Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
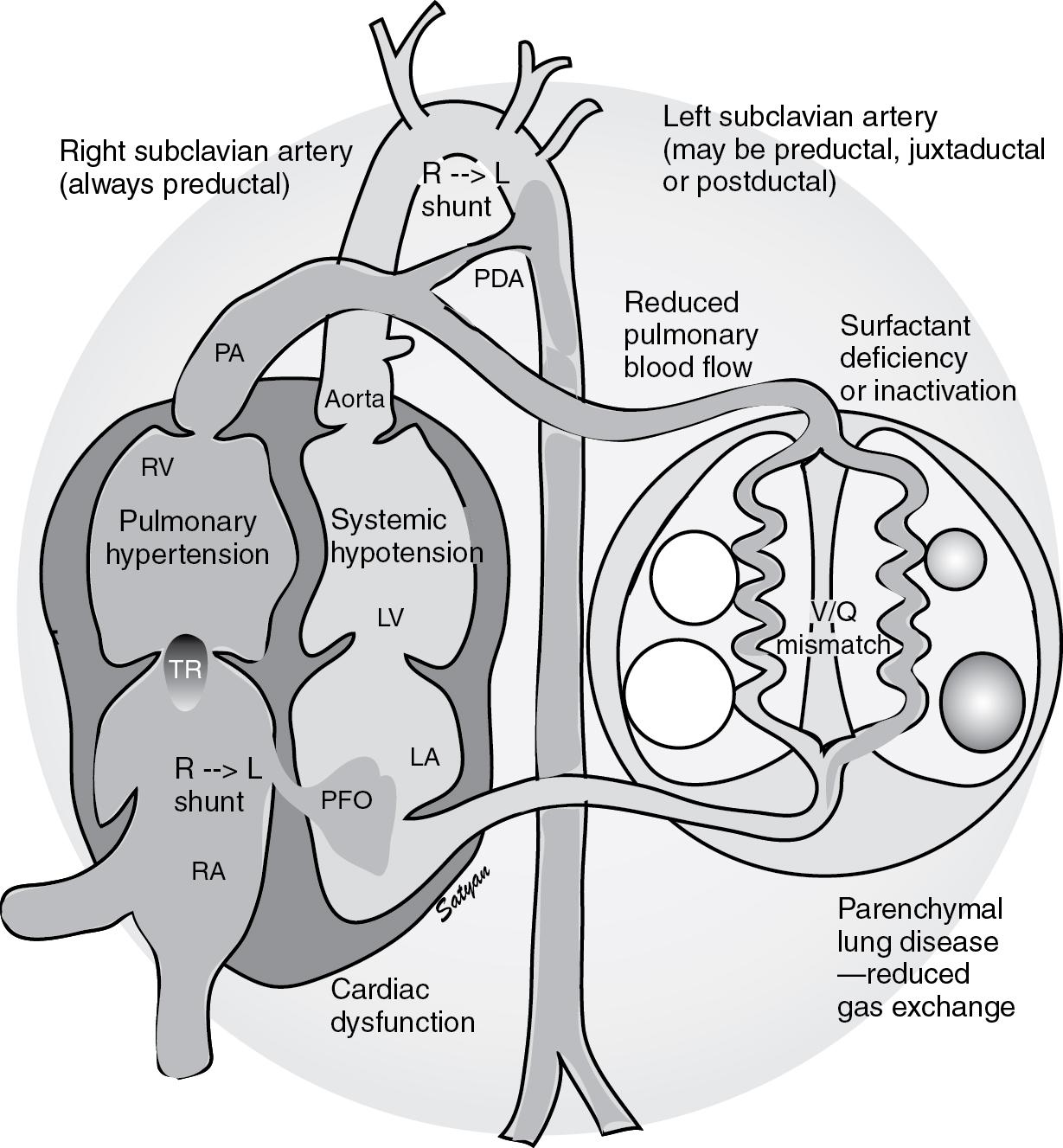
The fetus is in a state of physiologic pulmonary hypertension. The PaO 2 in the descending aorta of the fetus is approximately 20 to 25 mm Hg with an oxygen saturation (Sa o 2 ) of 55% to 58% ( Fig. 17.1 ). In spite of being “hypoxemic” (low PaO 2 levels relative to postnatal standards), the fetus can deliver adequate oxygen to its tissues and is not hypoxic. Oxygen delivery (DO 2 ) is dependent on cardiac output (CO) and oxygen content of the blood (CaO 2 ). The fetus maintains adequate oxygen delivery to its tissues by the following physiologic adaptations: (1) presence of fetal hemoglobin (HbF) with high affinity for oxygen, (2) higher hemoglobin levels in a term fetus compared with children and adults resulting in a higher CaO 2 at any given oxygen saturation level, and (3) high cardiac output (450 mL/kg/min from both ventricles). During fetal life, the placenta is the organ of gas exchange. The difference in oxygen saturation (a measure of oxygen uptake from the organ of gas exchange) between the umbilical vein (80%) and umbilical artery (58%) during fetal life (see Fig. 17.1 ) is similar to the difference between the pulmonary vein/aorta (95%–100%) and pulmonary artery (70%) in an adult.
Similar to the adult lung, the placenta receives approximately 40% to 45% of combined ventricular output during fetal life. The lungs receive 8% to 10% of combined ventricular output in fetal lambs and approximately 25% in near-term human fetuses ( ). After birth and following initiation of air breathing, pulmonary blood flow markedly increases ( ) resolving the fetal physiologic pulmonary hypertension ( ). In infants with an adverse event in utero or with abnormalities of pulmonary transition at birth, pulmonary hypertension persists into the newborn period, resulting in persistent pulmonary hypertension of the newborn (PPHN) leading to hypoxemic respiratory failure (HRF).
The following case studies discuss the presentation, clinical features, and management of PPHN and HRF.
A 32-year-old woman with gestational diabetes is admitted to a community hospital at 37 3 ⁄ 7 weeks’ gestation following rupture of membranes. She weighs 225 pounds and has a healthy 2-year-old boy born by cesarean section. A repeat cesarean section is performed for late decelerations. The baby weighs 8 pounds and 8 ounces (3855 g) and is brought to the nursery at 2 hours of age for hypoglycemia. He is noted to be tachypneic at 82 breaths per minute. His oxygen saturation (SpO 2 ) is 83% in room air, and he is placed in a hood with 30% oxygen. His SpO 2 continues to be low at 85%, and the inspired oxygen concentration is gradually increased to 45% to achieve saturation values in the 90s. A chest x-ray is obtained ( Fig. 17.2 ) and the regional perinatal center is called to request transfer.
