Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A number of techniques have been developed which exploit the shift in frequency of ultrasound when it is reflected from moving blood. This frequency shift is known as the ‘Doppler effect’. Five types of diagnostic Doppler instrument are usually distinguished:
Continuous wave (CW) Doppler
Pulsed wave (PW) Doppler
Duplex Doppler
Colour Doppler imaging (CDI; colour velocity imaging, colour flow imaging)
Power Doppler imaging.
The characteristics of an ultrasound beam, the propagation of ultrasound in tissue and the design of transducers as found in B-mode imaging are all relevant for Doppler techniques.
For all waves such as sound or light the Doppler effect is a change in the observed frequency of the wave because of motion of the source or observer. This is due either to the source stretching or compressing the wave or the observer meeting the wave more quickly or slowly as a result of their motion. In basic medical usage of the Doppler effect, the source and observer (receiver) are a transmitting and a receiving element usually positioned next to each other in a hand-held transducer ( Fig. 1-1A ). A continuous cyclic electrical signal is applied to the transmitting element and therefore a corresponding CW ultrasound beam is generated. When the ultrasound is scattered or reflected at a moving structure within the body, it experiences a Doppler shift in its frequency and returns to the receiving (detecting) element. Reflected ultrasound is also detected from static surfaces within the body but it has not suffered a Doppler shift in frequency. After the reflected ultrasound is received, the Doppler instrument separates the signals from static and moving structures by exploiting their different frequency.
Motion of the reflector towards the transducer produces an increase in the reflected ultrasonic frequency, whereas motion away gives a reduction. The system electronics note whether the detected ultrasound has a higher or lower frequency than that transmitted and hence extract information on the direction of motion relative to the transducer.
When the line of movement of the reflector is at an angle θ to the transducer beam, then the Doppler shift, f D , is given by:
where f t is the transmitted frequency, f r is the received frequency, c is the speed of ultrasound and u. cos θ (i.e. u × cosine θ ) is the component of the velocity of the reflecting agent along the ultrasonic beam direction. For a typical case of blood flow in a superficial vessel:
Transmitted frequency, f t = 5 MHz = 5 × 10 6 Hz
Velocity of sound in soft tissue, c = 1540 m/s
Velocity of blood movement, u = 30 cm/s
Angle between ultrasonic beam and direction of flow, θ = 45 °
The Doppler shift is therefore:
The shift in frequency is small and within the audible range. In an ultrasonic Doppler instrument, the electronics are designed to extract the difference in frequency, f D = f t – f r (the Doppler shift frequency). The instrument can therefore feed a signal of frequency f D to some output device such as a loudspeaker or frequency analyser.
So far we have considered an ultrasound beam being reflected from a structure moving at a fixed speed and hence generating a Doppler shift of one particular frequency. In practice, there are many reflecting blood cells and their speeds are different. The ultrasound signals returned to the detector from the different cells therefore have suffered different Doppler shifts and add together to give a complex signal containing a range of frequencies. The Doppler shift frequencies are extracted from the detected complex signal and can be fed to a loudspeaker where they can be interpreted by listening. High-frequency (high-pitch) components in the audible sound are related to high speeds, whereas low-frequency components correspond to low speeds. Strong signals, namely those of loud audible volume, correspond to strong echoes that have received a Doppler shift. Strong signals could be due to the detection of many blood cells, say in a large vessel, or to echoes from tissue. An output display called a spectral display or spectrogram is often used to portray the frequency content of Doppler signals.
In the PW Doppler technique, the electrical excitation signal is applied to the transmitter element at regular intervals as pulses, each containing typically 10 cycles, and therefore a corresponding train of pulses of ultrasound are transmitted, separated by non-transmission intervals of duration around 20 times that of each pulse. Regularly spaced echoes are then received back from a reflector and they can be regarded as samples of the signal which would be received if a continuous wave had been transmitted. If the reflector is moving the system electronics can extract a Doppler shift signal from the samples. The Doppler equation again applies to this Doppler shift and can be used to calculate the speed of the reflector.
A bonus of PW Doppler is that since pulsed ultrasound is employed, the range of the moving target may be measured from the echo-return time, as well as its speed from the Doppler shift. The range can be measured from one echo signal; however, the calculation of the Doppler shifts and hence speed of the reflector, typically requires 50–100 echoes. As in the CW case, a group of blood cells moving with different velocities produces a range of Doppler shift frequency components in the output signal.
