Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
| American College of Obstetricians and Gynecologists | ACOG |
| Abnormally invasive placenta | AIP |
| Cesarean delivery | CD |
| Color Doppler imaging | CDI |
| Federation International of Gynecologists and Obstetricians | FIGO |
| Intensive care unit | ICU |
| Magnetic resonance imaging | MRI |
| Massive obstetric hemorrhage | MOH |
| Multidisciplinary team | MDT |
| Odds ratio and confidence intervals | OR & CI |
| Placenta accreta spectrum | PAS |
| Postpartum hemorrhage | PPH |
| Randomized controlled trial | RCT |
The origin of the term placenta accreta is unknown, and there is no information in the medical literature on when it was first used and by whom. According to modern dictionaries, accreta is the feminine gender of the Latin word accretum, which combines the prefix ad, meaning to, and the verb crescere , meaning grow, increase, or expand.
Placenta accreta has been divided by pathologists into different grades according to the depth of placental tissue penetration into the uterine wall. These grades are: placenta accreta or adherenta when the villi simply adhere to the myometrium; placenta increta when the villi invade the myometrium; and placenta percreta when the villi invade the full thickness of the myometrium down to and beyond the uterine serosa into surrounding pelvic organs and vessels ( Fig. 21.1 ). This terminology has been and is still used by most pathologists. Placenta accreta has also been subdivided into total, partial, or focal, depending on the extent of the accreta area. It can be difficult to clinically differentiate between these categories, especially as they may coexist in the same placental bed ( Fig. 21.2 ).
Confusion also frequently occurs among clinicians regarding the difference between abnormally adherent placenta accreta and simple placental retention. The recent use by clinicians of the Victorian terminology “morbidly adherent placenta,” which was used at the end of the 19th century to describe placental retention, to now define both adherent and invasive cases of accreta placentation has added to confusion and led to wide variation in epidemiologic data. To avoid confusion in the present chapter, we will refer to placenta accreta spectrum (PAS), which includes the different grades of abnormal placental villous attachment and/or invasion into the myometrium beyond the physiologic decidual–myometrial junction zone.
All recent epidemiologic studies have shown a strong association between increasing cesarean delivery (CD) rates and the incidence of accreta placentation in subsequent pregnancies . It is difficult to obtain precise values on the prevalence and incidence of PAS due to major differences in study design and the lack of a standardized clinical definition of PAS owing to the absence in most population and cohort studies of detailed pathologic confirmation of the prenatal and clinical diagnosis at birth. Overall, PAS is still relatively uncommon with a prevalence of approximately 1 case per 800 births, but it is likely to become more common as CD rates increase around the world.
When PAS is present, the failure of the entire placenta to separate normally from the uterine wall after delivery is typically accompanied by massive obstetric hemorrhage (MOH) . Attempts to remove the accreta villous tissue provokes further bleeding and a cascade of ongoing hemorrhage, shock, and coagulation abnormalities requiring complex clinical management. This chapter reviews the pathogenesis, epidemiology, prenatal diagnosis, and management of this obstetric disorder.
In 1937, Irving and Hertig published a cohort of 18 cases, which they described clinically as “the abnormal adherence of the afterbirth in whole or in parts to the underlying uterine wall” and histologically as “the complete or partial absence of the decidua basalis,” signs that are still used today by clinicians and pathologists. They defined all their cases as adherenta where the villi were attached to the surface of the myometrium without invading it. They hypothesized that the lack of a plane of cleavage between the placental basal plate and the uterine wall leads to major hemorrhage if an attempt is made to forcibly remove villous tissue attached superficially to the myometrium. Their series did not include a single case of invasive PAS; thus their clinical and pathologic descriptions were limited to abnormally adherent accreta.
