Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
This chapter includes an accompanying lecture presentation that has been prepared by the authors: ![]() .
.
MRI provides valuable anatomic detail.
Significant advancements in MRI allow for more improved imaging diagnostic ability.
Diffusion-weighted imaging can provide information on the presence of ischemia and infarction among other neuropathologies such as hemorrhage, infection, and neoplasms.
MR angiography can provide detailed analysis of arterial and venous structures, and vessel wall imaging can complement this analysis, especially in the setting of aneurysm and vasculitis.
Perfusion and permeability imaging can aid in the diagnosis of intra-axial tumors in both the pre- and posttreatment phases.
Functional and diffusion tensor MRI can provide data regarding eloquent brain parenchyma and tracts as related to treatment planning.
MR spectroscopy can provide detailed information on the chemical composition of a parenchymal abnormality.
MR elastography can provide information on tumor stiffness and also shows promise with other pathologies such as Alzheimer dementia.
PET/MRI provides analysis of functional tumor extent and can help in diagnosis, treatment planning, and posttreatment follow-up.
Although the first MRI sequences provided largely anatomic information, many advances in scanning technique over the past 25 years increasingly reflect physiology in health and in disease. More than ever, MRI radiologists are able to suggest specific disease processes with greater confidence and accuracy, especially those that had been difficult to characterize using anatomic sequences alone. For example, perfusion and diffusion imaging allow rapid diagnosis of ischemia and infarction and can now identify the so-called area of ischemic penumbra or critical hypoperfusion that might be rescued with aggressive therapy. Integrity of white matter tracts can be established with diffusion tensor imaging (DTI), from which tractography permits graphic visualization of tract orientation and trajectory. Cerebrospinal fluid (CSF) flow patterns are demonstrated by performing cardiac-gated scanning to characterize bulk shifts in water. Blood oxygen level–dependent imaging exploits changes in flow and oxygenation within specific areas of the brain during specific tasks to demonstrate the site(s) and degree of brain activation. Spectroscopy offers a more refined understanding of brain metabolites than anatomic sequences and can therefore suggest areas of necrosis, areas of anaerobic metabolism, and regions of accelerated cell membrane turnover, or it can identify specific metabolites characteristic of specific disease processes. Vessel wall imaging can aid in the management of patients with aneurysm or vasculitis.
Water molecules, even within tissues that are macroscopically at rest, are constantly moving as a result of intermolecular collisions (Brownian motion, self-diffusion). Diffusion-weighted imaging (DWI) exploits differences in water diffusivity between voxels to generate image contrast.
Cellular and subcellular tissue structure, including cell membranes, macromolecules, and molecular transporters, affect mass transport so some molecules undergo relatively free diffusion, while others are relatively restricted. For instance, intracellular water typically has lower average diffusivity than extracellular water. Furthermore, in some locations, diffusivity of water in one direction may be higher than that in another direction. This phenomenon is referred to as anisotropy and is commonly observed in white matter tracts, where water diffuses more freely along the longitudinal axes of axons than perpendicularly. This preferential motion of water within many brain tissues differs from the more random (“isotropic”) motion in areas that lack significant microstructure, such as CSF. , Although the many pathologic states affect water diffusivity that may be revealed with DWI, the most prominent are ischemia, tumor, inflammation, and certain infections.
DWI techniques suppress signal in proportion to net flow of water molecules, thus producing contrast between volumes with different average diffusivity. Most commonly, this is achieved by application of magnetic gradients both before and after a refocusing pulse. The refocusing pulse inverts the magnetization direction, allowing removal of magnetic field inhomogeneities. In voxels containing molecules with relatively high net movement (e.g., areas of bulk water), this gradient scheme reduces the signal overall, and this high diffusivity results in a relatively “dark” diffusion appearance. In voxels containing water molecules with relatively little net motion, including pathologic states such as infarction of high cellularity, this results in a relatively increased signal, a state of low diffusivity that is depicted with a relatively “bright” diffusion appearance. Multiple factors related to gradient parameters (including amplitude, duration, and time separating the pulses) contribute to the degree of motion-related dephasing (as the protons regress to their resting state), reflected in the diffusion gradient strength (the so-called b-value ). Diffusion sequences in the brain most commonly employ b-values of 1000 sec/mm ; however, elsewhere in the body (including the spine), lower b-values may be employed to enhance signal and reduce artifact. Sequence parameters with higher b-values result in a higher degree of motion-related signal attenuation, which leads to higher contrast but lower signal-to-noise ratio (SNR). Typically, three or more acquisitions are performed, each with a different axis of the applied diffusion gradient. The images at each level are then combined on a voxel-wise basis into an “anisotropic” or “trace” image, one that is first-order unbiased by directionality of fiber tracts.
The amount of signal generated by a given voxel during a diffusion-weighted acquisition is also dependent on multiple factors other than the b-value. The most commonly used pulse sequences for DWI are T2-weighted echo planar imaging (EPI) acquisitions, meaning that tissues with long T2 decay times (such as edematous brain) will have relatively high signal, even when no diffusion sensitization is performed. EPI allows rapid reversal of the signal readout gradient, often in a sinusoidal fashion, thereby reducing imaging time and decreasing motion artifact. In fact, a “b0 image,” which is essentially a T2-weighted image without motion-probing gradients applied, is typically acquired as part of a diffusion acquisition and is necessary for calculation of apparent diffusion coefficient (ADC) maps. ADC maps better define the degree of diffusion restriction because they eliminate the underlying T2 bright signal (“T2 shine-through”) that is present regardless of degree of motion probing. ADC values are calculated automatically by imaging software using a mathematical formula and displayed as a sequence that directly correlates with the degree of water diffusion. Comparing the differences between two or more acquisitions with different magnetizations (b-values) yields a quantitative measure of diffusion displayed on the ADC maps. , In clinical practice, it is necessary to ensure that a “high” diffusion signal corresponds with “low” ADC values to definitively confirm the restricted diffusion that characterizes pathologic entities ( Fig. 12.1 ).
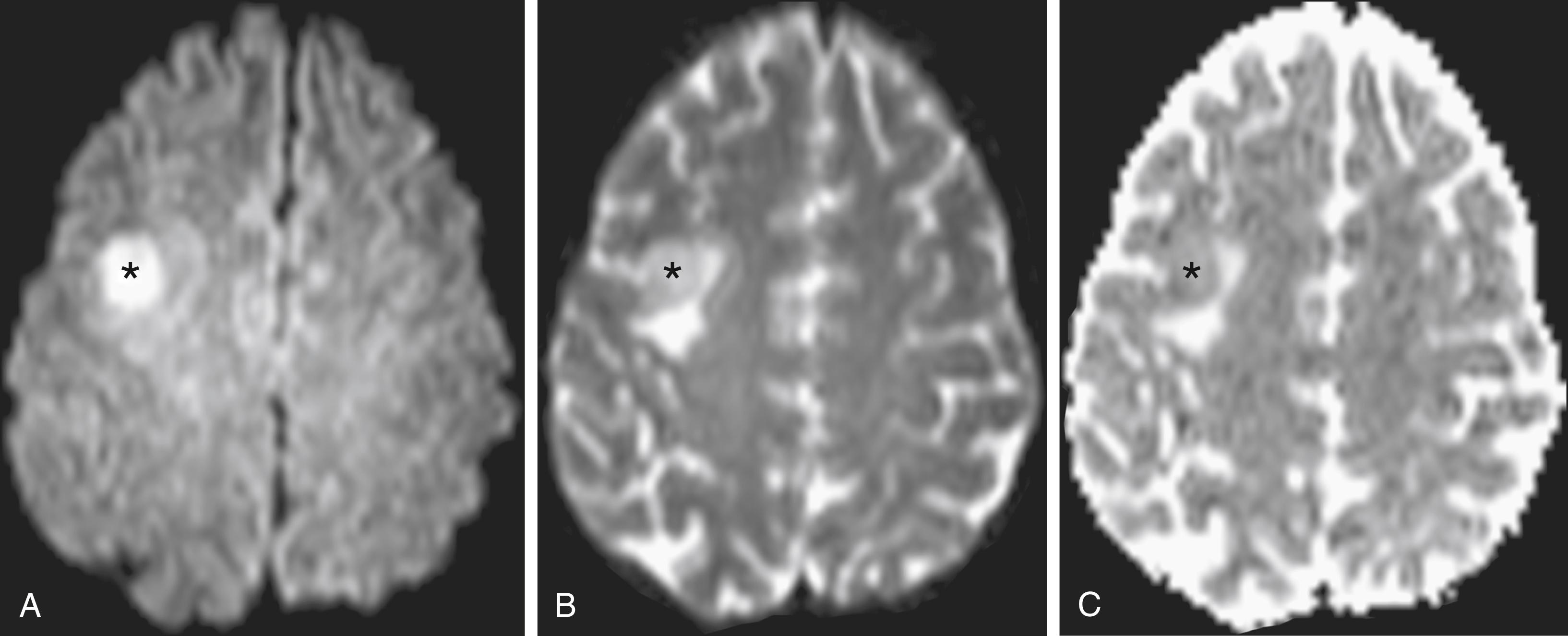
Recent advances have significantly improved resolution of and diminished artifacts associated with DWI, with resultant improvement in detection of small infarcts.
The normal brain has relatively homogeneous diffusivity, with minor differences between gray matter and white matter, rendering regions of pathologically restricted diffusion conspicuous. CSF appears hypointense on heavily diffusion-weighted sequences because of the high diffusivity of bulk water. However, on images acquired with lower b-values (as commonly employed in the spine), CSF can have an intermediate signal because its long T2 relaxation time balances dephasing signal loss.
In animal studies of infarct, DWI hyperintensity is appreciable within minutes of the onset of acute brain ischemia, long before other conventional sequences become conspicuous. The precise mechanism by which ischemia causes restricted diffusion is controversial, but the process likely includes an increased fraction of intracellular water as a result of dysfunction of ion transporters. Diffusion-restriction thresholds (values <500 mm 2 /sec) may predict the final infarct volume in acute stroke, and distinction of this established infarct core is of critical importance when considering intervention to rescue the surrounding at-risk hypoperfused tissue that does not yet have restricted diffusion.