It was noted above that the frequency of reflected ultrasound is shifted upward or downward depending on whether the motion of the reflector is toward or away from the transducer. A numerical example illustrates this point and emphasises the small changes in frequency that the instrument must distinguish. When 2 MHz ultrasound is reflected from an object travelling at 30 cm −1 toward the transducer, it returns to the receiver with a frequency of 2.00078 MHz, a shift of + 0.00078 MHz. If the object moves at 30 cm −1 away from the transducer, the ultrasound returns with a frequency of 1.99922 MHz, a shift of −0.00078 MHz. Virtually all Doppler instruments which measure velocity preserve this direction information.
Doppler blood flow instruments are required to be extremely sensitive and to be capable of detecting weak signals from moving blood in the presence of much stronger signals from static or moving tissues; the latter give rise to low-frequency Doppler shift ‘clutter’ signals. The magnitude of the scattered signal from blood is typically 40 dB below that received from soft tissues, i.e. the blood echo amplitude is typically one hundredth of the soft tissue echo amplitude. The dB (decibel) unit is a measure of the size of a signal relative to another signal; the second signal is often a reference signal or perhaps the input signal to an amplifier to which the output is compared. Blood flow signals may be detected even though the vessel is not clearly depicted, for instance in the fetal brain, or the renal artery of the neonate.
The transducer of a basic CW Doppler unit has two independent piezoelectric elements. Since the transmitting element is continually driven to generate a continuous wave of ultrasound, a second element is used to detect the reflected ultrasound. When a CW Doppler mode is implemented as part of an ultrasound system which uses array transducers, separate groups of array elements are used for transmission and reception. On extraction of the Doppler shift frequency a filter, the ‘wall thump’ filter, is often used to remove large, low-frequency components from the signal, such as those from slowly moving vessel walls. Typically in a Doppler unit operating at 5 MHz, Doppler shift frequencies below 100 Hz are removed by filtering. Basic CW Doppler instruments are small and inexpensive; CW Doppler mode facilities are incorporated into some array systems to allow them to detect high velocities (see section on Aliasing artefact, below).
The transmitted ultrasound field and the zone of maximum receiving sensitivity overlap for a particular range in front of the transducer ( Fig. 1-1A ). Any moving structure within this region of overlap will contribute a component frequency to the total Doppler signal. The shape of the region of overlap (the beam shape) can be considered as having a crude focus which depends on the field and zone shapes and on their angle of orientation to each other. In practice, the beam shapes are rarely well known for CW Doppler transducers. A 5 MHz blood flow instrument might be focused at a distance of 2 or 3 cm from the transducer and a 10 MHz device at a distance of 0.5–1 cm. CW Doppler instruments normally have ultrasonic output intensities (I spta ) of less than 10 mW cm −2 although they may be significantly higher when used in conjunction with duplex systems to measure high velocities.
A PW Doppler instrument, operating with 5 MHz ultrasonic pulses, may have a pulse repetition frequency (PRF) of 10 000 per second, i.e. 10 kHz. The highest velocity that the instrument can measure is directly proportional to its PRF (see Aliasing artefact, below); therefore the PRF is made as high as possible while still avoiding overlap between successive echo trains. A train of echoes is produced as a transmitted pulse passes through reflecting interfaces and regions of scattering targets. After amplification, successive echo signals from a specific depth are selected by electronic gating and the Doppler shift frequency is extracted as described above.
Pulsed Doppler devices can be used on their own by altering slowly the beam direction or the gated range depth while listening to the output, for example in transcranial blood flow studies. Identification of vessels is made easier by combining the PW Doppler mode with a real-time B-scan mode to form a duplex system; however, this obviously adds to the cost and complexity.
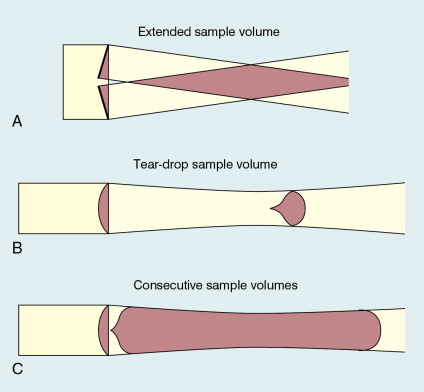
Since the ultrasound is pulsed and the excitation time is short, a stand-alone PW unit uses a single crystal transducer for transmission and reception ( Fig. 1-1B ). On setting the electronic gate to select a signal from a specific range, reflectors within a volume, known as the sample volume, contribute to the signal. The shape and size of the sample volume are determined by a number of factors: the transmitted pulse length, the beam width, the gated range length, and the characteristics of the electronics and transducer. The sample volume is often described as a tear drop in shape ( Fig. 1-1B ). Sample volume lengths are usually altered by changing the gated range length. In a blood flow unit for superficial vessels, the sample volume length may be as short as 1 mm, whereas in a transcranial device it can be 1 or 2 cm; however, the precise lengths are rarely known.