Several concepts have been proposed to explain the abnormal placentation in PAS, including a primary defect of trophoblast function, a secondary basalis defect due to a failure of normal decidualization, and more recently, abnormal vascularization and tissue oxygenation of an area of scar. Unlike the epithelial layers of the endometrium and uterine peritoneum, which heal by regeneration and recolonization of the scar area, the myometrium does not heal by regenerating muscle fibers, but by forming “foreign” substances, including collagen. The resulting fibrous tissue is weaker, less elastic, and more prone to injury than intact muscle. Myofiber disarray, tissue edema, inflammation, and elastosis have all been observed in uterine wound healing after surgery. Different surgical techniques such as single-layer versus double-layer closure of the myometrium, locked versus unlocked single-layer closure, or the suture material used for the closure may influence the healing process and the risks of subsequent uterine rupture, but data regarding the influence of these factors on the risk of PAS in subsequent pregnancies are sparse.
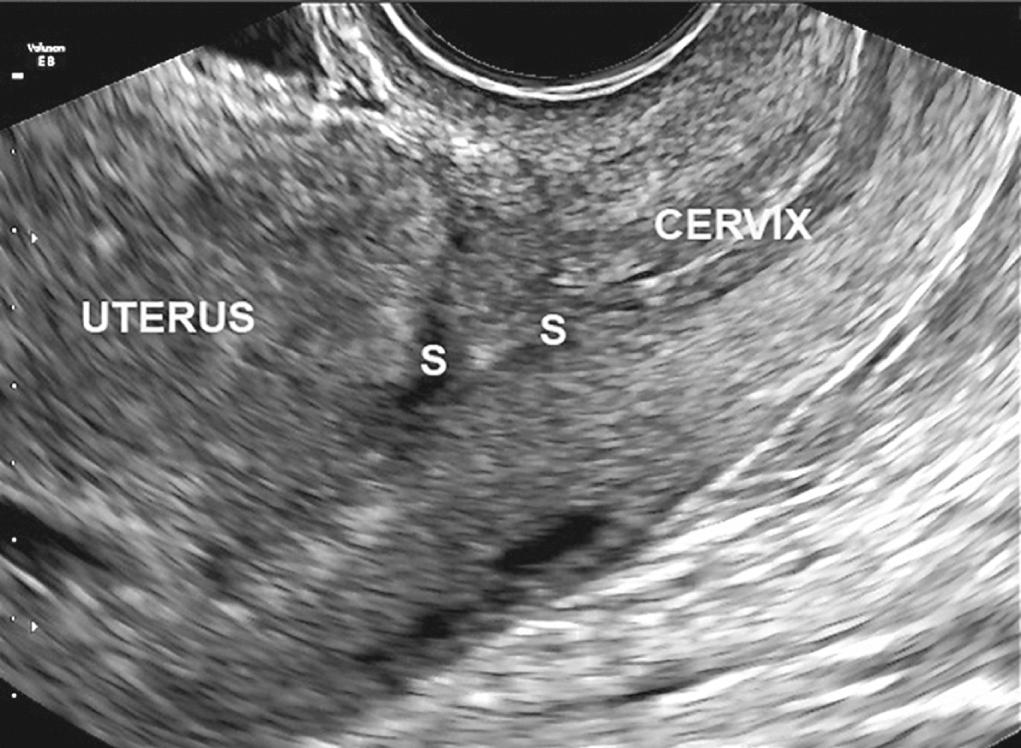
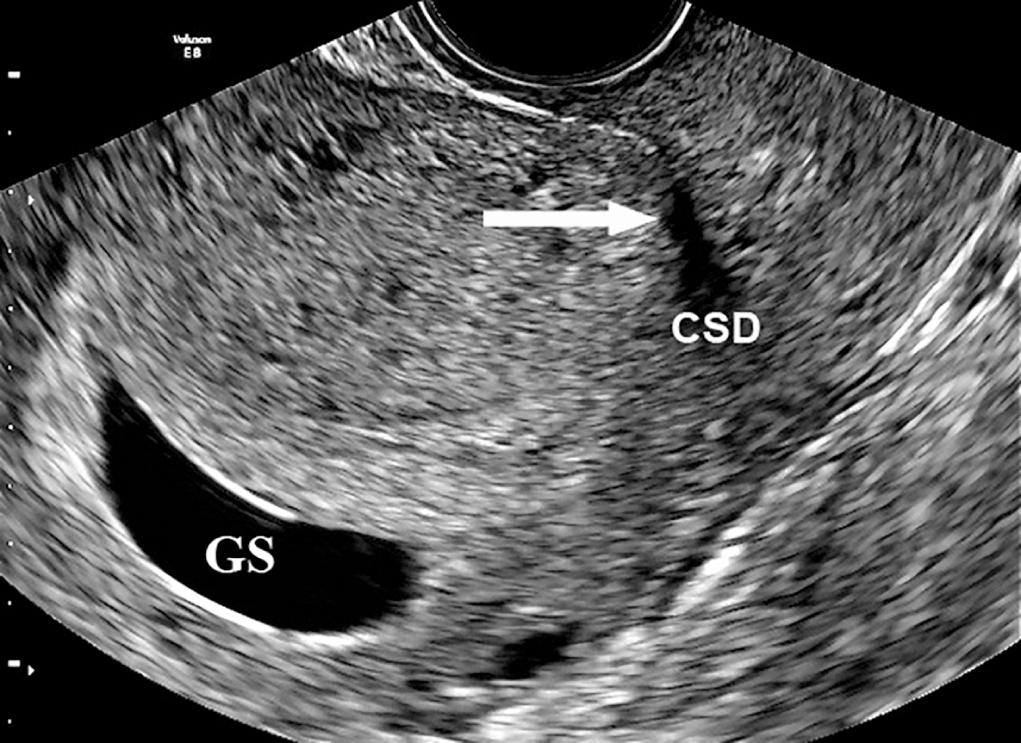
Large cesarean scar defects may lead to scar dehiscence with advancing gestation ( Fig. 21.3 and 21.4 ) and could even explain exceptionally rare cases of PAS leading to uterine rupture in the first half of pregnancy. Although this is an extremely rare complication of abnormal placentation, the mechanism of uterine rupture due to invasive PAS is likely similar to that of a tubal rupture in ectopic placentation. Scar dehiscence in the third trimester can also lead to false-positive diagnosis of invasive PAS when the lower edge of a low-lying placenta becomes visible under the uterine serosa at laparotomy. There is increasing evidence that some pregnancies diagnosed as “cesarean scar” pregnancies are a precursor to PAS, although epidemiologic evidence remains limited to a few retrospective cohort studies.
PAS is not exclusively a consequence of a prior uterine surgical scar, and extensive non-surgical endometrial damage such as that related to endometritis can lead to endometrial fibrosis and atrophy with secondary poor decidualization and development of PAS in a subsequent pregnancy. This may explain why before the advent of antibiotics a prior manual removal of the placenta with or without a uterine curettage was the main factor associated with PAS in subsequent pregnancies. Although fragments of myometrium are found in the products of conception in about one-third of specimens from uterine curettage for miscarriage, the observation that these procedures are not a major risk for subsequent PAS suggests that the trauma to the myometrium and endometrium is often relatively limited from a curettage procedure compared to that from a CD.