As infarcts age, ADC and DWI patterns evolve at different rates. Although both become abnormal within minutes (ADC dark and diffusion bright), ADC values normalize to match the isointensity of unaffected brain relatively rapidly, in most instances within 5 to 14 days. The ADC then continues to rise over the following weeks, remaining positive (bright) for the life of the patient. DWI normalizes more slowly, declining to isointensity relative to surrounding brain within about 4 to 5 weeks after the infarction, and ultimately dropping further into hypointensity in the setting of chronic encephalomalacia ( Fig. 12.2 ). ,
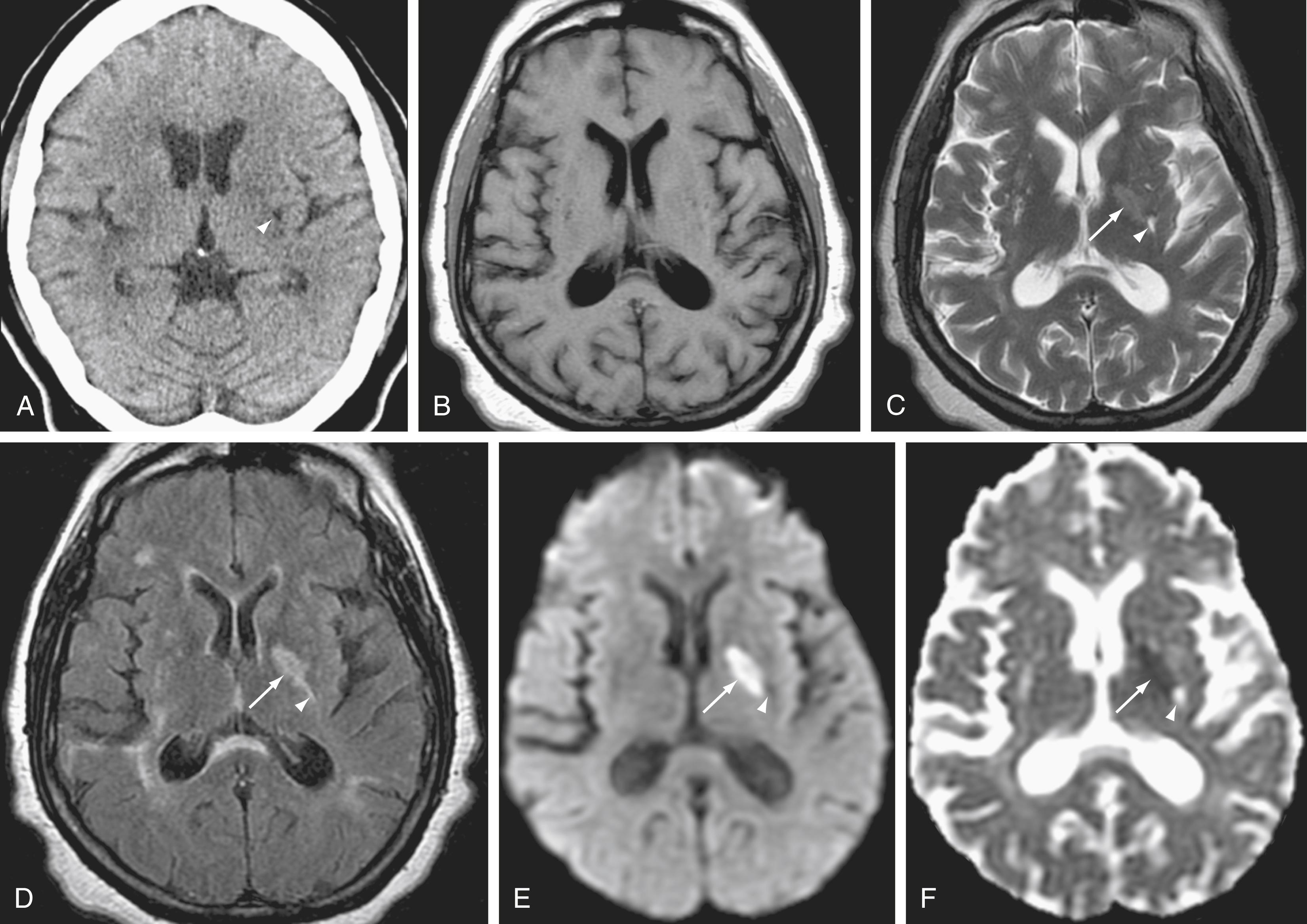
Many intracranial neoplasms have relative restricted diffusion compared with normal brain as a result of hypercellularity. Among histologic types that typically have restricted diffusion are lymphoma, medulloblastoma, and high-grade glioma. Epidermoid cysts also typically have an extremely high signal on DWI, key in their differentiation from arachnoid cysts. , As in ischemia, high signal on DWI can also be seen as a result of true restricted diffusion (see Fig. 12.1 ).
DWI can be used to predict the nature of cystic or necrotic cavities, most of which are bright on T2-weighted images with varying surrounding vasogenic edema. Even though many cavities do exhibit enhancement about the periphery, the presence of central diffusion restriction can be used to predict the macromolecules and inflammatory cells characteristic of abscess. This pattern differs from the low–central-diffusion, high-diffusivity pattern in necrosis, which is characterized by less restrictive debris.
Seizure activity can cause changes in water diffusivity as a result of transient increases in the fraction of water that is intracellular, presumably because of an imbalance between oxygen delivery and consumption. High signal on DWI often initially preferentially involves the cortical ribbon and pulvinar, and it almost always completely resolves, usually over days to weeks. Vasogenic edema, in contrast, can be seen as a late finding after a seizure. Restricted diffusion can be seen in the hippocampi after seizure and is associated with development of mesial temporal sclerosis. ,
Other common pathologic entities that typically have restricted diffusion compared with normal brain include demyelinating processes (including areas of active demyelination where a rim diffusion pattern is typical), herpes encephalitis, Creutzfeldt-Jakob disease, and leukodystrophies. It is also important to remember that acute parenchymal or extra-axial blood may have some degree of restricted diffusion, often seen about the margins of tumor resection cavities, where it is difficult to distinguish from infarct.
DWI sequences are, by design, sensitive to motion. Patient motion and physiologic brain motion resulting from pulse pressure and CSF motion may cause significant image degradation. To mitigate against these problems, fast acquisition techniques such as EPI and parallel imaging are commonly used. These and similar techniques are typically sensitive to chemical shift and susceptibility artifacts, the latter causing significant signal distortion, particularly adjacent to the skull base. Familiarity with these artifacts is critical to avoid misinterpretation of an artifact as pathology.
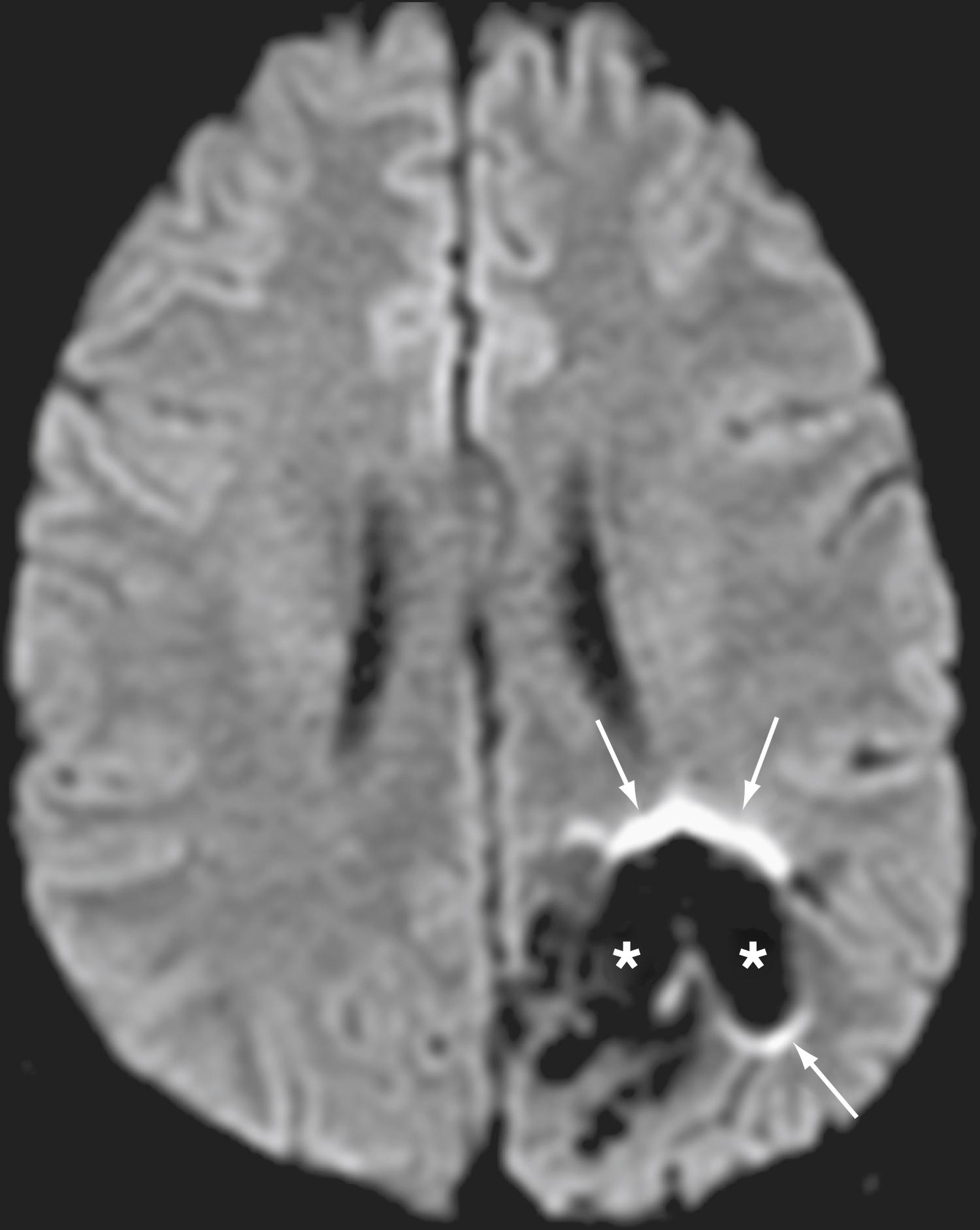
Ferromagnetic materials, such as orthopedic hardware, dental hardware, endovascular coils, or foreign bodies, create significant field distortion, particularly on gradient echo sequences commonly used in DWI, which can result in an image that is uninterpretable ( Fig. 12.3 ).