The ultrasonic output intensity of pulsed Doppler instruments varies considerably from unit to unit. The intensity (see Safety section, below) may typically be a few hundred mW cm −2 but can be as high as 1000 mW cm −2 , particularly when they are required to penetrate bone, as in transcranial Doppler. At present the most common use of stand-alone PW units is in transcranial examinations of cerebral vessels.
A summary of technical factors relating to the use of continuous wave and pulsed wave Doppler instruments is given in Box 1-1 .
Doppler beams are subject to the same physical processes in tissue as B-mode beams, i.e. attenuation, refraction, speed of sound variation, defocusing, etc.
Since stand-alone CW and PW units are used blind, the beam direction and also the sample volume in the PW case must be systematically moved through the region of interest to maximise both the volume and pitch of the audible Doppler signal.
PW Doppler is subject to the aliasing artefact in the measurement of high velocities, CW Doppler is not.
The sensitivity (gain, transmit power) of the Doppler unit should not be so high that noise detracts from the signal quality.
The instrument should be assessed on normal vessels where the blood flow pattern is known and the expected Doppler signal well understood.
The wall-thump filter should just be high enough to remove the strong low-frequency signal from vessel walls and any other moving tissue.
The final result in many cases should be a distinct display, called a ‘spectrogram’ or ‘sonogram’ (see section on the Spectrum analyser, below), with a clearly defined maximum-velocity trace.
Since the beam–vessel angle is unlikely to be known, the sonogram cannot be calibrated in velocity and the vertical axis remains as Doppler shift frequency.
Care should be taken to ensure good acoustic coupling between the transducer and the patient. Since there is no associated image it is not always apparent that a weak signal may be due to a lack of coupling agent.
If possible, information should be obtained on the shape of the sample volume for both CW and PW beams. The sample volume size can then be related to the size of the vessel under study. With CW Doppler there is very little depth discrimination. With PW Doppler the sample volume depth and size are set by the user.
There are three types of imaging used with Doppler techniques. The first, known as ‘duplex Doppler’, uses a real-time B-scanner to locate the site at which blood flow is to be examined then a Doppler beam interrogates that site. The second type creates an image from Doppler information, i.e. an image of velocities in regions of blood flow. Known as ‘colour Doppler’, ‘colour flow imaging’ or ‘colour velocity imaging’, it is normally combined with a conventional real-time B-scan so that both tissue structure and areas of flow are displayed. The third type of Doppler imaging is similar to colour Doppler, but generates an image of the power of the Doppler signal from pixel locations throughout the field of view and is known as ‘power Doppler imaging’ (power Doppler). A power Doppler image depicts the amount of blood moving in each region, i.e. an image of the detected blood pool.
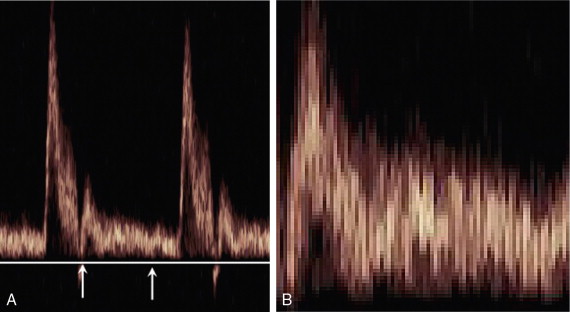
Duplex systems link CW or PW Doppler features and real-time B-scanners so that the Doppler beam can interrogate specific locations in the B-scan image ( Fig. 1-2 ). CW duplex is normally only used where very high velocities have to be measured without the aliasing artefact, for example in the estimation of the velocity of a jet through stenosed heart valves. The direction of the CW beam is shown as a line across the B-scan image. In the case of PW Doppler, markers on the beam line show the position of the sample volume. The Doppler beam is often directed across the field of view so that it does not intersect the blood flow at 90°.