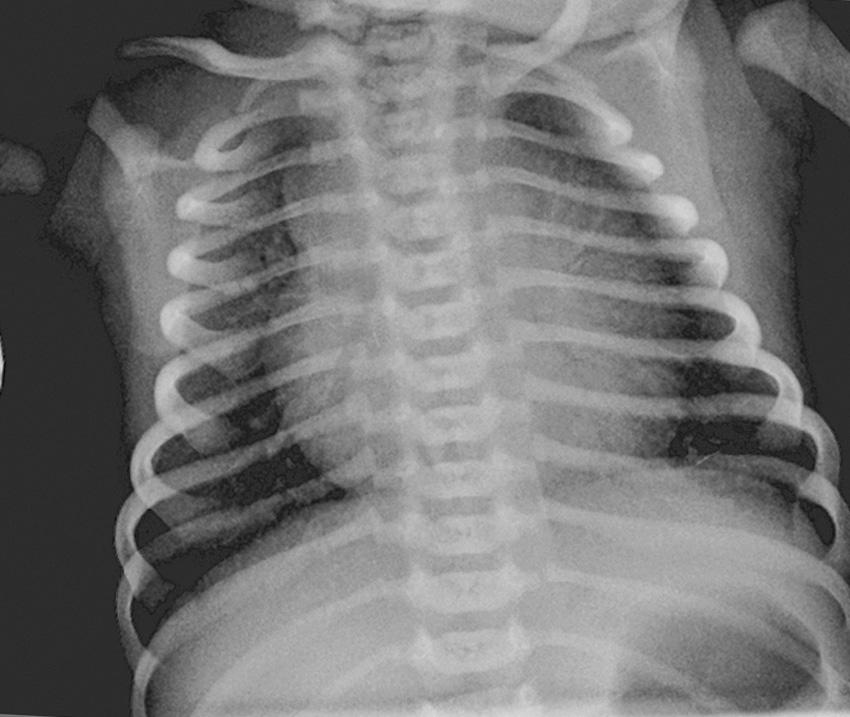
What is the most likely cause for hypoxemia and tachypnea in this infant?
Surfactant deficiency and respiratory distress syndrome (RDS)
Retained fetal lung liquid and transient tachypnea of the newborn (TTN)
Infection with pneumonia
Atelectasis
Cardiac failure
B. This infant has several risk factors for respiratory distress at birth.
Early term delivery (37–38 6 ⁄ 7 weeks postmenstrual age [PMA]) is associated with a higher risk of respiratory distress and admission to the neonatal intensive care unit (NICU) ( ). The American College of Obstetrics and Gynecology (ACOG) strongly recommends against induction and elective cesarean section before 39 completed weeks of gestation ( ) and has recently updated the guidelines for medically indicated late-preterm and early term deliveries ( ).
Maternal diabetes increases the risk of surfactant deficiency and RDS.
Delivery by cesarean section also increases the risk of respiratory distress. The additive effect of cesarean section on respiratory morbidity is higher at 37 weeks’ gestation compared with 39 or 40 weeks’ gestation. Vaginal delivery is associated with a rapid reduction in fetal pulmonary vascular resistance (PVR) at birth. Delivery by elective cesarean section ( ; ) delays the decrease in pulmonary arterial pressure ( Fig. 17.3 ) and increases the risk for PPHN ( ). Compared with matched controls, infants with PPHN are more likely to have been delivered by cesarean section ( ).
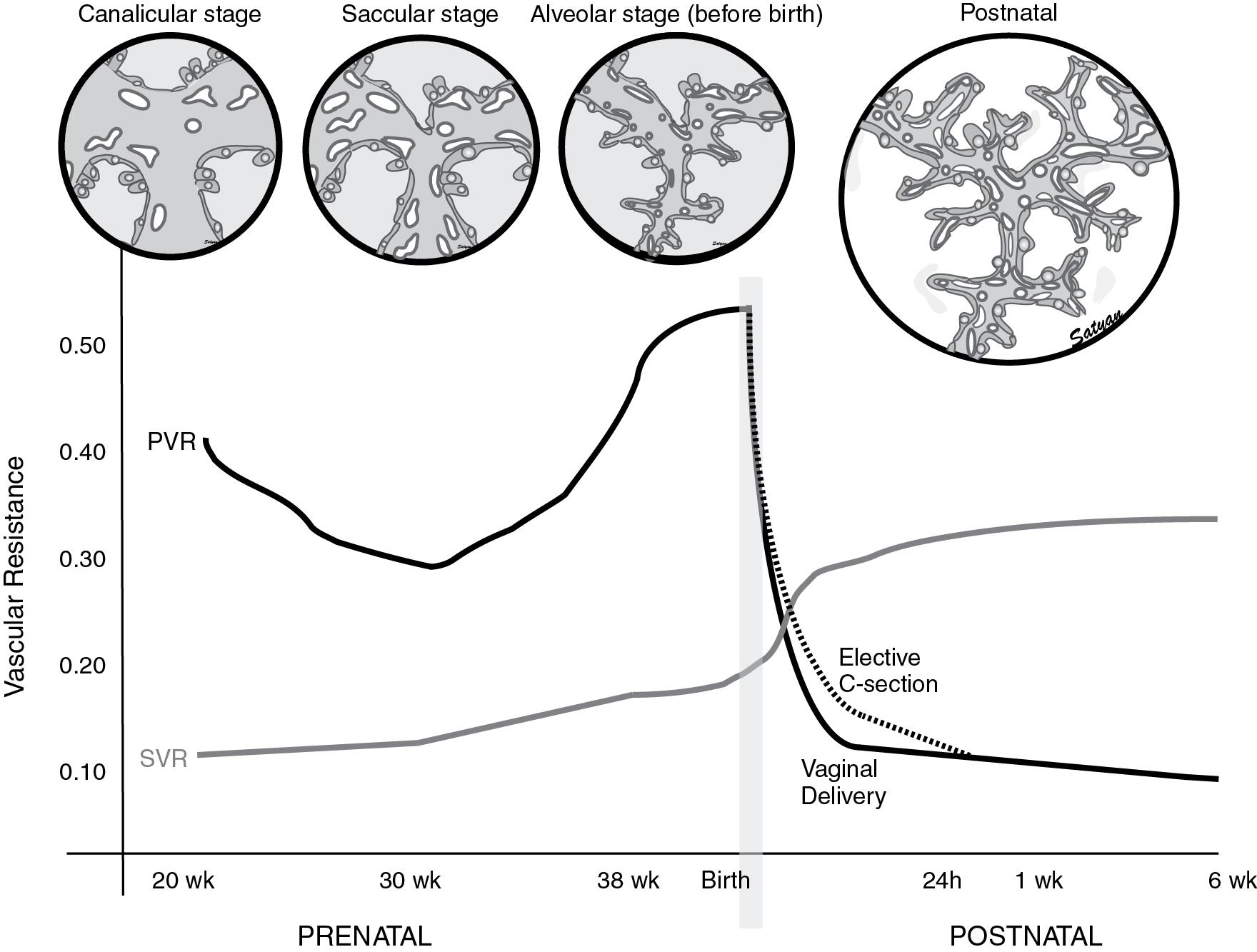
The entry of air into the alveoli with crying and breathing improves oxygenation of the pulmonary vascular bed, thereby decreasing PVR and increasing pulmonary blood flow ( ). The increase in pulmonary blood flow raises left atrial pressures above right atrial pressures, closing the foramen ovale. Removal of the low resistance placental bed from the systemic circulation at birth increases systemic vascular resistance (SVR). PVR falls to approximately half of SVR within a few minutes after birth. As PVR becomes less than SVR, flow reverses across the ductus arteriosus and oxygenated blood flows from the aorta to the pulmonary artery. The ductus arteriosus closes in the first few hours following birth in the newborn at term, largely in response to an increase in oxygen tension and clearance of circulating prostaglandin by the lungs, thus establishing the postnatal circulatory pattern. Vasodilator mediators such as nitric oxide (NO) and prostacyclin (PGI 2 ) are important mediators of pulmonary vascular transition at birth ( ).
The transport team arrives 3 hours later. The baby’s oxygen requirement has steadily increased to 95% oxygen by hood with saturations in the high 90s. Clinical examination reveals tachypnea (72 breaths/minute), grunting, and intercostal retractions. The arterial blood gas is as follows: pH 7.21, P co 2 66 mm Hg, and P o 2 52 mm Hg. His chest x-ray is repeated and is shown in Fig. 17.4 A. A decision is made to intubate the patient before transport.