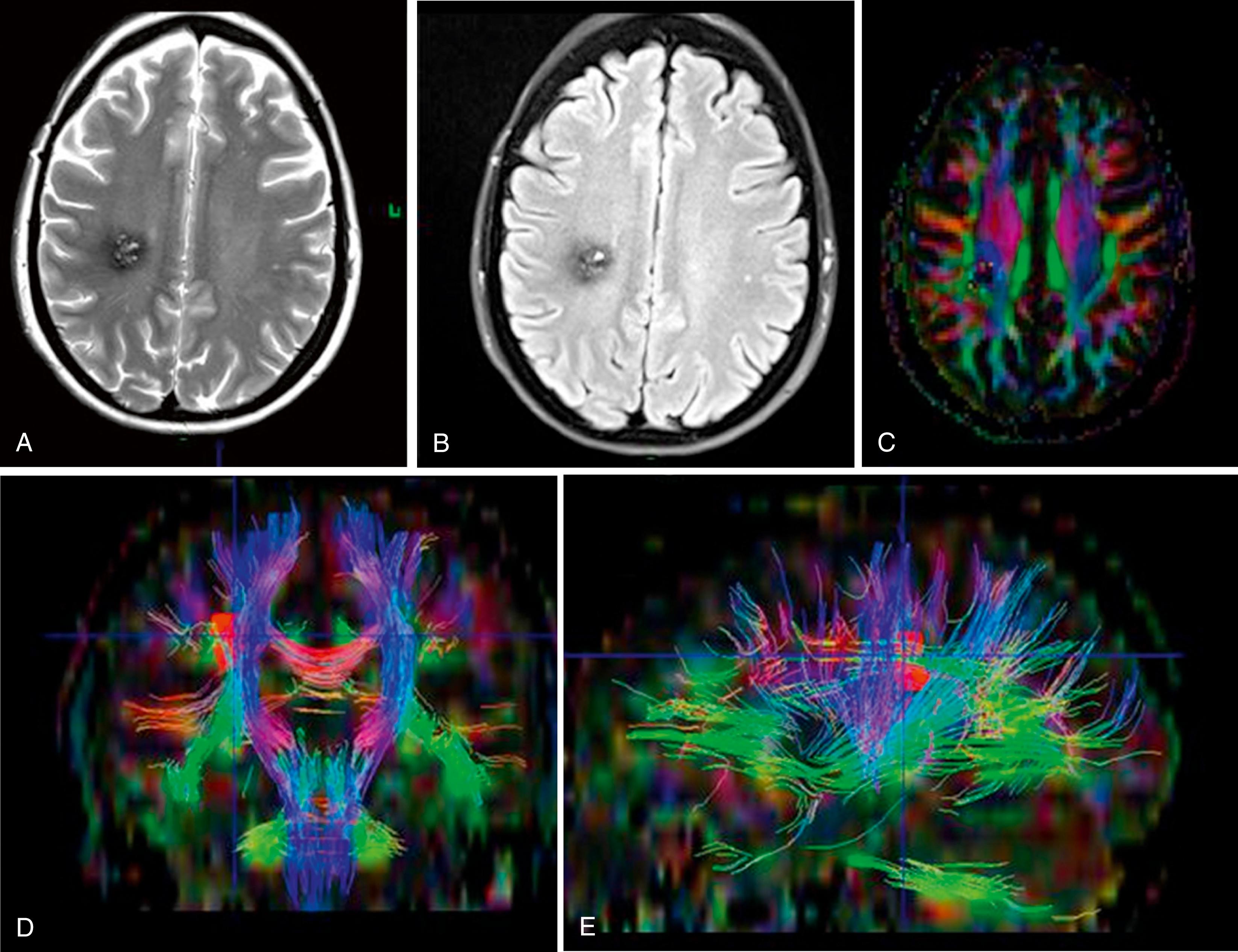
DTI is a newer technique that exploits diffusivity anisotropy (described in the “Diffusion-weighted Imaging” section earlier in this chapter) to infer information about the microstructure of the brain parenchyma. A “tensor” in the context of MRI is an array of numbers that associates a set of vectors with every voxel being imaged. These vectors describe the probability magnitude and directionality of diffusion at each voxel. To resolve ambiguity in diffusion that arises when only three directions are used (basic DWI), DTI will typically use at least six and perhaps many dozens of directions to better define orientations. Tractography, which is based on DTI data, is a technique that uses anisotropy data to infer the presence of continuous axons that traverse multiple voxels. This technique attempts to construct putative axon tracts. Typical algorithms for generation of tracts attempt to “grow” tracts from “seed” voxels, which can be generated randomly, based on heuristics, or manually by a human operator. Tracts are most often displayed as surface-shaded volume renderings in relation to selected cross-sectional images from the conventional MRI dataset.
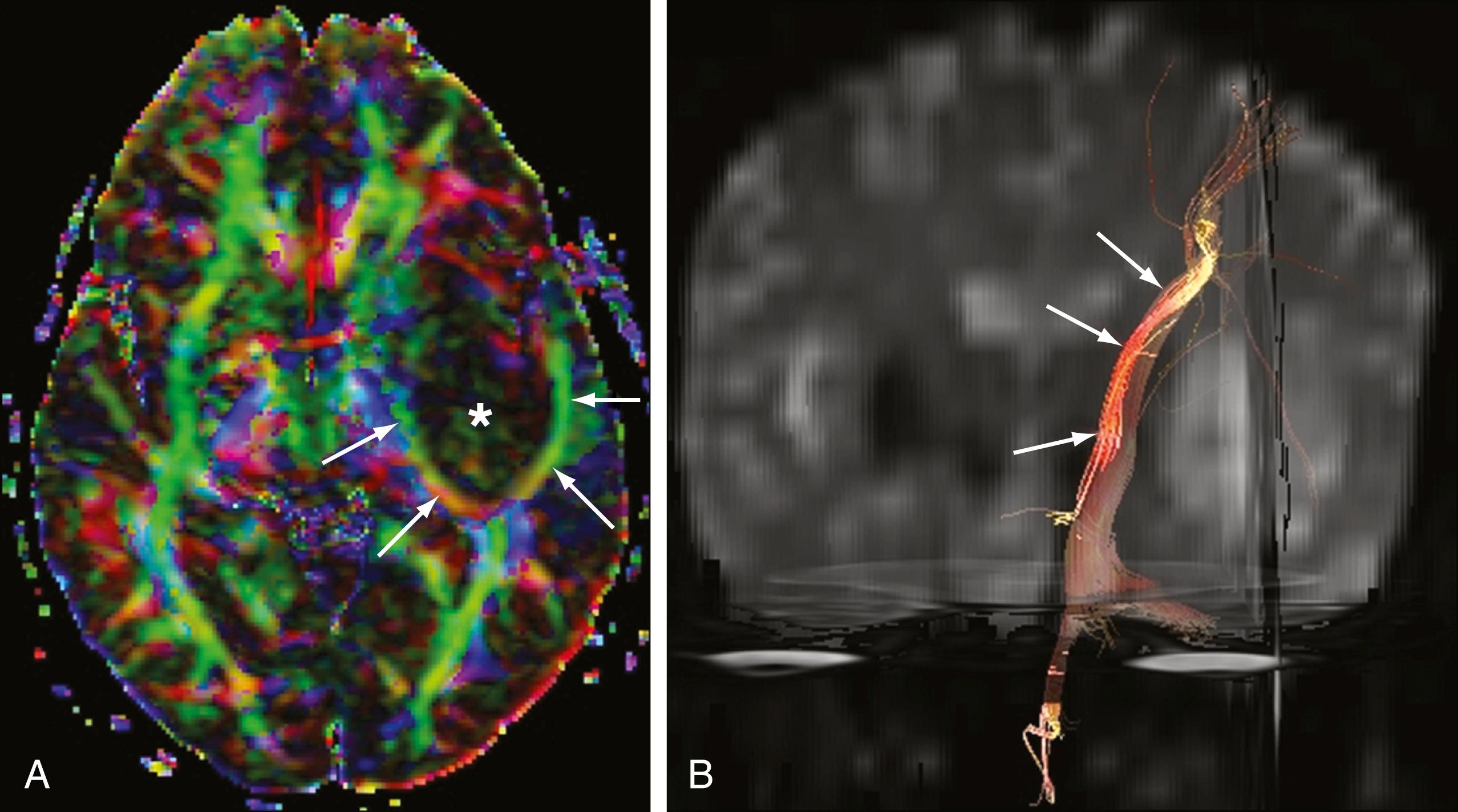
Fractional anisotropy is a term used to describe the degree to which the shape of the ellipsoid described by a voxel’s tensor deviates from a sphere. In the brain, anisotropy is typically presumed to result from the directionality of fiber tracts, and a decrease from expected anisotropy is thought to be related to a disruption of fibers. Fractional anisotropy maps can be viewed as stacks of two-dimensional (2D) images similar to conventional MRI. They can be color-coded to reflect directionality with respect to standard anatomic axes ( Fig. 12.4 ), with most clinical sites using a convention of red reflecting left-right, green reflecting anterior-posterior, and blue reflecting inferior-superior.
Diffusion kurtosis refers to a complex representation of diffusivity as it varies with respect to direction in a given voxel (and its deviation from normal Gaussian distribution). Calculation of kurtosis requires acquisition of diffusion-sensitive sequences in multiple additional directions (compared with conventional DWI), and results of kurtosis analysis can be displayed in various ways. ,
Tractography is perhaps most clinically useful in cases in which the locations of important fiber tracts are not well-defined by conventional anatomic sequences. This is particularly true when it is difficult to determine the relationship of tracts to pathologic structural lesions such as tumors or vascular malformations. The decision to surgically intervene in structural lesions, including those in the cord, may hinge on whether a tract is displaced or invaded ( Figs. 12.5 and 12.6 ). Compromise between procedure-related disability and the extent of tumor resection can be optimized based on knowledge of tract locations.
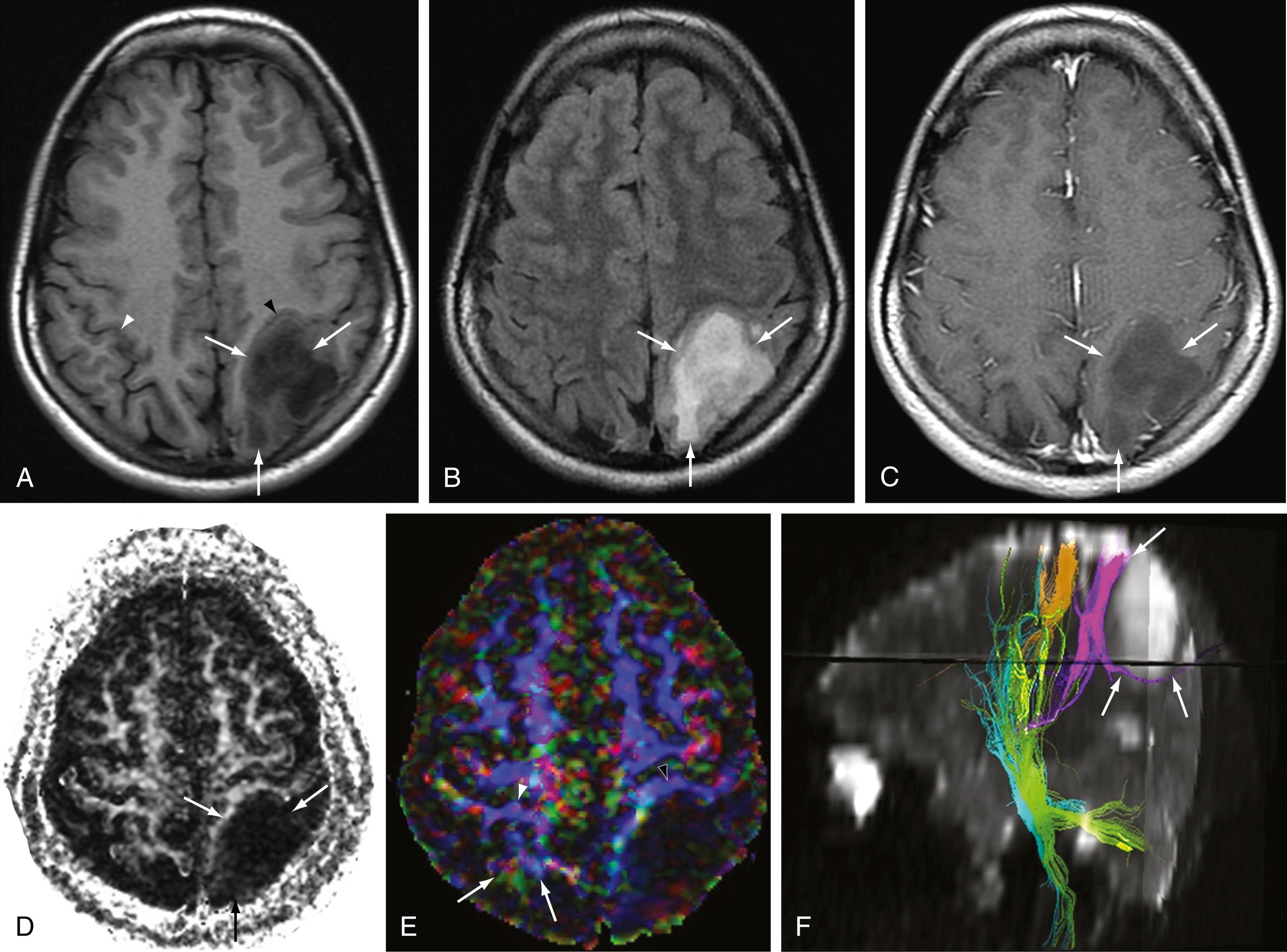
Abnormalities of fractional anisotropy and diffusional kurtosis have been shown to reflect changes in axonal integrity in a number of pathologic entities, including multiple sclerosis, , traumatic brain injury, neurodegenerative diseases, , and infarction. Although these techniques are currently infrequently used in routine clinical work, they are being actively pursued as sensitive proxies for disease activity, particularly as they relate to drug efficacy trials.