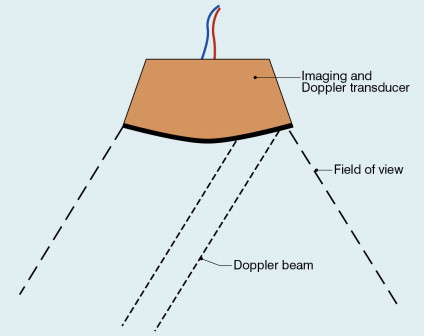
In duplex systems, the transmitted ultrasound frequency in the Doppler mode is often lower than that for the B-mode. The low Doppler beam frequency is to enable higher velocities to be handled before aliasing occurs, while the high B-scan frequency is to optimise resolution in the image. An example could be 5 MHz for Doppler and 7 MHz for B-mode when studies of superficial vessels are undertaken.
A summary of technical factors relating the use of duplex Doppler instruments is given in Box 1-2 .
The factors quoted above for CW and PW Doppler may also be relevant.
A spectrogram can be obtained from a known vessel and a known location within the vessel.
The Doppler beam may be refracted and not pass along the line shown in the B-mode image.
The beam–vessel angle may be measured manually, allowing blood velocity to be estimated.
Simple estimates of blood flow rate may be obtained from the measured diameter and mean velocity.
Spectral broadening due to the use of wide aperture transducers to give good focusing can cause large errors in the measurement of maximum velocity.
Since the Doppler beam is held fixed and the PRF is high, particular attention should be paid to the outputs of PW units in relation to safety.
Pulsed Doppler techniques require between 50 and 100 ultrasonic pulses to be transmitted in each beam direction for the determination of velocities of blood in a sample volume. It is therefore not possible to move the beam rapidly through the scan plane to build up real-time Doppler images of velocity of flow. Such imaging became possible when signal processing was developed which could quickly produce a measure of mean blood velocity at each sample volume from a small number of ultrasonic echo pulses. A technique called ‘autocorrelation processing’ of the signals from blood quickly gives the mean velocity in each small sample volume along the beam ( Fig. 1-1C ). This real-time colour Doppler imaging processes between 2 and 16 echo signals from each sample volume. In addition, the direction of flow is obtained by examining the signals for the direction of the shift as for CW and PW Doppler devices. Each image pixel is then colour-coded for direction in relation to the transducer and mean Doppler shift ( Fig. 1-3A ).
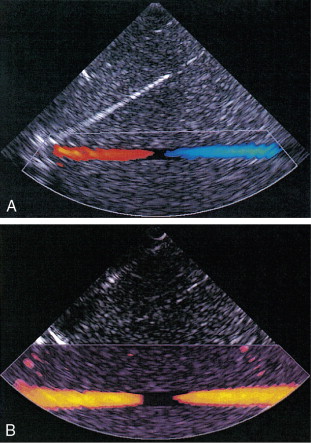
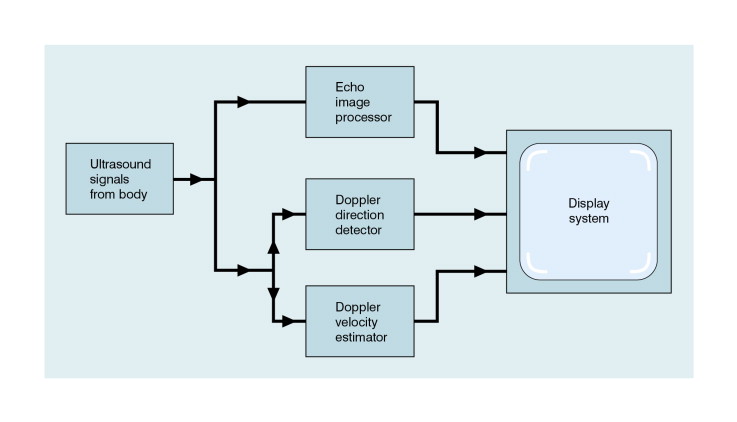
B-scanning and Doppler imaging are carried out with the common types of real-time transducer. Echo signals from the blood and tissues are processed along two signal paths in the system electronics ( Fig. 1-4 ). Going along one path, the signals produce the real-time B-scan image; going along the other path, autocorrelation function processing and direction flow sensing are employed to give a colour flow image. An important exclusion circuit in the autocorrelation path separates large-amplitude signals which arise from tissue and excludes them from the blood velocity processing. The B-mode and mean velocity images are then superimposed in the final display. Strictly speaking, the flow image is of the mean Doppler shift frequency and not the mean velocity, since the beam–vessel angles throughout the field of view are not measured. Colour shades in the image can indicate the magnitude of the velocity, for example light red for high velocity and dark red for low velocity. Turbulence, related to the range of velocities in each sample volume, may be presented as a different colour or as a mosaic of colours.