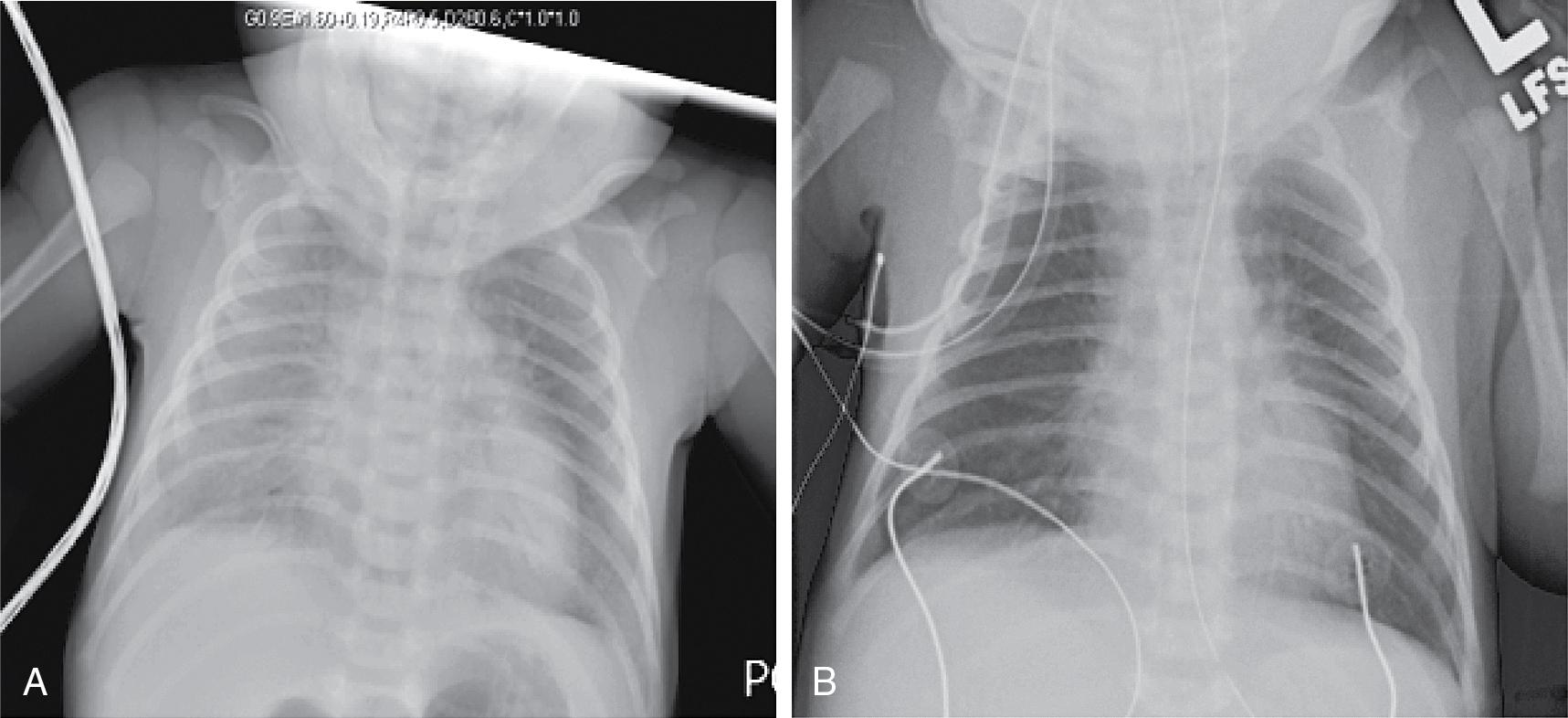
What is the most likely cause of hypoxemia and respiratory distress in this infant?
PPHN
Pneumonia
Absorption atelectasis
RDS/surfactant deficiency
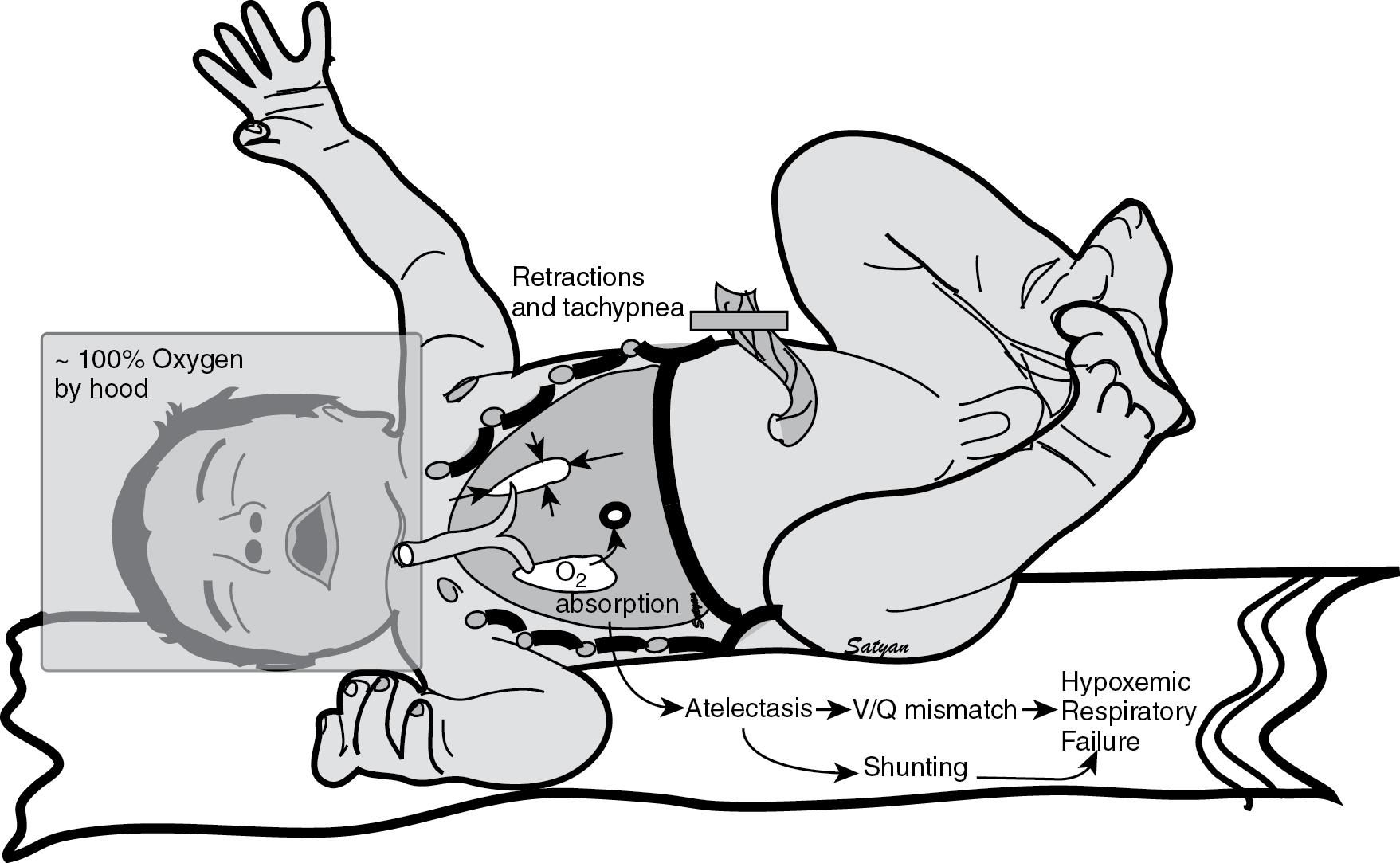
C. The infant is now grunting, which suggests that he is trying to maintain a normal functional residual capacity. Respiratory deterioration in this infant can be secondary to any of the choices mentioned above. However, the administration of high ambient concentrations of oxygen without positive pressure can result in absorption atelectasis ( Fig. 17.5 ). Unlike nitrogen (the predominant gas in room air), oxygen is easily absorbed from the alveoli into the bloodstream, leading to alveolar collapse. Because of nitrogen’s low solubility, very little diffuses across the alveolar capillary interface, and most of the inspired nitrogen stays in the alveolus helping to keep it open. When high fractional inspired oxygen is used, the concentration of nitrogen in the inspired gas and in the gas within the alveolus is decreased. Oxygen absorption atelectasis leads to ventilation–perfusion (V/Q) mismatch, shunting, and hypoxemia. Therefore it is important to avoid administration of high concentrations of inspired oxygen (above 50%–60%) without positive pressure (such as continuous positive airway pressure [CPAP]) in neonates with respiratory distress.