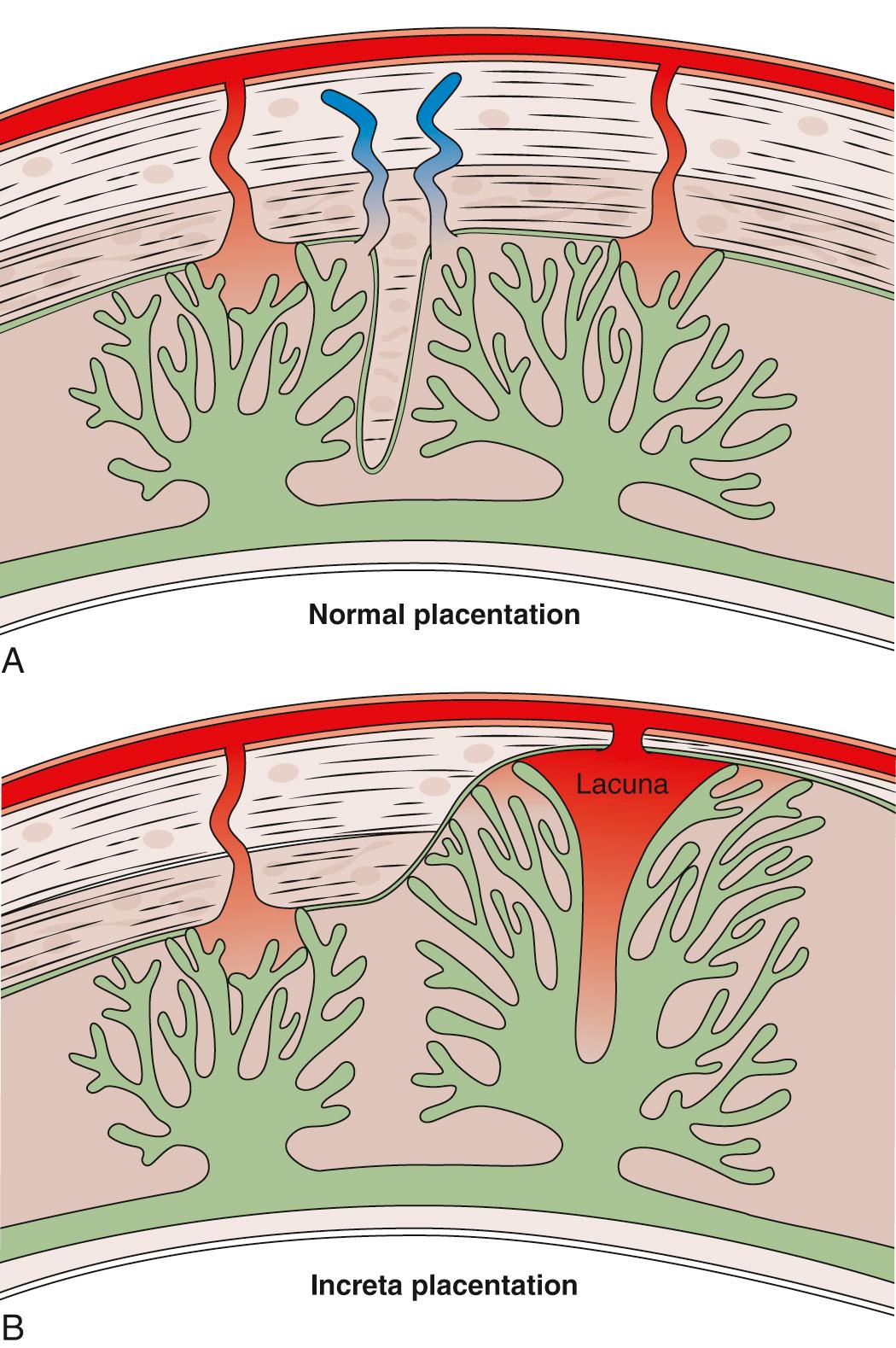
Histologic findings reveal that large and deep myometrial defects are often associated with the absence of re-epithelialization of the scar area . Leukocyte recruitment to the endometrium is observed during the normal secretory phase and has been reported to be increased following a CD. A prior CD increases not only the risk of PAS in subsequent pregnancies but also the risk of placenta previa, suggesting a tropism of the blastocyst for the scar area. Uterine artery resistance is increased, and the volume of uterine blood flow as a fraction of maternal cardiac output is decreased in women with a previous CD compared to women with a previous vaginal birth. Overall, these data suggest a possible relationship between a poorly vascularized uterine scar area and an increase in resistance to blood flow in the uterine circulation. This supports the concept of a primary deciduo-myometrium defect in PAS, in which the myometrium and its vasculature below the junctional zone are exposed to the migrating extra-villous trophoblast and villous tissue ( Fig. 21.5 ). The loss of the normal plane of cleavage and the attachment of villi into the wall of radial and arcuate arteries may explain the clinical symptoms of invasive placentation ( Table 21.1 ).