Doppler images typically contain about 64 genuine lines of information and 128 consecutive sample volumes along each line. The frame rate varies from 5 to 40 frames per second, depending on the depth of penetration and the width of the field of view. As in B-scanning, the appearance of the image is usually improved by inserting additional lines or frames whose data are calculated from the genuine lines, a process known as interpolation. Alteration in flow can occur rapidly over the cardiac cycle, therefore a cine-loop store of say the last 128 frames is of value for review purposes. Doppler spectrograms can be made by selecting the appropriate beam direction and sample volume location in the image and then switching to the PW or CW mode. PW and CW Doppler techniques provide more detailed information on blood velocities than colour Doppler, so spectral information is still of value.
A summary of technical factors relating to the use of colour Doppler imaging is given in Box 1-3 .
The mean Doppler frequency shift is the quantity which is presented in a colour-coded form in each pixel. When the colour bar is labelled in velocity the beam–vessel angle has been assumed to be zero throughout the image.
The velocity component along the Doppler beam is heavily dependent on the angle between the direction of flow and the beam direction (the cosine θ dependence). The colours depicted in the image are therefore heavily angle dependent.
The flow pattern on the colour Doppler display can be related to the structures shown in the B-mode image.
Colour Doppler imaging is a pulsed technique so aliasing is a problem.
A good colour Doppler machine is one which discriminates well between signals from tissue and those from blood.
The colour Doppler field of view box should be adjusted to cover only the region of interest and therefore maximise the frame rate.
The velocity range covered by the colour scale should be carefully matched to the velocities expected in the study.
A cine-loop is useful for the review of fast-changing blood flow patterns.
A change in the colour registered in a vessel from, say, blue to red may not mean a change in the direction of flow along a vessel. It may merely mean the beam–flow angle has changed from less than 90° to more than 90°.
The power of the Doppler signal from each small sample volume in the field of view may be displayed rather than the mean frequency shift ( Fig. 1-3B ). The power of the signal from each point relates to the number of moving blood cells in that sample volume. The power Doppler image may be considered to be an image of the blood pool. The power mode does not measure velocity or direction and therefore the image shows little angle dependence, nor does it suffer from aliasing; however, it obviously presents less information about blood flow. The attraction of power Doppler images is that they suffer less from noise than velocity images, as the power of the background noise for any sample volume with no blood flow signal is less than the power of the background noise plus Doppler signal when blood flow is present. The background noise may be used to set a threshold above which signals are accepted for Doppler flow. Noise from sample volume regions lacking blood flow is therefore reduced in the power image by a threshold detector. However, when the same signal is used in the velocity imaging mode, the noise will produce a mean velocity value which the machine will treat as a genuine blood velocity and which will therefore appear in the image. The power Doppler mode is therefore less prone to noise and hence more sensitive and can be used to detect small vessels. Further sensitivity can be obtained by averaging power images over several frames to reduce spuriously distributed noise even more. In velocity imaging there is interest in showing quick changes in blood flow and hence less averaging is used.
Power Doppler imaging often provides a more complete image of the vasculature than velocity imaging. This has made it popular in clinical use and it is commonly used initially to locate regions of interest prior to investigation by colour Doppler or duplex methods. It is also possible to use the direction information in the signal to colour-code the corresponding power image.
A summary of technical factors relating to the use of power Doppler imaging is given in Box 1-4 .
In the power Doppler image, the power of the Doppler signal at each pixel is colour-coded.
No velocity information is displayed.
The power Doppler signal is direction insensitive; so the display is the same regardless of whether the blood is flowing toward or away from the transducer. However, some systems do display different colours depending on the direction of blood flow by including some directional Doppler information.
The power Doppler image is insensitive to angle except near 90° where the Doppler signal may fall below the clutter filter, which cuts out low Doppler shifts, and no signal is displayed.
There is no aliasing, because the frequency information (i.e. velocity) is not estimated from the Doppler signal.
The power Doppler image is very sensitive to movement of the tissue or probe (the flash artefact). Some machines incorporate a flash filter to try to reduce this effect.