The baby is intubated by the transport respiratory therapist and placed on a conventional ventilator with a positive end expiratory pressure (PEEP) of 6 cm H 2 O, and peak inspiratory pressure (PIP) of 18 cm H 2 O at a rate of 35 breaths per minute. A dose of surfactant is administered. A subsequent x-ray, obtained before transfer, shows improved aeration ( Fig. 17.4 B). The oxygen concentration is reduced to 35% with a right upper extremity (preductal) SpO 2 of 94%. On the baby’s arrival to the Children’s Hospital, the echocardiogram shows normal cardiac anatomy with left-to-right shunting at the patent ductus arteriosus (PDA) and patent foramen ovale (PFO). An arterial blood gas drawn from the umbilical arterial line is as follows: pH of 7.49, Pa co 2 of 31 mm Hg, and PaO 2 of 60 mm Hg. The ventilator rate is weaned to 30 breaths per minute and the PIP is lowered to 16 cm H 2 O after delivery of adequate tidal volumes is verified. The neonatologist discusses with the transport team and the residents the importance of avoiding hypocapnia and the effect of blood gases and ventilator settings on pulmonary blood flow.
All of the following statements regarding the effect of pH, P o 2 , and P co 2 on pulmonary and cerebral circulation are correct EXCEPT:
Hypoxia causes pulmonary vasoconstriction and cerebral vasodilation.
Acute metabolic acidosis increases PVR and dilates the cerebral circulation.
Acute respiratory acidosis increases PVR and dilates the cerebral circulation.
Decreasing mean airway pressure (Paw) and reducing lung volume below functional residual capacity (FRC) increases PVR.
Marked increase in Paw reduces cardiac output and cerebral blood flow.
B. The systemic (cerebral) circulation responds to changes in blood gas parameters in a different manner compared with the pulmonary circulation ( Fig. 17.6 ). Hypoxia constricts pulmonary vessels and increases PVR. Normoxia dilates pulmonary vessels and decreases PVR, but hyperoxia does not result in additional vasodilation ( ; ; ). The change point below which PVR increases is the alveolar PAO 2 that corresponds to a PaO 2 of 50 mm Hg in neonatal animals without pulmonary hypertension and 60 mm Hg in neonatal animal models of PPHN. Conversely, cerebral vasodilation occurs in response to decreased arterial oxygen content. Acidosis (both respiratory and metabolic) leads to pulmonary vasoconstriction, and alkalosis (both respiratory and metabolic) causes pulmonary vasodilation. The combination of acidosis and hypoxia results in exaggerated pulmonary vasoconstriction. Maintaining an arterial pH over 7.30 will reduce pulmonary vasoconstriction in response to hypoxia ( ).
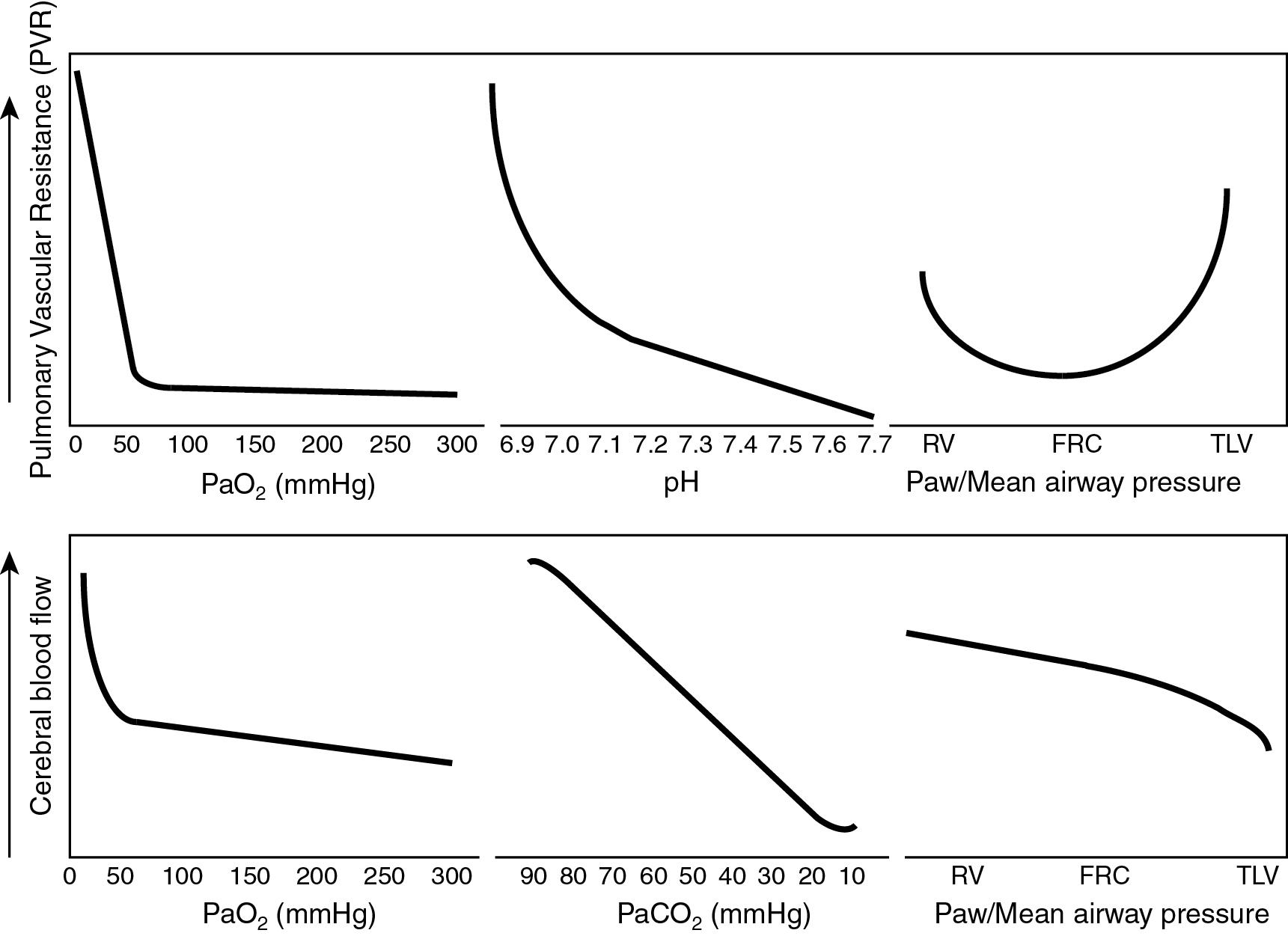
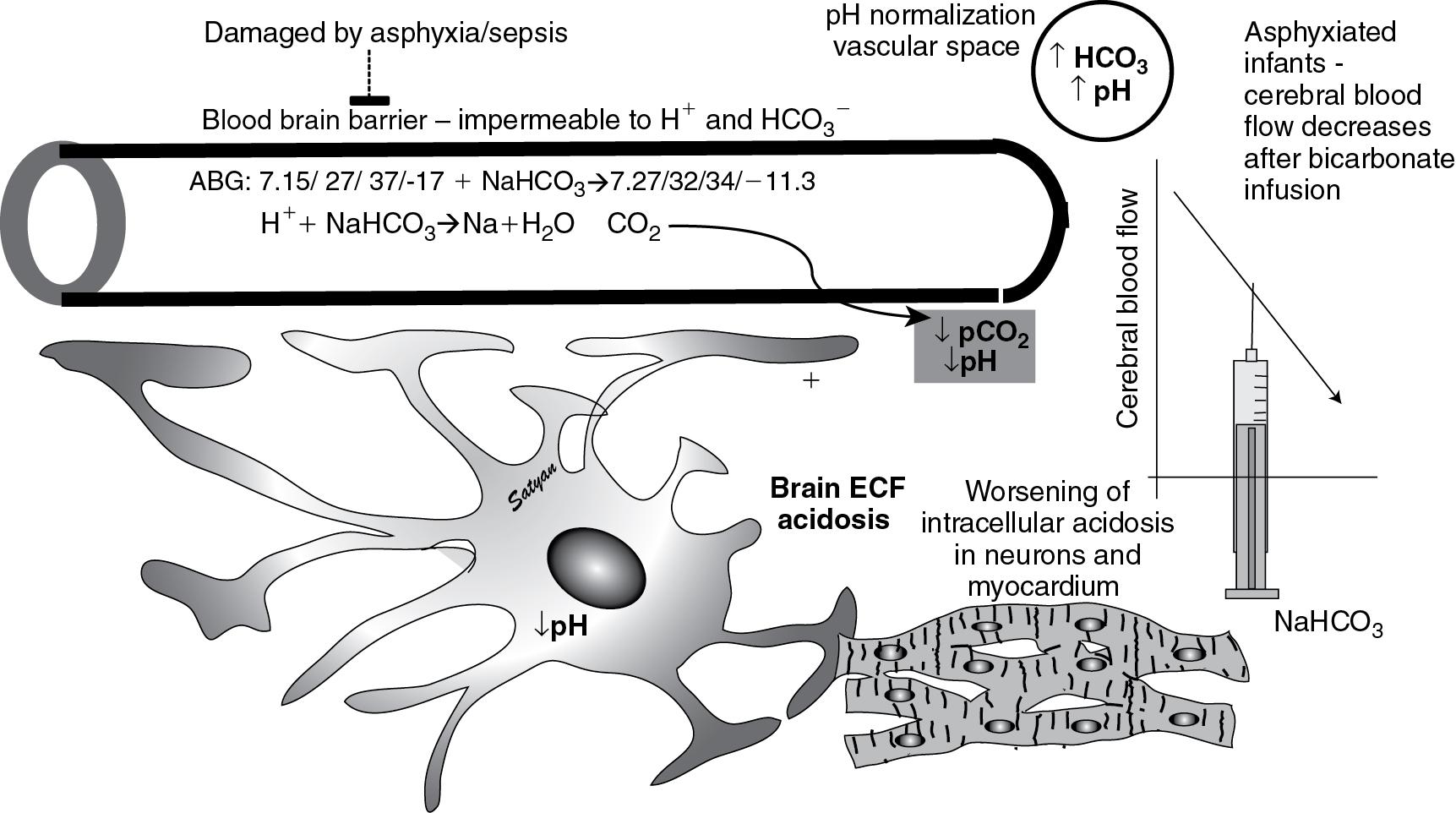
Cerebral blood flow, on the other hand, decreases with respiratory alkalosis (approximately 4% reduction in flow per mm Hg decrease in Pa co 2 in adults and a slightly lower degree in neonates). Changes in Pa co 2 mediate alteration in cerebral blood flow by changing perivascular fluid pH in the brain ( Fig. 17.6 and Fig. 17.7 ). Acute metabolic acid–base disturbances do not result in changes in cerebral blood flow, as H + ions do not cross the blood–brain barrier. However, in neonates with severe birth asphyxia, a rapid infusion of sodium bicarbonate causes cerebral perivascular alkalosis (owing to a leaky blood–brain barrier) and reduces cerebral blood flow ( ). Increasing Paw reduces venous return, thereby decreasing cardiac output and cerebral blood flow. The effect of Paw on PVR is dependent on lung volume. At FRC, PVR is at its lowest and increases with atelectasis and hyperinflation ( Fig. 17.6 ).