Many algorithms for tractography require one or more user-defined “seeds,” and the results can vary dramatically based on small differences in seed placement. For this reason, tractography is very operator-dependent and requires knowledge of neuroanatomy and the clinical goals and intent of the DTI. Additionally, data noise has a profound impact on calculated putative tracts.
DTI tractography in its basic form depends on the assumption that a single ellipsoid can accurately describe diffusivity within a voxel. However, this assumption becomes invalid in many instances where fibers cross or change orientation within a voxel. As a result, tractograms encountering these conditions may become prematurely truncated by tracking software algorithms. More complex representations of diffusivity, such as Q-ball imaging and high-angular-resolution diffusion imaging (HARDI), address these issues to some extent, but at the cost of diminished SNR and increased acquisition time.
Similar to DWI, DTI is very sensitive to patient motion and susceptibility artifact. These issues are particularly problematic in the spinal cord.
Evaluation of the intracranial vascular system is important in the diagnosis and planning of treatment of many vascular-related disease processes such as aneurysms, arteriovenous malformations (AVMs), infarctions, and venous sinus thrombosis. Although the “gold standard” for evaluation of many vascular entities remains digital subtraction angiography (DSA), magnetic resonance angiography (MRA) offers an alternative for assessment of intracranial vessels that is noninvasive and does not require the use of ionizing radiation. The three main varieties of MRA used clinically are time-of-flight (TOF), phase-contrast (PC), and contrast-enhanced (CE) techniques. Each source data set is typically post-processed by a technologist and/or radiologist before interpretation to yield maximum-intensity projections that can be rotated in space for a three-dimensional (3D) effect ( Fig. 12.7 ). Maximum intensity projection projects the voxels with the highest attenuation value through the 3D source imaging volume onto a 2D image. This allows the viewer to see longer segments of vessels in one image slice, thereby allowing easier detection of vascular stenosis and large vessel occlusion; however it may reduce the conspicuity of aneurysms, which would be best evaluated on the source images.
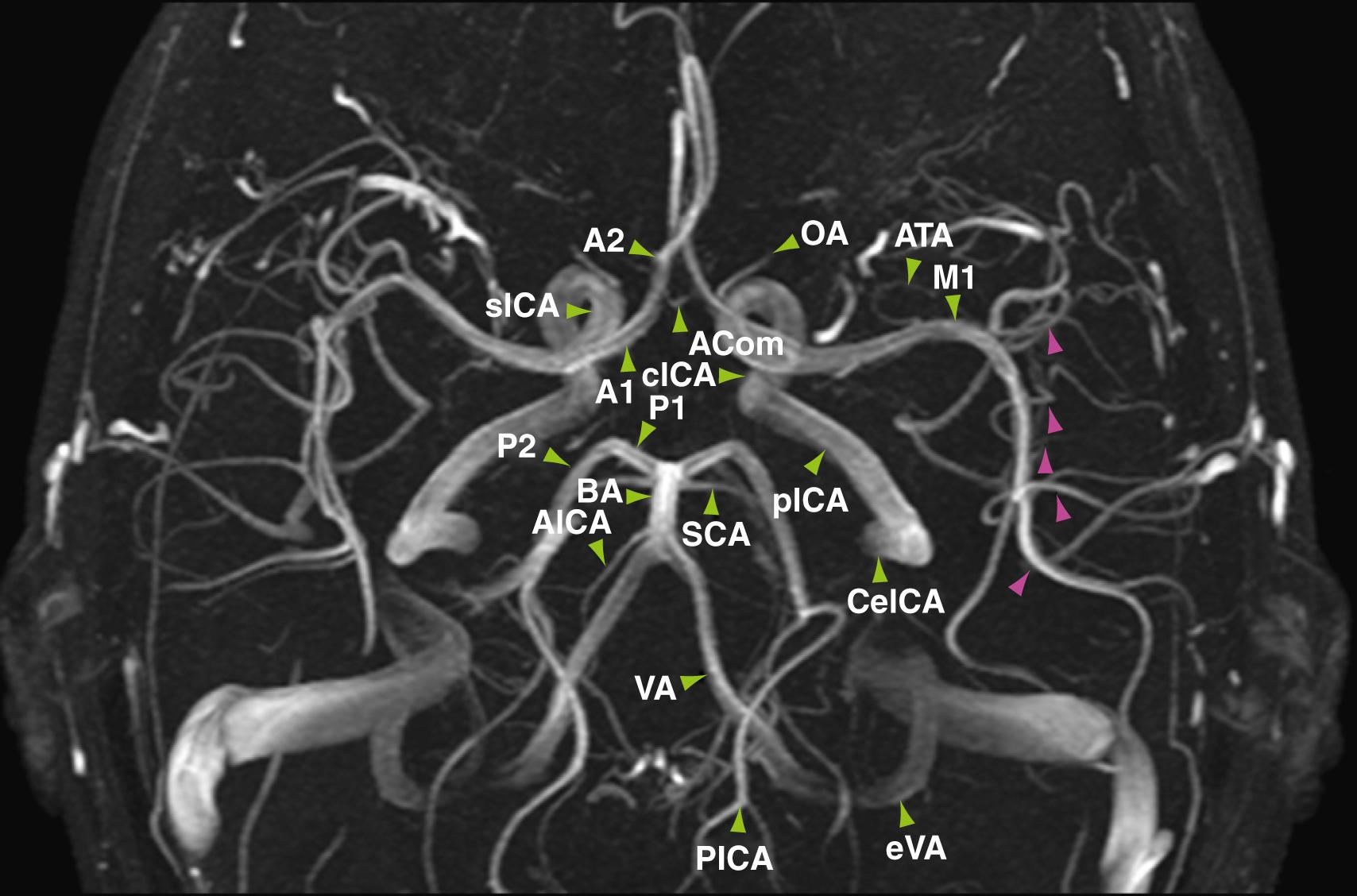
TOF MRA is performed by applying repetitive pulses to stationary tissues in a discrete volume of the brain, which results in saturation or suppression of signal in these tissues. Blood flowing into the volume has not been saturated and is therefore fully magnetized; this in-flowing blood therefore provides the only significant signal within the imaging slab. The intrinsic contrast that is derived from flowing blood eliminates the need for injection of contrast material to visualize the intracranial vessels. The typical basic TOF MRA pulse sequence consists of a gradient-recalled echo sequence, which is acquired with either a 2D or 3D technique. 2D TOF images are obtained as contiguous or slightly overlapped sections, whereas 3D TOF images are derived from one or more overlapping 3D volumes. Of these two methods, 3D TOF is now more commonly used than 2D TOF because of its superior spatial resolution.
In PC MRA, vascular contrast is obtained by applying a bipolar phase-encoding gradient using a velocity-encoding (VEnc) factor. The VEnc factor represents a velocity that is predefined by the operator, measured in cm/sec, and chosen to represent the highest velocity likely to be encountered in a specific vessel. Phase shifts in moving spins or flowing blood are obtained when gradients with opposing polarities are applied twice during a single radiofrequency excitation. This method results in nulling of the signal from stationary tissue while exploiting the signal from flowing blood. Visualization of blood flow depends on the velocity and direction of flow. In specific encoding orientations, middle gray usually represents no flow into or out of the presented plane, whereas progressively increasing shades of white or black indicate directionally encoded higher velocities up to the predefined VEnc factor. Blood that flows at velocities higher than the VEnc factor will demonstrate aliasing, in which signal intensity “wraps around” to the other end of the grayscale spectrum and will therefore appear opposite to what may otherwise be expected. In other words, if flow in a certain direction matches the VEnc factor, it will be encoded as white, but any greater velocity in that direction will actually appear as black. Because arterial flow is more rapid than venous flow, the typical arterial VEnc factor intracranially measures greater than 60 to 80 cm/sec, whereas slow flow within veins and venous sinuses may be best imaged with a VEnc factor of 20 cm/sec or less. Images generated from this technique provide not only the direction of flow but also the magnitude of flow. Therefore PC MRA can provide more information than TOF MRA.
CE MRA uses a high-resolution T1-weighted technique in which the vessels are accentuated by a tight time-critical intravascular bolus of gadolinium chelate. The 2% to 3% concentration of gadolinium in blood by volume causes a marked T1 shortening effect in vessels, with a resultant increase in intraluminal signal. As CE MRA is dependent on contrast concentration, it provides anatomic information related to lumen diameter and the concentration of contrast material in vessels, rather than the physiologic information reflecting the flow rate as calculated with TOF and PC techniques. Advantages of CE MRA over non-CE techniques include higher SNR, decreased susceptibility to artifacts caused by pulsatility and flow, shorter image acquisition times, and temporal resolution that illustrates patterns of flow over time. The need for improved temporal resolution led to the development of time-resolved CE MRA, identified by different vendors with such names as TRICKS (time-resolved imaging of contrast kinetics), TREAT (time-resolved echo-shared angiography technique), and TWIST (time-resolved imaging with stochastic trajectories). These sequences improve temporal resolution by repetitively acquiring the center of the radiofrequency spectrum along with varying peripheral portions during each acquisition; data missing from unsampled regions are interpolated by using data obtained at other points in time. This development enables more rapid 3D acquisitions before, during, and after transit of a contrast bolus, thereby allowing identification of the arterial, capillary, and venous phases. , The generation of numerous full-volume sets over time allows technologists or physicians to create cine movies of entire volumes or specific territories over time.
The angiographic modality with unparalleled spatial and temporal resolution is DSA. However, manipulation of catheters in the aorta or in the carotid or vertebral arteries, even by an experienced operator, is associated with up to a 1.3% risk for a cerebral event. , Therefore in clinical practice most institutions still use noninvasive techniques such as computed tomographic angiography (CTA) or MRA for preliminary evaluation of the vascular tree in the clinical setting of ischemic disease and infarction, aneurysm, AVM, dural arteriovenous fistula (AVF), and veno-occlusive disease.