A number of agents have been considered which can enhance the scattering of ultrasound from blood and hence could be employed as echo-enhancing or contrast agents. Ophir and Parker have reviewed contrast agents. From this review and more recent experience it has become obvious that agents in the form of encapsulated microbubbles are by far the most likely to be successful echo-enhancing agents in the immediate future. This is due to the large difference in acoustic impedance between the gas in the bubbles and the surrounding blood. In addition, bubbles of a few microns in diameter have a fundamental resonance frequency of a few megahertz. For example, 4 μm diameter bubbles resonate at 4 MHz, which is well within the range of medical ultrasound systems. Bubbles of these dimensions are important since, even with only very thin wall encapsulation, they are able to pass through the capillaries of the lung into the systemic circulation. An investigation by a committee of the American Society of Echocardiography concluded that contrast echocardiography carried a minimal risk for patients and that there were few residual or complicating side-effects. Other studies have confirmed these conclusions; however, more work is required on new agents as they become available.
The development around 1990 of contrast agent microbubbles that can be used by percutaneous venous injection was the breakthrough which gave rise to the current high level of activity in this field. Table 1-1 gives examples of agents which are currently under commercial development. Large gas molecules are encapsulated in some agents to reduce the rate of diffusion and so increase the lifetime of the bubbles. Typically the lifetime in blood ranges from 2–3 min up to 20–30 min. An attraction of contrast agents is the ability to increase the signal obtained from small blood vessels which are difficult to detect by conventional Doppler methods, such as cerebral or renal vessels. There is also interest in perfusion studies, for example to observe and measure the wash-in and wash-out of agent in the myocardium in a manner analogous to nuclear medicine studies.
| Agent | Manufacturer | Type of Agent | Capsule | Gas | Bubble Size | Concentration |
|---|---|---|---|---|---|---|
| DEFINITY/ LUMINITY |
Lantheus Medical Imaging |
Microsphere | Lipid | Octafluoropropane | Mean diameter 1.1–3.3 μm (98% < 10 μm) |
10 × 10 8 μ bubbles/mL |
| OPTISON | GE Healthcare | Microsphere | Albumin | Octafluoropropane | Mean diameter range 3.3–4.5 μm (95% < 10 μm) |
5–8 × 10 8 μ bubbles/mL |
| SONOVUE™ | Bracco | Stabilised microbubble |
Phospholipids | SF 6 | Mean diameter 2.5 μm (90% < 6 μm) |
2–5 × 10 8 μ bubbles/mL |
Enhanced scattering is obtained if a bubble is insonated with ultrasound of a frequency equal to that of the fundamental resonance frequency of the bubble. At low power (that is low ultrasonic wave pressure amplitudes) the oscillations of the bubble are about its centre and are directly in proportion to the size of the pressure fluctuations in the ultrasound wave. However, at higher powers the oscillations become distorted and ultrasound at frequencies different from that of the incident wave is generated by the bubbles. These frequencies are known as harmonics and are simply related to the fundamental resonance frequency of the bubble, so that the second harmonic frequency is twice the fundamental frequency. There is considerable interest in detecting and using the second harmonic, since tissue does not produce this effect to any great extent and the second harmonic signal comes predominantly from the echo-enhancing agent in the blood vessels. Both pulse–echo and Doppler systems have been designed to pick out the second harmonic component in the ultrasound returned to the transducer and use it to enhance the signal from the agent in blood, possibly by as much as 20–30 dB. These systems are being evaluated in clinical practice.
The scattering from contrast agents can also be enhanced if the acoustic pressure fluctuations in the beam are large enough to damage the microbubbles, causing them to leak. An unencapsulated gas bubble then forms next to the original one; however, since it has no outer shell, the scattering from it is undamped and can be around 1000 times higher than that from the encapsulated bubble. This effect has been exploited in a technique known as ‘intermittent’ or ‘transient’ imaging, which allows time for the damaged bubbles to be replaced between sweeps of the ultrasound beam.
A summary of technical factors relating to the use of microbubble contrast agents is given in Box 1-5 .
Contrast agents are still being developed but some technical points of importance include:
Check the age and shelf life of the agent.
Microbubble contrast agents are fairly fragile. The handling instructions should be carefully followed.
The ultrasonic beam is capable of destroying some microbubbles; higher transmit power will therefore not necessarily produce a stronger echo signal.
Several contrast agents can be insonated at high acoustic pressures to give enhanced backscatter due to bubble destruction. This phenomenon is also known as ‘acoustically stimulated emission’. Aspects of its safety still need to be fully evaluated.
Determine the physical phenomena which are expected to be present under the operating conditions and which will enhance the echo signal, e.g. harmonic scattering, free bubble creation and bubble destruction.
When the Doppler signal with the information on blood velocities has been obtained, it has to be interpreted. It is essential to obtain good-quality signals in order to be able to detect disease.