Chest x-rays are often helpful in the diagnosis of infants with HRF and some of the characteristic x-ray appearances for common neonatal respiratory disorders ( Box 17.1 ) are described here. These descriptive terms are often used in the NICU and may provide a clue to the diagnosis ( Box 17.2 , Snow White and the Radiology Seven Dwarfs).
T ransient tachypnea of the newborn/retained fetal lung fluid
A spiration syndromes (meconium, blood or amniotic fluid, and a sphyxia)
C ongenital anomalies (congenital diaphragmatic hernia)
Hy aline membrane disease/RDS
Pne umonia or sepsis
A ir leaks (and A ntidepressant use by mothers)
Grainy - Respiratory distress syndrome or pneumonia
Hazy - Respiratory distress syndrome, pneumonia, pulmonary hemorrhage, or edema
Patchy - Pneumonia or atelectasis
Fluffy - Meconium aspiration syndrome
Bubbly - Pulmonary interstitial pneumonia (small bubbles) and congenital diaphragmatic hernia (large bubbles)
Blacky - Idiopathic PPHN (black-lung PPHN) and pneumothorax
Streaky - Transient tachypnea of newborn
(Snow) Whiteout - Chylothorax, pleural effusion, massive atelectasis
Grainy: This is a classic description of RDS caused by surfactant deficiency. Grainy, translucent appearance of the lung fields is caused by the presence of microatelectasis interspersed with areas of hyperexpansion. In the absence of surfactant, small alveoli have a tendency to collapse, owing to a higher surface tension, and inspired air empties into larger alveoli. Large alveoli have lower surface tension and continue to get larger (Laplace’s law), leading to a translucent, ground glass, or grainy appearance. The lungs are low volume (in nonintubated infants), and air bronchograms (air in large airways superimposed on a background of granularity) may be present.
Streaky: A streaky appearance is characteristic of TTN with retained fetal lung liquid. Streaky interstitial shadowing associated with fluid in the horizontal fissure is the classic appearance of TTN. The lungs are usually well expanded or have an increased volume. This is caused by peribronchial cuffing and air trapping in expiration leading to hyperexpansion of the lungs ( ).
Black lung: In “black-lung” or idiopathic PPHN without parenchymal lung disease, lung fields are dark because of decreased pulmonary vascularity. The lung fields may also appear black in infants with a pneumothorax secondary to anterior layering of lucent air in the pleural space.
Fluffy: In meconium aspiration syndrome (MAS), lung fields are overinflated with fluffy infiltrates in both lung fields. Aspirated meconium may exert a ball-valve effect leading to expiratory obstruction and air trapping.
Bubbly: Lung fields appear bubbly in pulmonary interstitial emphysema where air extravasates into the wall of the bronchi. A bubbly appearance may also be seen on the side of herniation in congenital diaphragmatic hernia (CDH) caused by the presence of air in the intestines.
Hazy: The lung fields can appear hazy in infants with pneumonia, RDS, and pulmonary hemorrhage.