| Description | |
|---|---|
| Grade 1 | Abnormally Adherent Placenta (Placenta Adherenta or Creta) |
| Clinical criteria | At vaginal delivery:
If laparotomy is required:
|
| Histologic criteria |
|
| Grade 2 | Abnormally Invasive Placentation (Placenta Increta) |
| Clinical criteria | At laparotomy
|
| Histologic criteria | Hysterectomy specimen or partial myometrial resection of the increta area shows placental villi within the muscular fibers and sometimes in the lumen of the deep uterine vasculature. |
| Grade 3 | Abnormally Invasive Placentation (Placenta Percreta) |
| Grade 3A | Limited to the Uterine Serosa (Perimetrium) |
| Clinical criteria | At laparotomy
|
| Histologic criteria | Hysterectomy specimen showing villous tissue within or breaching the uterine serosa. |
| Grade 3B | With Urinary Bladder Invasion |
| Clinical criteria | At laparotomy
|
| Histologic criteria | Hysterectomy specimen showing villous tissue breaching the uterine serosa and invading the bladder wall tissue or urothelium. |
| Grade 3C | With Invasion of Other Pelvic Tissue/Organs |
| Clinical criteria | At laparotomy
|
| Histologic criteria | Hysterectomy specimen showing villous tissue breaching the uterine serosa and invading pelvic tissues/organs. |
These data indicate that significant damage to the uterine wall is associated with a secondary failure of decidualization in the area of the scar, which has an impact on both implantation and placentation. The decidual defect may have an adverse effect on early implantation by creating conditions for preferential attachment of the blastocyst to scar tissue and facilitating abnormally deep invasion of the villous tissue into the uterine wall and beyond.
When Irving and Hertig published their cohort in 1937, only one of their 18 cases had been previously delivered by cesarean section. By 1966, when Luke et al. published their series of PAS, 9 of their 21 cases had a previous history of CD. With the rapid increase in CD rates over the last two decades, a prior CD has become the main risk factor for the development of PAS in subsequent pregnancies, while other factors are now responsible for a relatively small proportion of accreta placentation. The risk of PAS increases with the number of prior CDs. A recent systematic review and meta-analysis reported summary odds ratio (OR) of 2.95 (95% CI, 1.32 to 6.60) for placenta accreta after a CD. Due to high interstudy heterogeneity in methodology and lack of availability of some data, the study could not perform further analysis to stratify risk by the number of prior CDs. In other studies, when the risk of PAS has been stratified by the number of previous CDs, the ORs for PAS disorders increased from 8.6 (95% CI, 3.5 to 21.1) after one prior CD to 17.4 (95% CI, 9.0 to 31.4) after two previous CDs, and to 55.9 (95% CI, 25.0 to 110.3) after three or more prior CDs. A multicenter study of 30,132 women who underwent non-labored CD in 19 academic hospitals in the United States between 1999 and 2002 found that 143 had PAS disorders and that the absolute risk increased from 0.24% after one prior CD to 6.74% after six or more previous CDs. The recent Nordic Obstetric Surveillance Study (NOSS) found that the risk of invasive PAS (after exclusion of cases of adherent PAS and/or simple placental retention) increased seven-fold after one prior CD.