A cerebrovascular accident, or stroke, is a major cause of morbidity and mortality. It is the third most common cause of death in the United States, with approximately 795,000 cases annually. The majority of these cases are caused by atherosclerotic disease, which compromises arterial flow as a result of stenosis, progressive occlusion or embolus, or some combination thereof. This can lead to ischemia and potentially infarction of the brain tissue involved. MRA has proven itself a valuable method to study the major arterial intracranial vessels to evaluate the effect of atherosclerotic disease. Specifically, 3D TOF MRA has been used as a screening method in stroke patients because it is minimally invasive and provides a reasonable assessment of the degree of vessel occlusion. However, TOF MRA provides suboptimal visualization of vessels containing slow or turbulent flow, and in these instances such vessels may be better seen with the use of intravascular contrast. For example, CE MRA has been shown to offer better visualization of the internal cerebral and middle cerebral arteries, which exhibit artifactual narrowing on 3D TOF MRA because of slow or turbulent flow. Although such limitations exist, TOF MRA remains an important noninvasive tool for evaluation of intracranial arterial circulation in patients with acute neurological symptoms.
Detection of intracranial aneurysms can be achieved with noninvasive techniques such as MRA and CTA. 3D TOF MRA has been shown to have an overall sensitivity of 95% and a specificity of 89% for the detection of intracranial aneurysms ( Fig. 12.8 ). The sensitivity for detection of aneurysms larger than 3 mm is greater than that for aneurysms 3 mm or smaller (94% versus 38%) on older 1.5T scanners, but aneurysms as small as 1 mm can be detected at higher field strengths such as 3T. Both TOF MRA and CE MRA are optimized to show vessel lumens; apical thrombus may remain undetected unless MRA is correlated with conventional sequences that are more sensitive for nonflowing blood. ,
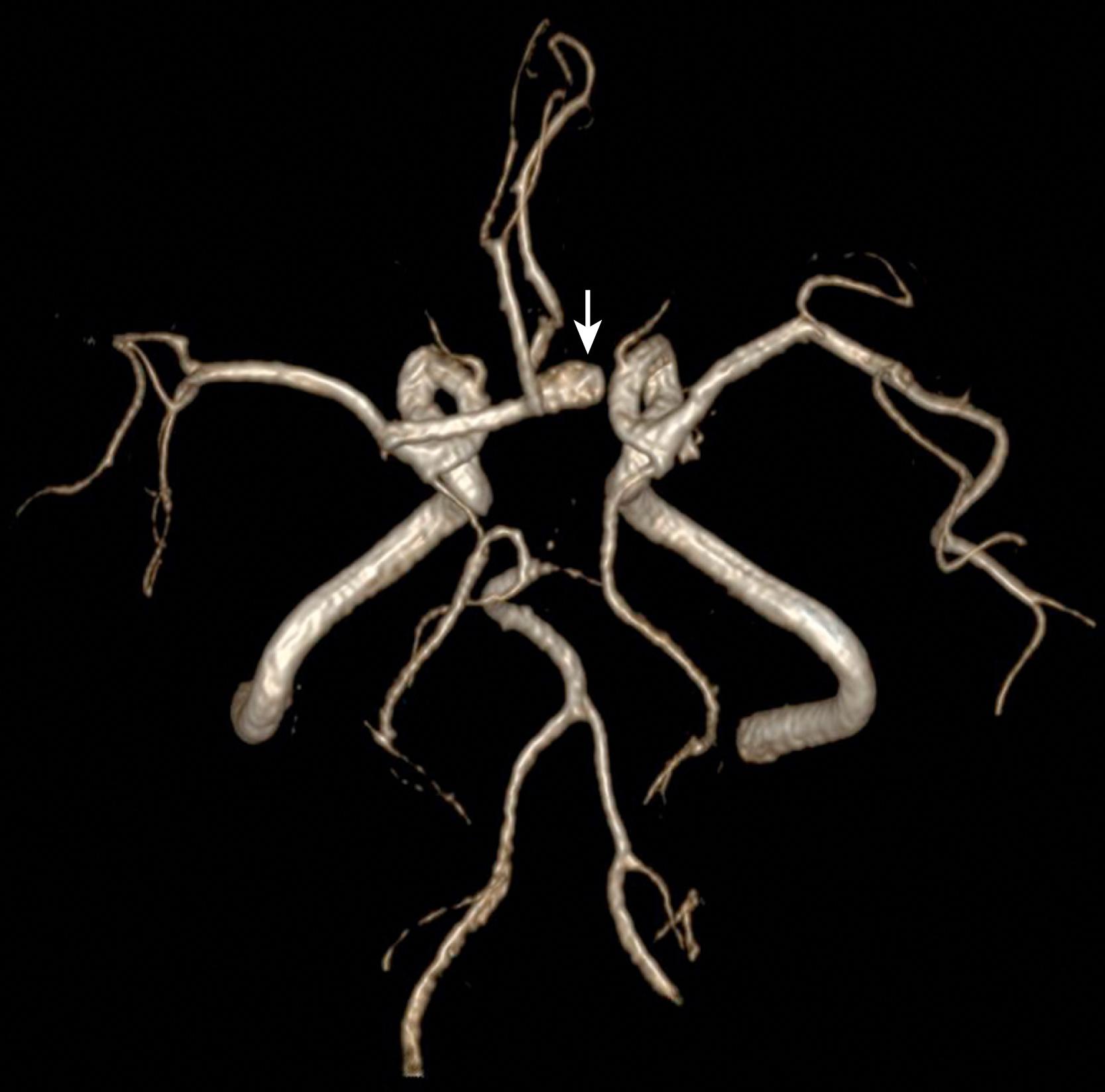
Studies have shown similarities and differences between 3D TOF MRA and CE MRA in the detection of aneurysms. One study demonstrated no significant difference in the quality of the images and equal detection rates for both techniques. Another study showed that 3D TOF MRA detected more aneurysms than CE MRA, , whereas a third study indicated a sensitivity of 100% and a specificity of 94% for CE MRA, hence suggesting that CE MRA may be superior to the 3D TOF technique. Without clear consensus regarding which modality is superior, both provide relatively accurate noninvasive methods for the detection of aneurysms.
MRA has also been studied for the follow-up of intracranial aneurysms treated by coil embolization. This patient population has historically been difficult to image because of either streak artifact or magnetic susceptibility that may be seen on CT and MRI, respectively, as a result of the coil material. Serial studies have been performed with the use of both 3D TOF and CE MRA after treatment to evaluate for delayed aneurysm configuration because recanalization is estimated to occur in 10% to 40% of patients. Studies evaluating residual flow in coil-treated aneurysms have shown a sensitivity and specificity of 81% and 90.6%, respectively, for 3D TOF MRA and 86.8% and 91.9%, respectively, for CE MRA. Furthermore, it has been suggested that CE MRA may be slightly more sensitive in evaluating residual slow flow. DSA still remains the gold standard for evaluation of residual filling in treated aneurysms, but studies have shown an 86% to 94% agreement between 3D TOF MRA evaluation and DSA. In a large prospective 3T series that demonstrated equivalence between 3D TOF MRA and CE MRA for evaluating aneurysm occlusion, coils were substantially better seen with 3D TOF compared with CE MRA. Thus the use of 3D TOF at 3T may be preferred over CE MRA for follow-up of coiled intracranial aneurysms.
AVMs and AVFs are typically evaluated with DSA because of its excellent spatial and temporal resolution, and the opportunity for subsegmental injection. However, MRA techniques may also provide an accurate noninvasive method for preoperative planning and evaluation. 3D TOF MRA and PC MRA allow characterization of flow in abnormal vessels, but they do not provide information on the hemodynamics of vascular malformations. , Because definition of the architecture in and around malformations (e.g., arterial feeders, nidus size, and draining veins) is crucial in the evaluation of AVMs, advances in temporal resolution have had a large impact on noninvasive evaluation of these abnormalities. Indeed, ultrafast CE MRA and time-resolved CE MRA techniques have been shown to define relationships between lesions and the vasculature better than TOF and routine CE MRA techniques. , It should be noted that aneurysms associated with AVMs are important angioarchitectural features that may be poorly detected by MRI/MRA, and demonstration of such aneurysms remains a distinct advantage of CTA over MRA. Specific types of time-resolved CE MRA techniques such as TRICKS/TWIST MRA have been developed to provide hemodynamic information that allows the ability to detect processes previously undetectable on noninvasive imaging, such as early venous drainage ( Fig. 12.9 ). For example, in a small series of 40 patients, time-resolved MRA at 3T was shown to be a reliable technique for the evaluation and surveillance of dural AVFs.
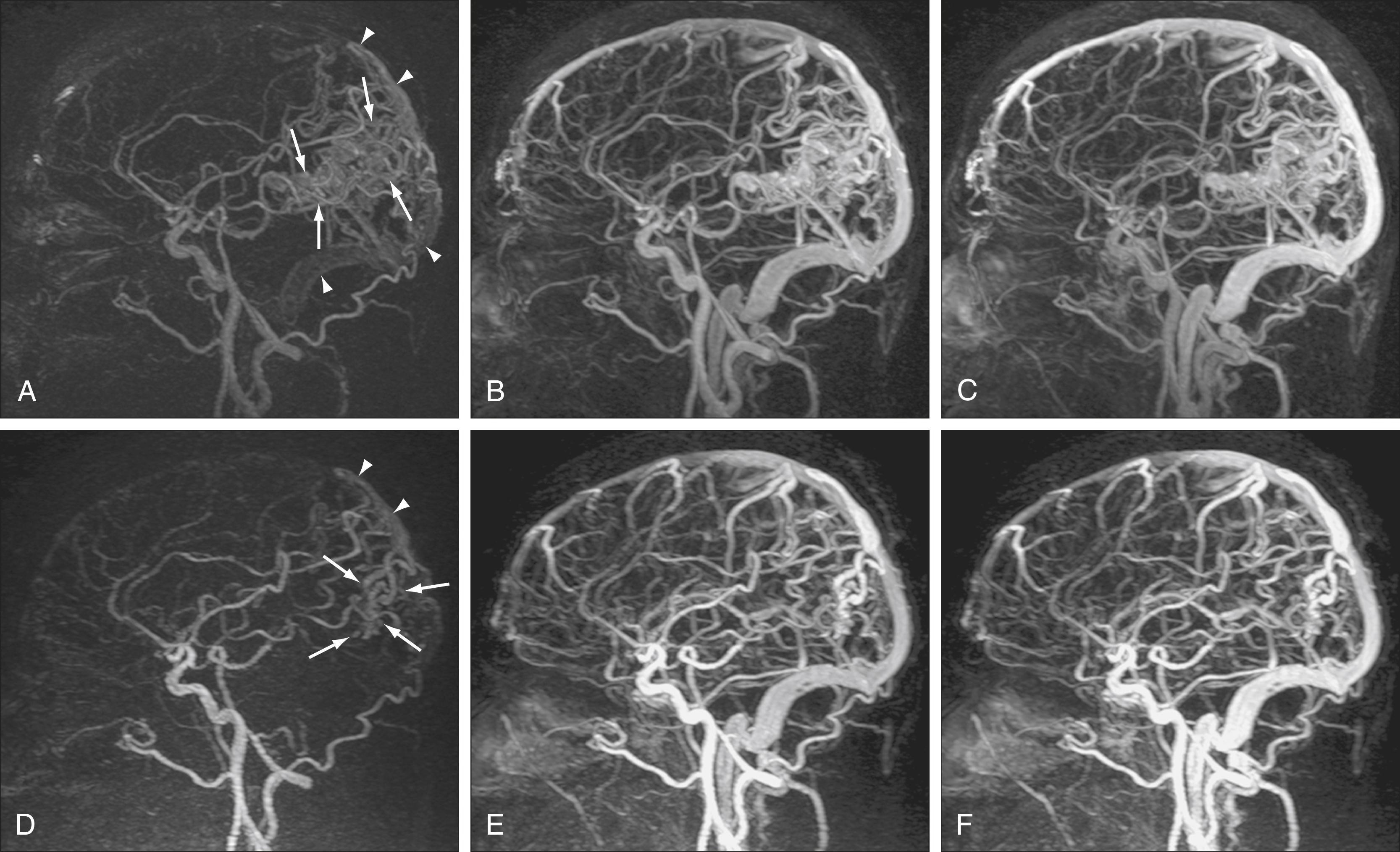
MRA techniques can be applied to study the intracranial venous system. Although arterial flow provides a robust signal on 3D TOF sequences, the slower flow in veins provides a more limited signal with 3D techniques. 2D TOF techniques are used instead because they offer excellent sensitivity for slow flow and less saturation effects than with 3D TOF techniques ( Fig. 12.10 ). , 2D TOF MRA best depicts flow perpendicular to the scanning plane, so areas of turbulent or tortuous flow (e.g., from the straight sinus through the transverse sinus through the sigmoid sinus) may require imaging in more than one acquisition plane to indicate flow patterns adequately. In addition to TOF magnetic resonance venography (MRV), 2D or 3D PC MRV may be used because these techniques can indicate not only the rate of flow but also the directionality of flow. However, the dependence of PC MRV on operator-defined VEnc parameters may complicate its use, with incorrect assumptions at the time of scanning potentially leading to artifacts on the final images.