Patchy: A patchy (nonhomogenous opacities) chest x-ray is suggestive of pneumonia. It is important to recognize that pneumonia caused by early onset sepsis from group B streptococcal infection and gram-negative infections such as Escherichia coli can mimic other neonatal respiratory disorders such as RDS or MAS.
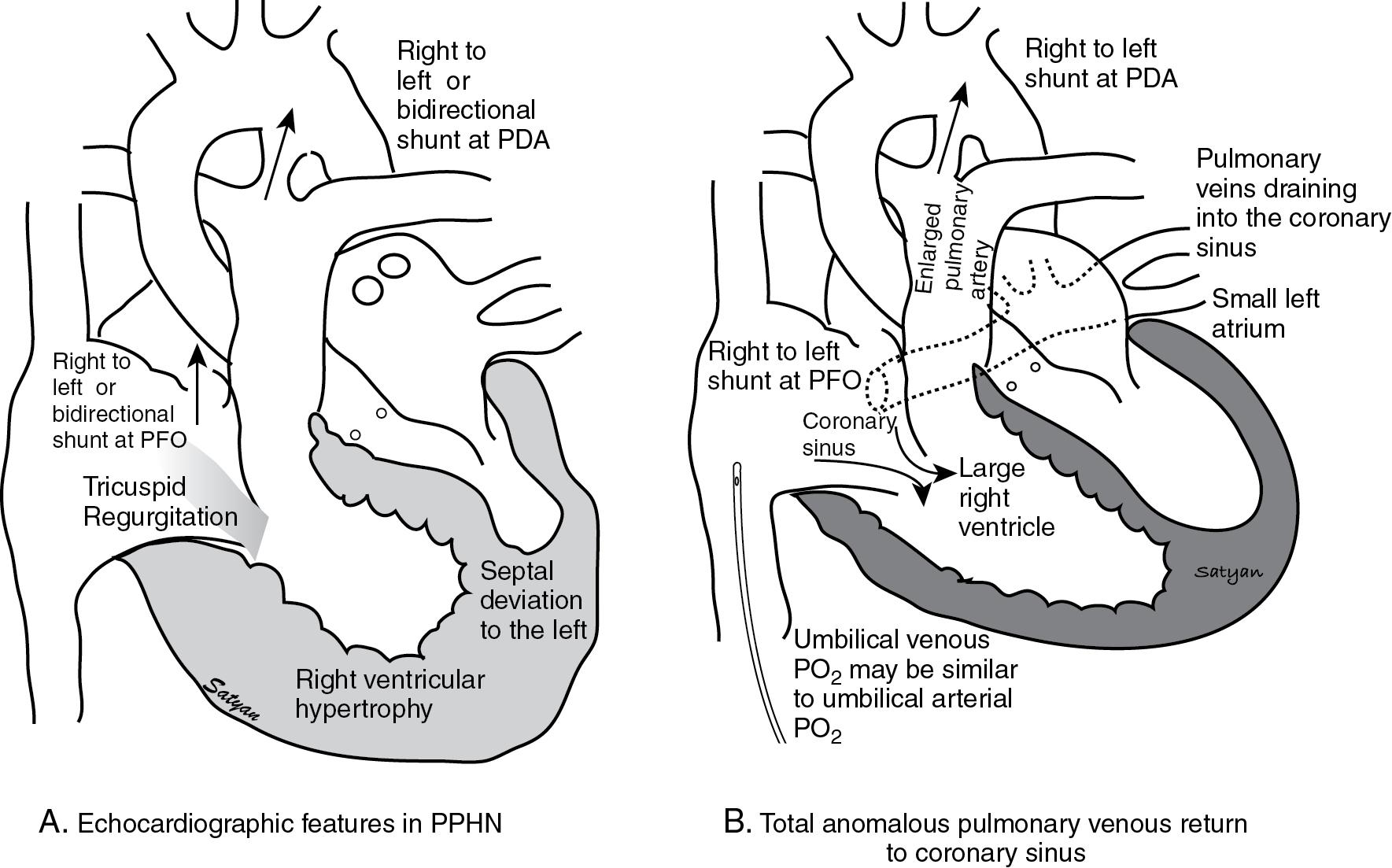
White-out appearance: A white-out appearance may be seen in diffuse extensive atelectasis of the lung. A similar appearance may be seen in obstructed total anomalous pulmonary venous return (TAPVR). The typical “snowman in a snowstorm” appearance is caused by pulmonary edema from obstruction of the pulmonary venous return associated with a supracardiac form of TAPVR. Clinically, TAPVR may occasionally be confused with PPHN, and a chest x-ray may be helpful in narrowing the diagnosis. However, an echocardiogram is necessary to confirm the diagnosis.
An understanding of the basic principles of gas exchange and transport is crucial to assessing severity of PPHN and HRF. Fig. 17.8 reviews the changes in oxygen levels through the process of oxygen transport from inspired gas to the mitochondria. This figure also illustrates the concept of systemic oxygen delivery (calculated by multiplying cardiac output with the difference between arterial and venous oxygen content A−V DO 2 ).
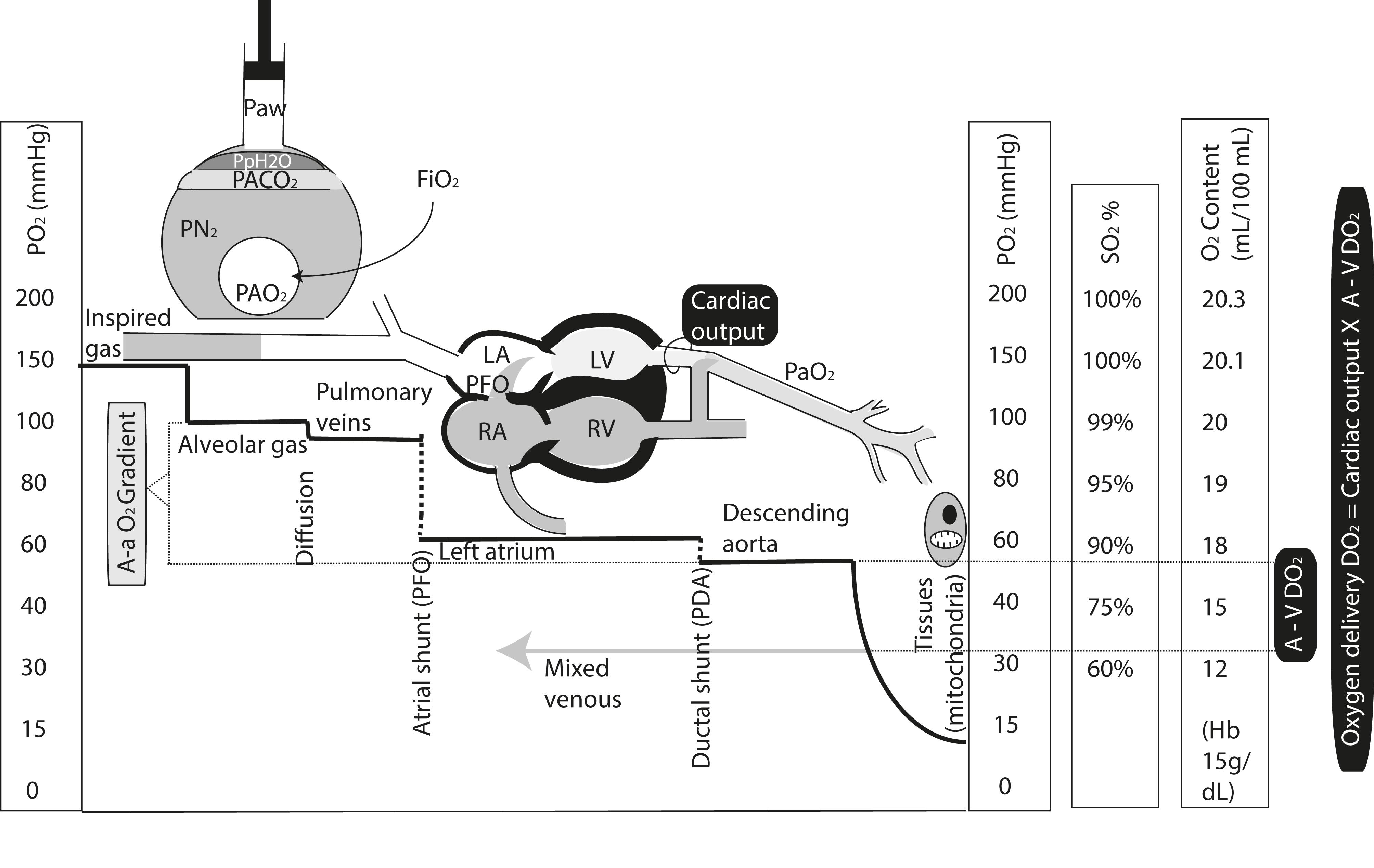
List the factors that determine oxygen delivery to the tissues in the order of importance.