The single most important risk factor for PAS disorders, found in around half of all cases, is a placenta previa. Moreover, the risk of a previa implantation increases with the number of prior CDs . A retrospective cohort study of 399,674 women who gave birth to two consecutive singletons between April 2000 and February 2009 in England as well as a meta-analysis of 37 previously published studies from 21 countries indicate that the rate of placenta previa at second births for women with vaginal first births was 4.4 per 1000 births, compared to 8.7 per 1000 births for women with CDs at first birth (OR, 1.60; 95% CI, 1.44 to 1.76). In the meta-analysis, the overall pooled random effects OR was 2.20 (95% CI, 1.96 to 2.46) for previa after prior CD. Overall, these epidemiologic studies indicate that following a single CD, there is a 50% increase in the risk of placenta previa in a subsequent singleton pregnancy, and following two CDs, there is a two-fold increase in risk of placenta previa compared to women with a history of two vaginal deliveries.
The risk of PAS increases with both the number of prior CDs and the presence of a placenta previa. The risk increases from about 4% in women presenting with a placenta previa and no previous history of CD to 50% to 67% in women with previa and with three or more prior CDs. The UK national case-control study reported that the incidence of PAS increases from 1.7 per 10,000 to 577 per 10,000 births in women presenting with a placenta previa and a prior CD. The study also found a 3.2-fold increase in the risk of placenta accreta after more than one prior CD in women presenting prenatally with low-lying/placenta previa. A large multicenter US cohort study found that for women presenting with placenta previa and prior CDs, the risk of accreta is 3%, 11%, 40%, 61%, and 67% for first, second, third, fourth, and fifth or more cesareans, respectively. The risk of PAS is independent of other maternal characteristics such as parity, body mass index, tobacco use, and coexisting hypertension or diabetes. A decision-analytic model built using data on national birthing order trends and cesarean and vaginal birth after CD rates in the United States between 1995 and 2005 found that if primary and secondary CD rates had continued to rise as they have in recent years, by 2020 the CD rate would be 56.2%, and there would be an additional 6236 cases of placenta previa, 4504 cases of PAS, and 130 maternal deaths annually. Poisson regression models were recently used to assess the relative incidence of PAS among repeat versus primary CDs using the 2000 to 2011 US Nationwide Inpatient Sample data. The authors found that compared with women with a primary CD, women who underwent a repeat CD were 2.13 (95% CI, 1.98 to 2.29) times more likely to have PAS in the subsequent pregnancy.
Any form of damage, even small, to the integrity of the endometrial-myometrial junctional zone following curettage, myomectomy, endometrial resection, or endometrial ablation has been associated with PAS in subsequent pregnancies ( Box 21.1 ). Uterine anomalies, adenomyosis, and submucous fibroids have also been associated with PAS in primiparous women. These rare cases suggest that intramyometrial implantation and development of villous tissue are not always secondary to major uterine surgery and may explain sporadic cases of PAS disorders observed before the 20th century. The high prevalence of these uterine conditions, in particular fibroids and adenomyosis, in the general population and the lack of clear evidence of their association with invasive placentation suggest that they are probably not a major risk factor for PAS disorders.
Endometritis
Major uterine anomalies
Adenomyosis
Submucous fibroids
Myotonic dystrophy
Cesarean section
Uterine curettages
Manual removal of the placenta
Cornual ectopic pregnancy
Myomectomy
Hysteroscopic surgery (endometrial resection)
IVF procedures
Uterine artery embolization
Chemotherapy and radiotherapy
Intrauterine contraceptive device
IVF , In vitro fertilization.
The OR for PAS disorders in pregnancies resulting from in vitro fertilization is 3.1 (absolute risk: 8.2 per 10,000). Similar to advanced maternal age, the association between PAS and use of standard assisted conceptions techniques is most likely due to confounding factors such as multiparity, risk of previa, and the risks of prior uterine surgery. The risks of PAS associated with new fertility treatment techniques such as endometrial scratching remain to be evaluated. A multicenter observational study of 176 women with prior myomectomy indicated no increase (0%; 95% CI, 0% to 1.98%) in the risk of PAS disorders in subsequent pregnancies.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here