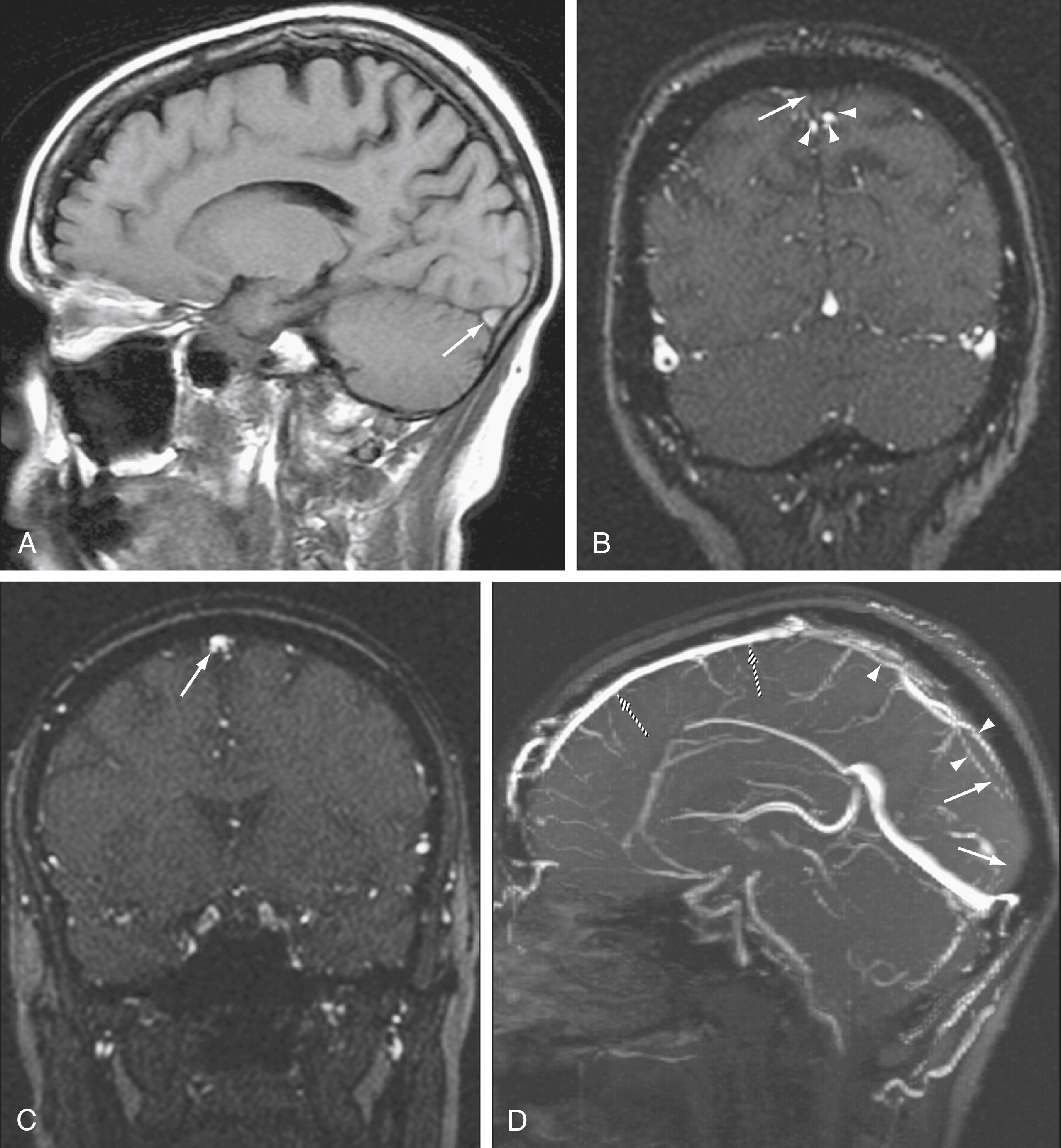
MRV can be enhanced by exploiting the paramagnetic effects of intravenous gadolinium. CE MRV has been shown to provide significantly better visibility of venous structures, including deep cerebral veins, than is possible with the TOF technique. Selected venous structures studied in a small group of patients demonstrated visibility of 99% with CE MRV and 75% with TOF MRV. CE MRV can also be helpful in reducing the effects of turbulent flow on visualization of the venous sinuses.
Various MRA techniques can serve as alternatives to DSA imaging, although limitations in both spatial and temporal resolution still render DSA the gold standard in evaluating most neurovascular pathologies. However, MRA is used widely because it is noninvasive (or minimally invasive if gadolinium is injected). Therefore it is important to be familiar with the limitations of the various vascular MR techniques.
Spin dephasing can occur when blood flow is complex or turbulent and when vessels are in close proximity to tissues with short T1 properties, such as fat and subacute hemorrhage (methemoglobin). This configuration may result in loss of signal on TOF and PC MRA. Slow flow can also result in a similar loss of signal on these imaging sequences because of spin saturation. , Flow saturation effects can be diminished by using the “multiple overlapping thin slab acquisition” (MOTSA) technique for 3D TOF MRA ( Fig. 12.11 ). Most institutions use three or four such slabs in an MRA acquisition through the head; this technique improves flow signal in vessels but lengthens imaging times.
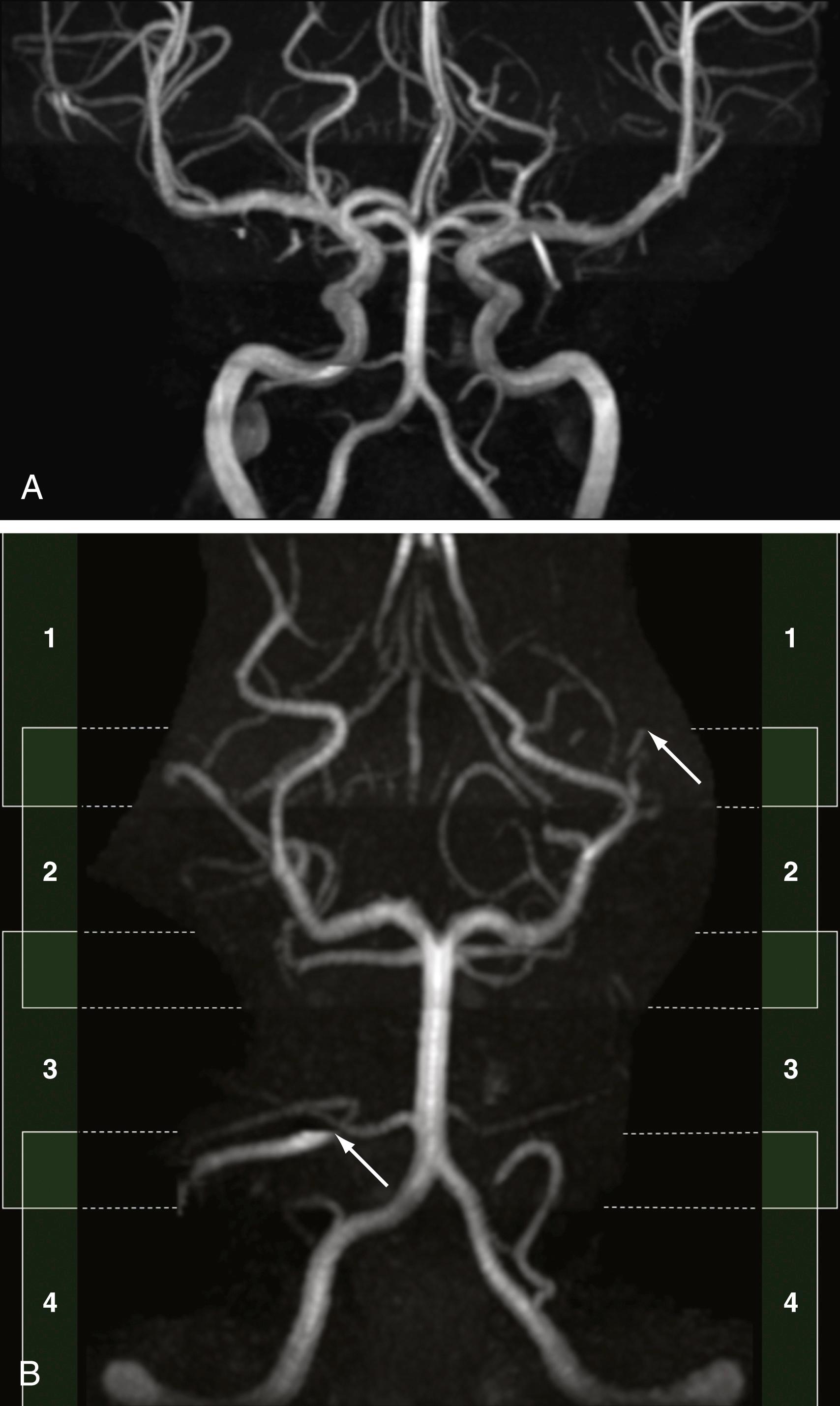
Velocity aliasing can be seen with PC MRA when true velocities exceed the VEnc parameter of the imaging sequence. This may result in the perception of motion opposite the actual direction of blood flow; however, experienced radiologists will recognize the abrupt transition from black to white (or vice versa) as artifactual. In addition, factors such as longer acquisition times, lower spatial resolution, and artifacts related to pulsatile flow have limited the use of PC MRA compared with TOF and CE MRA. Therefore some facilities may reserve PC MRA to answer specific questions about flow directionality rather than including it as a standard imaging sequence.