A Doppler signal may be analysed into its frequency components in order to give a display of the velocities of the blood cells at each instant ( Fig. 1-5 ). Short time intervals of the Doppler signal are analysed, for example a segment of 5 ms duration. This produces an instantaneous spectrum of the frequencies in the sample volume for that time period. If an angle correction is then applied, this spectrum will represent the range of velocities in the sample volume. The frequencies in each spectrum are displayed along a vertical line on which the power of each frequency component is presented as a shade of grey. The consecutive velocity spectra are then displayed as side-by-side grey-shade vertical lines. In this way a spectral display, or spectrogram, is built up ( Fig. 1-6 ). Note the difference between the instantaneous velocity spectrum which tells us about the pattern of velocities in the sample volume at that instant and the spectral display, or spectrogram, which shows how the velocity pattern varies with time. Spectrograms are generated in real time during the clinical examination and it is usually possible to store a few seconds of the trace in an analyser for subsequent review.
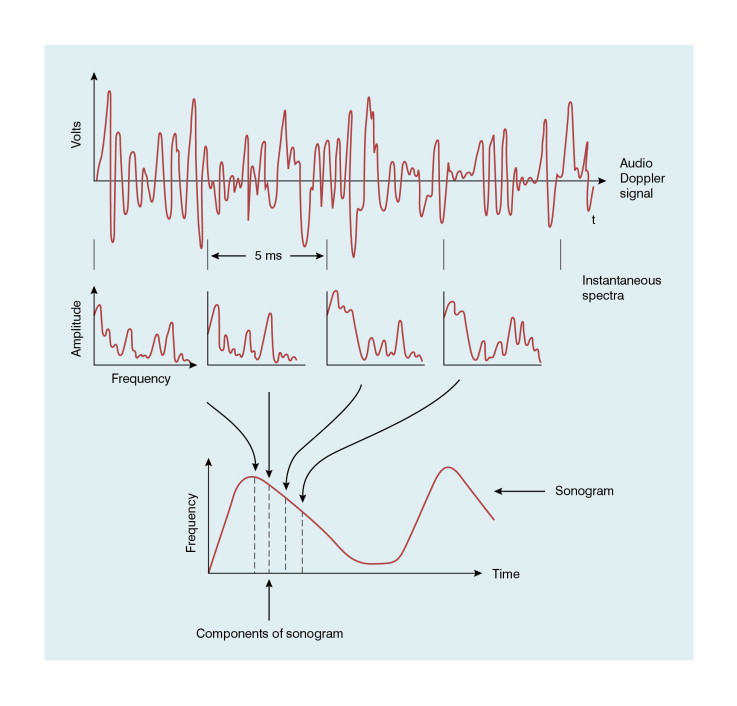
The temporal resolution in a spectrogram, that is the smallest discernible time interval, equals the length of the portion of Doppler signal used to produce each instantaneous spectrum and is typically 5–10 ms. The frequency (or velocity) axis of the spectrogram usually has about 100 scale intervals each of 100 Hz. The Doppler signal is therefore resolved into frequency components separated by 100 Hz, the frequency resolution of the spectrogram.
It is often desirable to make measurements on a spectrogram, for example an estimate of the time between two events or of the maximum velocity during systole or diastole. Measurement is performed by placing a cursor on the relevant points of interest then a variety of calculations can be performed by the system computer. Indices related to the spectrogram shape and hence to normality or abnormality of flow velocities can be calculated within the analyser and displayed on its screen. These are discussed later in this chapter. The oscillatory shape of a spectrum or a trace derived from it is often referred to as a waveform.
A summary of technical factors relating to spectral analysis is given in Box 1-6 .
Arrange the frequency and time scales to best display the detailed information in the sonogram by adjusting velocity scale, baseline and sweep speed.
Arrange the grey tones, or colours, of the spectral display to give the best ‘image’ quality by adjusting the Doppler gain.
Treat with caution information from weak signals in a noisy background.
A clear spectrogram is required before the maximum frequency can be traced.
It is usually best to make measurements over at least five heart cycles. However, if this is not possible, perhaps due to respiratory movement, useful information can be obtained from one or two cardiac cycles.
Make use of the total storage capacity of the analyser and use the scroll facility to select the most suitable part of the spectrogram to make relevant measurements.
Check thoroughly the reliability and accuracy of automatic tracing techniques and calculations derived by the ultrasound system.
Note whether the vertical axis has been calibrated in velocity by allowing for the beam–vessel angle.