Dissolved oxygen
Hemoglobin concentration
Hemoglobin saturation
Cardiac output
D , B , C , A. The cardiac output and hemoglobin concentration are the most important factors determining systemic oxygen delivery. The essential goal of management of PPHN is to maintain adequate delivery of oxygen to the tissues.
The severity of PPHN and HRF and response to treatment are objectively assessed by using six indices ( Figs. 17.8 and 17.9 ).
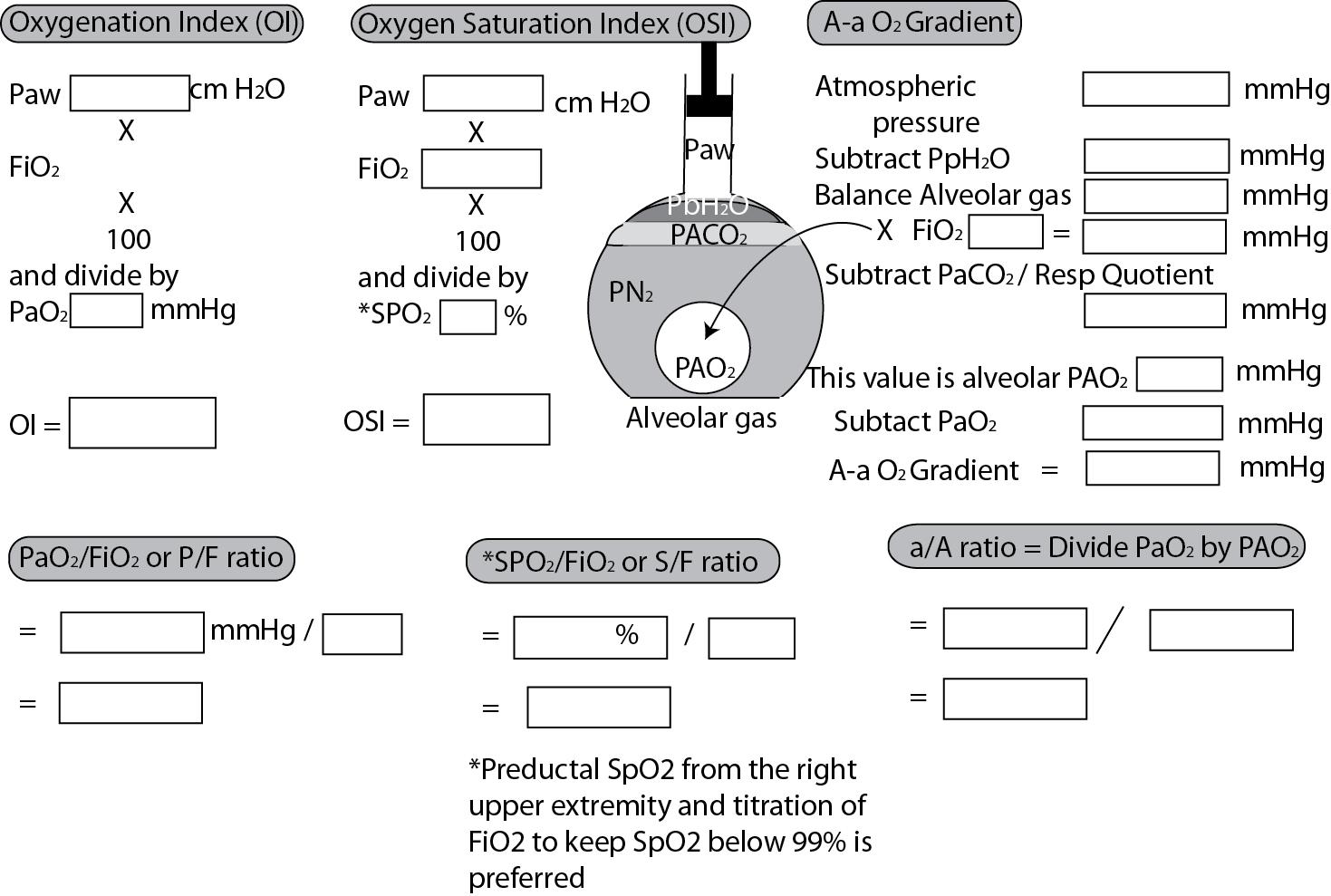
Oxygenation index (OI): This index includes factors that determine oxygenation (Paw and concentration of inspired oxygen) in the numerator and arterial oxygen tension in the denominator. This is one of the few indices that includes ventilator pressure and is commonly used in clinical trials to evaluate therapy in PPHN/HRF. The formula for calculating OI is given here and is illustrated in Fig. 17.9 with an example.
Some units assess the severity of PPHN/ HRF based on OI ( ).
Mild − OI ≤15
Moderate − OI 15 to ≤25
Severe − OI 25 to ≤40
Very severe − OI >40 (with possible need for extracorporeal membrane oxygenation [ECMO])
Oxygen Saturation Index (OSI): Many neonatal units prefer to monitor infants on invasive mechanical ventilation using noninvasive measures such as oxygen saturation by pulse oximetry (SpO 2 ) in the absence of indwelling arterial lines ( ). OSI is calculated as Fi o 2 × Paw × 100 divided by SpO 2 . Preductal SpO 2 values, preferably in the low to mid 90s (by adjusting Fi o 2 ) will result in clinically meaningful values for OSI. High SpO 2 values (especially 100%) reduce the accuracy of OSI as the range of PaO 2 associated with 100% SpO 2 can vary widely along the top flat portion of the oxygen–hemoglobin dissociation curve. Within reasonable limits, OSI values are approximately half of OI values.
Alveolar-arterial oxygen gradient (A-a gradient or A-aDO 2 ): This number estimates the gradient in the partial pressure of oxygen from the alveolus (PAO 2 with an uppercase “A” to denote alveolus) to the aorta (PaO 2 with a lowercase “a” to denote arterial) and is calculated as follows:
Please refer to Fig. 17.9 for a detailed explanation.
a/A ratio: This is a ratio of arterial to alveolar P o 2 and is commonly used in translational studies.
