Fine-cut CT of the face with direct or reformatted coronal and sagittal views and 3D reconstruction is used to diagnose facial trauma and to direct nonsurgical and surgical treatment. With current CT scanning technology, plain films are not necessary and provide less information. An exception is the Panorex, which is used by many physicians as an adjunct or primary study for mandible fractures and to assess teeth and their roots in particular.
Because of its rich blood supply, even questionable tissue should be salvaged in treating facial lacerations and avulsions. The robust perfusion of facial tissue provides resistance to infection, and repair can be done after a longer delay than would be safe elsewhere on the body. Although there is no strict cutoff, primary repair is generally done up to 24 hours after injury. Even grossly contaminated wounds or those from animal bites are irrigated extensively, debrided, and closed primarily. If there is the possibility of facial nerve injury, this is confirmed by the physical examination finding of weakness or absence of function of a portion of the muscles of facial expression. It is important to recognize a facial nerve laceration so that the distal cut ends can be identified with a nerve stimulator and tagged if they are not to be repaired immediately. Identification of distal stumps by nerve stimulation is not possible after a few days because conduction ceases. Parotid duct injuries should be identified and treated acutely to prevent the formation of sialocele or salivary fistula. In a sharp laceration or penetrating injury to the cheek, a parotid duct injury can be confirmed by direct visualization or injection of dye. This is done by cannulating the Stensen duct on the mucosal surface of the cheek and injecting a small amount of methylene blue dye. Extravasation of the dye into the wound indicates a parotid duct laceration, and repair over a stent should be done in the operating room.
Current concepts in facial fracture treatment rest on craniofacial techniques to provide surgical exposure of the craniofacial skeleton, anatomic reduction of fractures, and rigid bone fixation with low-profile titanium plates and bone grafting techniques. Failure to reconstruct the bony facial skeleton invariably results in shrinkage and tightening of the facial soft tissue envelope, a sequela that is almost impossible to correct secondarily.
Treatment of forehead fractures involves assessment of the frontal sinus and cranial base. The approach is dictated by injury to the anterior or posterior table of the frontal bone or skull base and whether there is a dural injury or injury to the nasofrontal ducts that drain the frontal sinuses into the nose. Fractures of the upper midface include malar (zygoma) fractures, nasoorbitoethmoid fractures, and orbital fractures. There is considerable overlap in this region. For example, malar fractures occur in association with orbital fractures to a varying degree because the zygoma, in addition to producing cheek projection and determining facial width, is also part of the orbit. Treatment of fractures of the lower midface, the maxilla, focuses on the restoration of the preinjury dental occlusion. It is important to determine the patient’s preoperative occlusion; the relationship of the upper and lower teeth is described by the Angle classification.
Maxillary fractures are classified using the LeFort system based on the level at which the midface is separated from the rest of the craniofacial skeleton. Repairs focus on the restoration of facial height and projection. With significant comminution or bone loss, bone grafting may be required to maintain the appropriate position of the maxilla in space. Rigid plate and screw fixation obviates the need for prolonged maxillomandibular fixation. Fractures of the mandible are treated by reduction and rigid fixation using restoration of occlusion as the principle intraoperative and postoperative goal. Many mandibular fractures are treated with open reduction and internal fixation, which may make maxillomandibular fixation unnecessary. Certain fractures, like those in younger patients or favorable fracture patterns, are best treated closed, and the decision to pursue an open or closed approach depends on the fracture location and orientation.
The same principles of fracture repair apply in the pediatric patient with some differences. Early treatment within one week is necessary, given the rapid healing in children, and fixation is complicated by the presence of permanent teeth embedded in the maxilla and mandible that are easily damaged by hardware. Resorbable hardware is frequently used for children but does not have the mechanical strength required for most adult fractures. However, recent advances have allowed treatment of certain adult fractures such as orbit fractures with resorbable hardware, but this is still considered an off-label use.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here