Studies have reported high sensitivity and specificity, ranging from 94% to 96%, for 3D TOF MRA in the evaluation of intracranial stenosis. However, this MRA technique has a tendency to overestimate the degree of stenosis. A small study demonstrated overestimation of stenosis in 37% of diseased intracranial arterial segments with 3D TOF MRA when compared with DSA Even though such measurement errors do limit the positive predictive value of TOF MRA in evaluating intracranial stenosis, this technique is still frequently used because of the high negative predictive value with a noninvasive technique.
MRV is best reserved for conditions in which there is concern about the patency of large or medium-sized veins. Even with this caveat, flow on TOF MRV may overestimate poor flow if the MRV is acquired in a plane parallel to the venous flow. This is a common limitation in the interpretation of these images and often mandates closer assessment of the source images. In instances in which a concern for true thrombosis persists, the acquisition plane may be modified, or PC MRV or CE MRV may be performed.
CSF flow studies have been used to evaluate a variety of conditions related to defined or suspected hydrocephalus. In particular, cardiac-gated PC MRI provides a method for both qualitative and quantitative assessment of intracerebral and cervical CSF flow. The technique is a reliable and rapid tool available for characterization of CSF flow dynamics.
As in PC MRA, the PC technique can be used to quantify the rate and directionality of CSF flow. A similar bipolar gradient is applied to stationary tissue to cancel the magnetization from stationary spins and null the signal from these tissues. CSF that moves through the plane of acquisition between two separate gradient pulses will experience a net phase shift that is proportional to its velocity along a selected axis. , The VEnc factors used in PC CSF studies are typically lower than those used for PC MRA, averaging 10 to 15 cm/sec. Sagittal imaging is usually acquired in the midline sagittal plane, although other orthogonal or oblique planes can be prescribed if needed. For example, axial PC CSF imaging can be performed about the foramen magnum to demonstrate the pattern of flow around the medulla, which may be particularly useful in evaluation of flow in Chiari malformations.
PC CSF study differs from PC MRA in its use of cardiac gating. As a result, this technique permits segregation of magnitude and direction of flow at different points in the cardiac cycle. Sixteen phases are typically acquired through each cycle, with triggering cues derived from a pulse oximeter and each cycle generally defined by an electrocardiographic R wave. In patients with a normal sinus rhythm, adequate data are acquired through the cardiac cycle. However, certain arrhythmias may compromise gating and limit signal, particularly in the later phases.
PC MRI has been used to evaluate CSF flow in a wide range of cerebral disorders, including communicating hydrocephalus, normal-pressure hydrocephalus (NPH), and aqueductal stenosis. Analysis of CSF pulsation in the axial plane at the foramen magnum has improved our understanding of the role of CSF flow disturbances in patients with symptomatic Chiari I malformations. PC MRI has been used to demonstrate elevated peak systolic velocities through the subarachnoid space and foramen magnum in these patients compared with normal subjects.
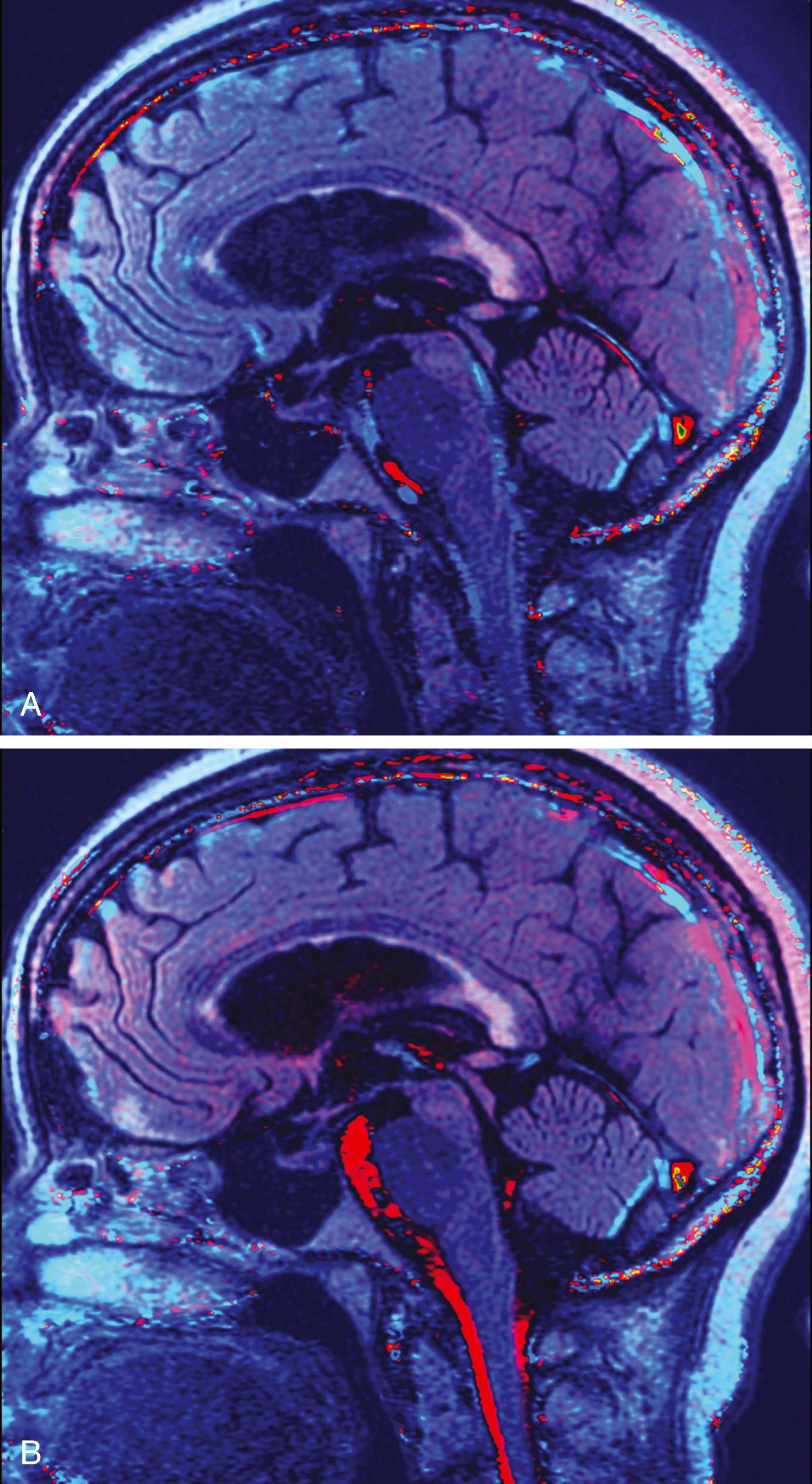
PC MRI sequences are usually analyzed in both static and cine formats. Typically, CSF flow sequences will generate magnitude, phase, and three orthogonal PC flow-encoded sets. Of greatest utility are the directional series that characterize flow within a structure. In the case of midline CSF flow from the third ventricle through the fourth ventricle outlet foramina, the most useful direction would conform most closely to the z -axis ( Fig. 12.12 ). Shades representing one direction versus another may vary by scanner vendor; in practice, radiologists typically refer to the structures with known flow direction (e.g., the deep venous system) to determine which shades represent which directions of flow.
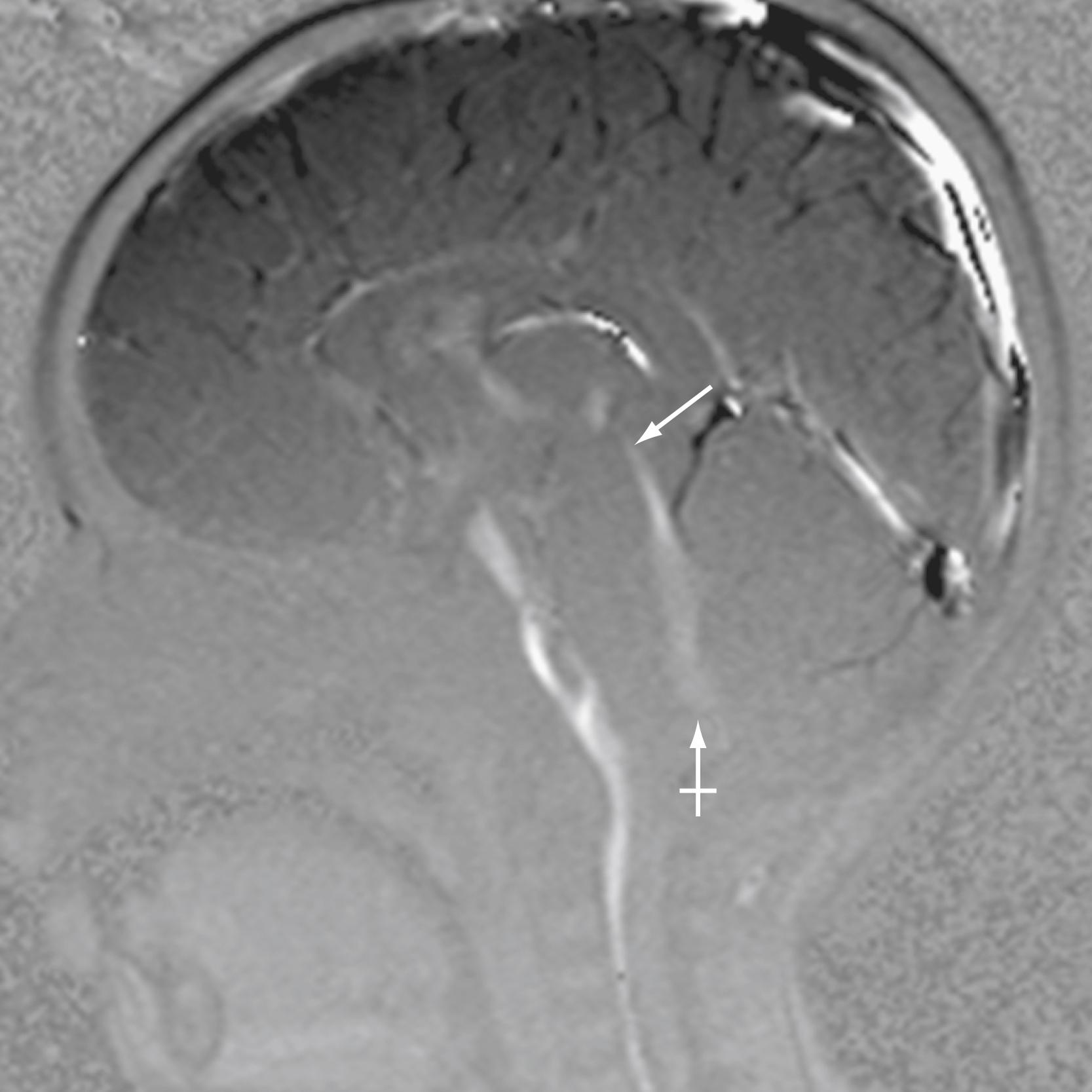
During a normal cardiac cycle, there is rhythmic movement of not only CSF but also brain parenchyma. Cardiac pulsations are transmitted through the intracranial arteries and capillaries and result in motion within the CSF. The relationship between the cardiac cycle and CSF is perceived as normal oscillatory CSF flow. During systole, expansion of the cerebral arterial vessels creates a pressure wave within the subarachnoid CSF. This pressure wave is transmitted through the intracranial subarachnoid space and results in outflow of CSF through the foramen magnum into the compliant spinal CSF space. In diastole, the arterial relaxation and resulting change in intracranial pressure cause CSF to flow back into the cranium through the foramen magnum. , , PC MRI techniques can be used to demonstrate the pulsatile flow within the CSF compartments and have been used to demonstrate the mechanical coupling between blood flow and CSF flow. Cine CSF flow studies have shown that normal CSF pulsations exhibit a timed sequence of oscillatory flow at specific sites in the ventricular system and subarachnoid spaces. The full length of the aqueduct can often be outlined by upward and downward waves of CSF pulsation ( Fig. 12.13 ). CSF flow within the aqueduct is directed caudally during systole and cranially during diastole, with an approximate flow velocity of 3 to 5 cm/sec.