P/F ratio: This is commonly used in pediatric and adult critical care units to assess severity of acute lung injury (ALI) and/or acute respiratory distress syndrome (ARDS) and is obtained by dividing PaO 2 by fraction of inspired oxygen (Fi o 2 ). The patients are categorized based on the severity of hypoxemia: mild (>200 mm Hg to ≤300 mm Hg), moderate (>100 mm Hg to ≤200 mm Hg), and severe (PaO 2 /Fio 2 ≤100 mm Hg).
S/F ratio: This index is calculated by dividing preductal SpO 2 by Fio 2 . The limitations described in the section on OSI also apply to this index (see Fig. 17.9 ).
A 38-year-old woman at 34 3 ⁄ 7 weeks’ gestation with poorly controlled diabetes mellitus and a previous history of stillbirth at 36 weeks is being evaluated at the high-risk antenatal clinic. An episode of prolonged fetal bradycardia is noted, and an emergency cesarean section is performed. The baby needs CPAP in the delivery room for mild grunting and is transferred to the newborn nursery in room air. The infant is tachypneic and has preductal saturations in the mid-80s. He is given 50% oxygen by hood with improvement in saturations to the mid-90s. The initial chest x-ray shows expansion to seven ribs, bilateral haziness with mild granularity, and fluid in the horizontal fissure. The baby is transferred to the NICU for further evaluation.
What is/are the most likely diagnoses?
RDS
Retained fetal lung fluid and TTN
Early onset sepsis with pneumonia
Pulmonary hypoplasia
A and B. This is a common scenario that presents to clinicians in the NICU and well-baby nursery. This baby has clinical symptomatology that straddles both RDS and TTN. However, late-preterm gestation and maternal diabetes put this infant at greater risk of RDS. The presence of low lung volumes with granularity on chest x-ray suggests that RDS is the predominant cause of hypoxemia in this infant. This infant is born prematurely without labor and some degree of retained lung liquid should be expected.
Following admission to the NICU, the baby is placed on nasal CPAP 4 to 6 cm H 2 O in 40% oxygen. The initial capillary blood gas is as follows: pH 7.28, P co 2 55 mm Hg, and P o 2 62 mm Hg. The baby continues to be tachypneic with moderate retractions and the CPAP is increased to 6 and then to 8 cm H 2 O. The oxygen requirement gradually increases to 65% to maintain SpO 2 in the mid-90s. The arterial blood gas at 4 hours of age is as follows: pH 7.22, Pa co 2 60 mm Hg, PaO 2 48 mm Hg, and base deficit of 4 mEq/L. The chest x-ray is repeated and shows decreased lung volumes and increased granularity. A complete blood count (CBC) with differential count and blood culture are obtained. The baby is started on ampicillin and gentamicin. The white blood cell count is 18,000/mm 3 with an immature to total neutrophil ratio of 0.11, hematocrit 55%, and platelets 246,000/mm 3 . The oxygen saturation values are 92% and 82% in the right upper and lower limb, respectively.
What is the next step in the management of this infant?
Request an echocardiogram to rule out cyanotic congenital heart disease (CHD) or PPHN
Start inhaled NO via CPAP because this infant has clinical symptomatology consistent with PPHN
Endotracheal intubation and surfactant replacement
Maintain the current level of CPAP (6–8 cm of water) and repeat an arterial blood gas in 1 hour
Noninvasive positive pressure ventilation (NIPPV) via nasal cannula
C. This infant has untreated RDS as evidenced by the clinical picture of respiratory distress with elevated Pa co 2 and increasing oxygen requirement. This infant has predisposing risk factors for RDS such as late-preterm gestation and maternal diabetes. The chest x-ray appearance confirms the diagnosis. The SpO 2 in the right upper limb is higher than that in the lower limb. This suggests shunting across the ductus arteriosus from right (pulmonary artery) to left (aorta). Preductal oxygen saturations are often obtained from the right upper extremity (the right subclavian artery always arises proximal to the ductus arteriosus, whereas the left subclavian artery occasionally can arise at or after the insertion of the ductus arteriosus; see Fig. 17.10 ). The presence of a lower SpO 2 in the lower limb compared with the right upper limb is indicative of a right-to-left ductal shunt. An echocardiogram will be helpful if the infant does not respond to intubation, surfactant replacement, and alveolar recruitment.
The baby is intubated and receives a dose of surfactant. The initial ventilator settings are PIP of 20 cm H 2 O, PEEP of 4 cm H 2 O, pressure support of 12 cm H 2 O, Paw of 10 cm H 2 O, rate 40 breaths per minute, and inspiratory time of 0.35 seconds. There is minimal improvement in oxygen requirement following administration of surfactant. Umbilical arterial and venous lines are placed, and the blood gas at 8 hours of age has a PaO 2 of 45 mm Hg in 70% oxygen.
An echocardiogram is performed, which shows a structurally normal heart with a right ventricular systolic pressure of 55 mm Hg. Right-to-left shunting is noted both at the foramen ovale and PDA ( Fig. 17.11 A). Inhaled NO is started at 20 ppm. The baby is reevaluated 15 minutes following initiation of inhaled NO and noted to have a persistent oxygen requirement at 70%. A repeat arterial blood gas at 10 hours of age shows pH of 7.22, Pa co 2 of 58 mm Hg, Pa o 2 of 64 mm Hg, and a base deficit of 5 mEq/L. The chest x-ray shows poorly inflated lungs with bilateral ground-glass opacities.
What is the most likely reason for poor response to inhaled NO in this infant?
Missed diagnosis of cyanotic CHD
Inadequate alveolar recruitment
Surfactant protein B deficiency
Pulmonary venous hypertension
B. The most likely reason for not responding to inhaled NO is inadequate alveolar recruitment. The persistent underinflation on chest x-ray is evidence of inadequate respiratory support. Increasing the PEEP, PIP, or inspiratory time are common strategies to increase Paw and oxygenation during conventional ventilation. The use of high-frequency ventilation with adequate Paw can also achieve lung recruitment. It is unusual for cyanotic CHD to present with elevated Pa co 2 . Surfactant protein B deficiency is a very rare condition and presents with intractable respiratory failure in term infants. Pulmonary venous hypertension is usually secondary to obstruction to pulmonary venous drainage, mitral stenosis, or left ventricular dysfunction.
The baby is placed on a high-frequency oscillator with the following settings: Paw of 12 cm H 2 O, amplitude 30, frequency 12 Hz, and 50% O 2 . Inhaled NO is continued at 20 ppm. A chest x-ray is repeated and shows inflation to eight ribs, symmetric bilateral opacification, and normal heart size.
For inhaled NO to be effective, it has to be delivered to the site of action (i.e., to the vascular smooth muscle cell in the pulmonary arteriole through the respiratory tree). Failure to achieve lung recruitment before initiation of inhaled NO is a common cause for failure to sustain an oxygenation response.
The baby is given another dose of surfactant, and the lungs are recruited by increasing the Paw to 16 cm H 2 O with prompt improvement in oxygenation. The inspired oxygen concentration is weaned to 30%. The Paw is decreased to 14 cm H 2 O. A repeat blood gas is as follows: pH of 7.33, Pa co 2 of 40 mm Hg, and PaO 2 of 98 mm Hg.
Subsequently, supplemental oxygen is weaned to 21%, and inhaled NO is weaned off over the next 20 hours. The baby is switched over to the conventional ventilator the following day and extubated 24 hours later to 30% oxygen by nasal cannula.
A 32-year-old gravida 2, para 0 mother is admitted in labor at 40 weeks’ gestation with rupture of membranes. Meconium-stained amniotic fluid (MSAF) is noted, and induction of labor with oxytocin is commenced. Fetal tachycardia and loss of variability are observed, and an emergency cesarean section is performed.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here