To obtain reproducible results, try to use the same beam–vessel angle for all examinations, e.g. 60°.
A good-quality audible Doppler signal will result in a good-quality sonogram. Degradation of a sonogram is due to electronic and acoustic noise which should only be a problem when the gain of the Doppler unit or analyser is very high for the detection of weak signals. Noise in sonograms creates considerable difficulties for the automatic calculation of quantities and they should therefore be used with caution. If the automatic mode cannot deal with the noise, then the traces should be drawn manually making use of the eye’s ability to distinguish the true signal from the noise. It should also be remembered that the vertical axis of a sonogram can only be labelled as velocity after the beam–vessel angle has been measured.
Waveform indices are derived from a combination of a few dominant features of the waveform. Indices that have the same or similar names in the literature are occasionally defined differently, so a first step is to check the definition of any index to be used. In practice only two classes of index are used to any great extent, those related to the degree of diastolic flow and others related to spectral broadening. The variation in time of the maximum velocity displayed in a spectrogram is commonly used as a source of data for the derivation of an index ( Fig. 1-7 ). Since the maximum velocity is not always clearly apparent in a spectrogram, some analysers produce a trace which is closely related to the maximum velocity trace. One example is a trace showing the upper velocity boundary below which the velocity components contain seven-eighths of the power of the Doppler signal.
The mean velocity waveform (average velocity waveform) is also employed ( Fig. 1-7 ). To calculate the mean velocity at each instant, the values of velocity and the intensities of the signal for each velocity component in the instantaneous spectrum are used. The mean velocity is used together with the vessel cross-sectional area to calculate blood flow rate. However, it is difficult to measure mean velocity accurately and there are several other problems associated with calculating flow rates; these are discussed further in Chapter 2 .
Since the beam–vessel angle may not always be known, the waveforms or spectrograms will not be corrected for angle. Indices are therefore defined involving ratios of velocities. In such a ratio, the angle factors appear on both the top and bottom and hence cancel each other out, so that the index is independent of beam angle. Errors are also reduced by averaging the calculated indices over several heart cycles.
A number of the most commonly encountered indices are briefly discussed below:
The A/B ratio is defined as the ratio of two specified velocities, e.g. maximum velocities, at two points in the cardiac cycle ( Fig. 1-8 ). It is usually employed where there is no reverse flow in the waveform.
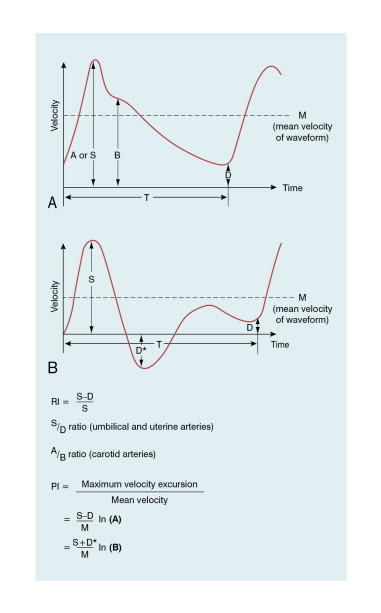
In Fig. 1-8 :
High resistance in the distal vessels produces low diastolic flow in the supplying artery and results in a high value for this index; a low resistance results in a low value as there is higher diastolic flow. It is also known as the Pourcelot index.
The pulsatility index (PI) is defined as:
This ratio is used in vessels where reverse flow may occur, for example in the lower limbs ( Fig. 1-8 ). The PI may typically have a value of 10 for the normal common femoral artery but be around 2 when proximal disease severely dampens the waveform.
As defined above, the PI index is heart rate dependent. To avoid this, PI can be calculated over a specified time from the start of systole, e.g. for the first 500 ms. The pulsatility index is then labelled ‘PI(500)’.
The damping factor is defined as the ratio of pulsatility indices at two sites along an artery. It quantifies the damping of the waveform downstream along a diseased vessel.
The numerical value of this index increases as disease becomes more severe, a value of 2 being typical of a high degree of damping. This index is mentioned for completeness, it is not widely used.
Turbulence increases the range of blood cell velocities in a vessel. One index to quantify this broadening of the spectrum of velocities is:
Conclusions with regard to the presence of turbulence should be made with caution and only after familiarity has been gained with the patterns for laminar and plug flow for the particular instrument being used. Other technical factors can cause spectral broadening: for example ‘geometrical spectral broadening’, which results from the range of Doppler angles which any given blood corpuscle subtends to different points on the face of the transducer.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here