Alterations in normal CSF flow dynamics can be seen in a variety of neurological conditions. Normal oscillatory CSF can be affected when soft tissue impinges on or affects the foramen magnum, as seen in symptomatic Chiari I malformations. Abnormal flow can also occur in patients with Dandy-Walker malformations, arachnoid cysts, or fourth ventricular outlet obstruction. CSF pulsation waves in these cases appear to be confined to the isolated space without detection of any significant CSF flow across the point of obstruction ( Fig. 12.14 ). A mass that partially obstructs CSF outflow may also alter the time course of CSF pulsations.
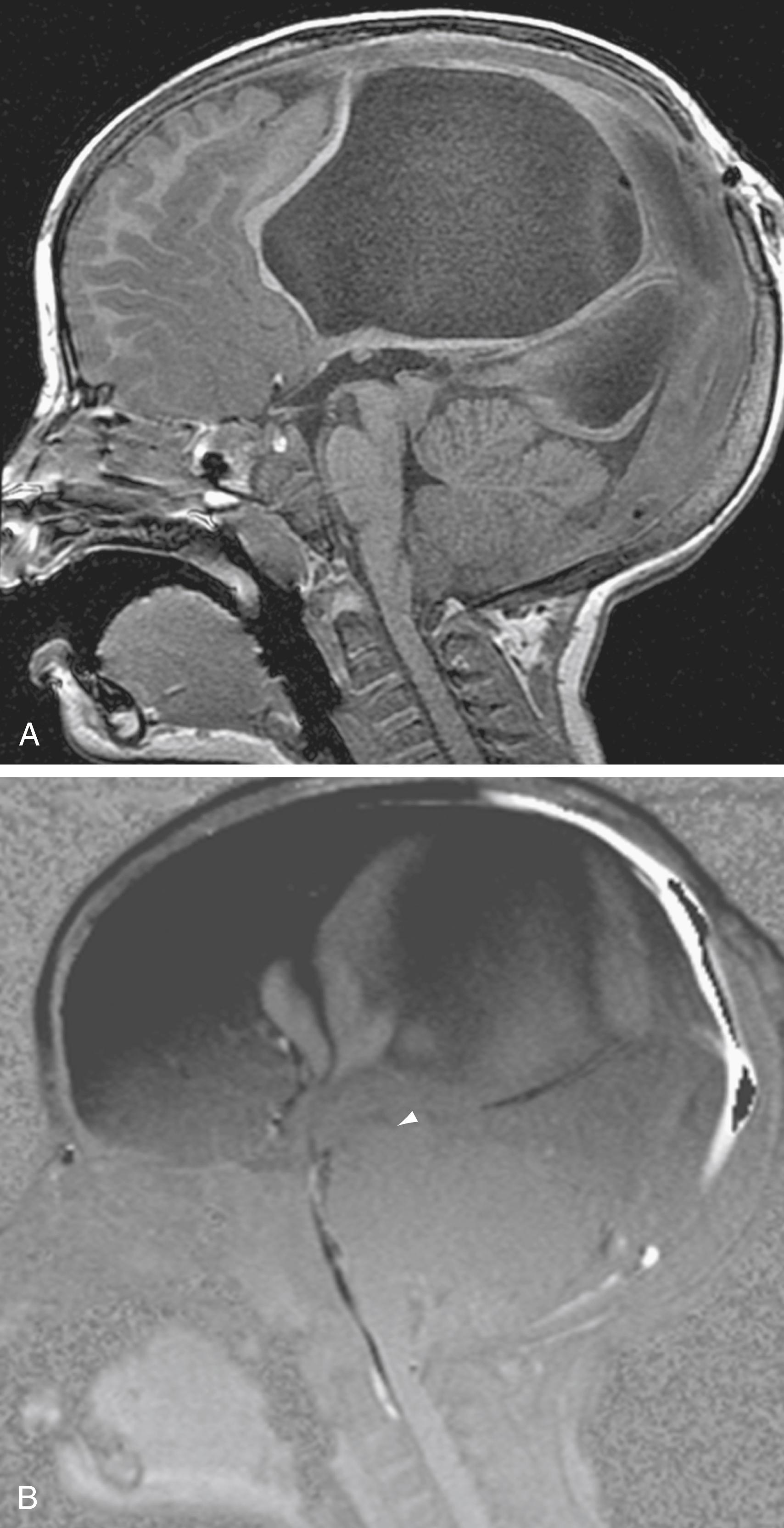
An imbalance between intracranial blood flow and CSF flow has been suggested to be responsible for certain neurological disorders, including NPH. PC MRI has provided a means for the measurement of CSF flow at the aqueductal level. This has allowed improvement in diagnosis and management of patients with communicating hydrocephalus. It has been shown that a small group of symptomatic patients with CSF stroke volume greater than 42 μL per cycle may benefit from CSF shunting ; in normal volunteers the aqueductal stroke volume averages 39.51 ± 18.21 μL per cycle (range, 8.68 to 71.7). Quantification of the peak flow rate has likewise been suggested as an appropriate means for detecting abnormal aqueductal flow. However, it has not proven to be a reliable parameter for differentiating patients with hydrocephalus from normal individuals.
The use of PC MRI has also had important implications in the management of aqueductal stenosis or compression and the management of posterior third ventricle masses. The diagnosis of impaired flow through the aqueduct as the cause of hydrocephalus can direct management to endoscopic third ventriculostomy, which has shown success in the treatment of obstructive hydrocephalus. , Although conventional MRI may demonstrate signs of aqueductal stenosis such as triventricular dilation or downward bulging of the third ventricle, evaluation with these signs alone is subjective and highly dependent on observer experience. PC MRI offers a reliable, reproducible, and accurate method for the diagnosis of aqueductal compromise in patients with suspected obstructive hydrocephalus by demonstrating the absence of CSF flow ( Fig. 12.15 ).
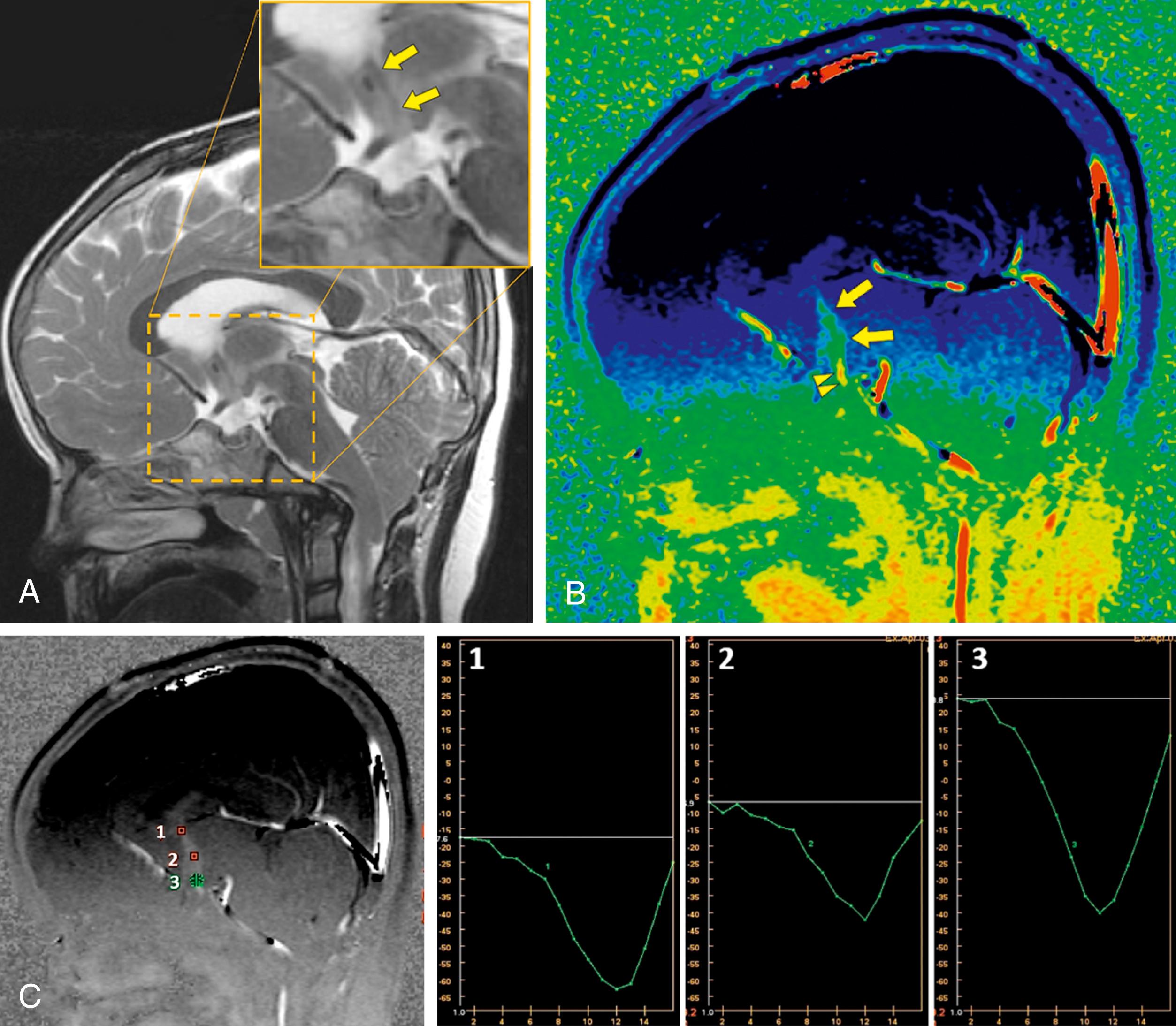
The accuracy of CSF flow evaluation can be limited if the operator does not select the proper VEnc factor. If CSF velocity exceeds the preselected VEnc parameter, aliasing or phase wraparound may occur. It is important to understand that the information acquired with PC MRI techniques without the use of true cardiac gating is an approximation of true CSF flow. The images obtained do not represent specific data points within the cardiac cycle but rather are approximations of preselected intervals with respect to the heart rate. In addition, time-related measurements may be compromised in patients with certain arrhythmias.
Knowledge of certain pitfalls in PC MRI can greatly enhance diagnostic accuracy. For example, absent CSF pulsation may not indicate aqueductal obstruction; in some situations, slow flow of CSF in general (e.g., with obstruction at the level of the foramen of Monro by a colloid cyst) may result in reduced CSF flow downstream that could be interpreted as impaired flow through the aqueduct. In addition, CSF pulsations can appear to be discontinuous in a patent CSF space when there is converging flow, turbulence, or aliasing of pulsations.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here