Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Regional anesthesia is only successful when local anesthetic is inserted in close proximity to the targeted nerves. From the inception of regional anesthesia over a century ago, several techniques have been designed and available to facilitate the correct placement of local anesthetic, including the paresthesia-seeking approach, peripheral nerve stimulator, and most recently ultrasound guidance.
There are no data to support the superiority of one nerve localization technique—paresthesia, nerve stimulation, ultrasound—over another with regard to reducing the risk of nerve injury.
Ultrasound imaging can elucidate the structure of peripheral nerves and adjacent anatomic structures for regional block. Peripheral nerves have a characteristic honeycomb echotexture, formed by the internal pattern of connective tissue and nerve fibers.
Ultrasound provides real-time imaging for needle tip placement and drug injection. Successful local anesthetic injections clarify the border of the nerve and track along the nerve path and its branches. Ultrasound guidance results in more consistent procedure times for peripheral nerve blocks and can be applied to many regional anesthesia procedures. Anatomic variation in nerve position and course, which is a potential source of block failure, can be directly visualized.
Lipid emulsion bolus and infusion improve the success of resuscitation from cardiac arrest because of local anesthetic toxicity if given immediately after a local anesthetic overdose.
Prior to regional blocks checklists are now being performed in an effort to make rare adverse events even less common, thereby improving patient safety.
The authors would like to thank Ram Jagannathan, MD, MBBS, for his help with imaging. Additionally, we would like to acknowledge Dr. Adam B. Collins for providing figure 46.36.
This chapter contains content from two chapters in the 8th edition text. The editors, publisher, and returning contributors, Drs. Sandra L. Kopp and Andrew T. Gray, would like to thank Drs. Terese T. Horlocker and Denise J. Wedel for their contribution in the prior edition of this work. It has served as a foundation for the current chapter.
Peripheral nerve blocks can be performed using a variety of guidance techniques. Recently, ultrasound has gained popularity for regional anesthesia because it allows direct imaging of peripheral nerves, the block needle, and injection distribution. This chapter is a focused update of two chapters on peripheral nerve blockade from the previous edition. The sections to follow contain a selective description of the more common peripheral nerve blocks utilized in clinical practice.
The paresthesia-seeking technique has a long, successful history as a simple method that requires little specialized equipment. A paresthesia is elicited when a needle makes direct contact with a nerve. Paresthesia-seeking techniques are reliant on patient cooperation and participation to guide the needle and local anesthetic injection accurately; therefore only small doses of sedation medication are recommended. Paresthesia techniques have been criticized for causing patient discomfort, although clinical studies have not shown a significant increase in neurologic complications with this technique. Caution should be used when initiating the injection of local anesthetic to ensure that the needle is not intraneural. There is controversy in the literature regarding the use of B-bevel (blunt bevel or short bevel) needles versus sharp needles regarding the incidence and severity of nerve injury if the needle inadvertently punctures or pierces the nerve. Because B-bevel needles have a blunt tip, which is likely to push the nerve aside, they are much less likely to penetrate the nerve; however, when an injury does occur, it appears to be more severe. In contrast, sharp needles are more likely to penetrate the nerve, but the injury appears to be less destructive. Success with the paresthesia technique is highly dependent on the skill of the practitioner and requires a thorough understanding of anatomy. This technique was slowly replaced in the 1980s when peripheral nerve stimulation was introduced. Currently, no single technique has been shown to be superior with respect to incidence of neurologic complications.
Peripheral nerve stimulators deliver small pulses of electric current to the end of a block needle to cause depolarization and muscle contraction when the tip of the needle is in close proximity to a neural structure. This technique allows for localization of a specific peripheral nerve without requiring the elicitation of a paresthesia, thus allowing patients to be more sedated during block placement. It is necessary to attach the cathode (negative terminal) to the stimulating needle and the anode (positive terminal) to the surface of the patient because cathodal stimulation is more efficient than anodal stimulation. Most current-stimulating needles are coated with a thin layer of electrical insulation along the needle shaft with the exception of the tip. This allows for higher current density at the tip of the needle. Higher current output (>1.5 mA) is more likely to stimulate neural structures through tissue or fascial planes and can be associated with painful, vigorous muscle contractions. After localization of the correct motor response, the current is gradually decreased to a current of 0.5 mA or less. A motor response at a current of approximately 0.5 mA is appropriate when used to facilitate the location for injection of local anesthetic or catheter placement. Immediately following injection of local anesthetic or saline (ionic solutions), the current density at the needle tip will rapidly dissipate and the evoked motor response is eliminated (the Raj test) .
The stimulating current pulse can be modified to produce a sensory response. The short-duration impulse commonly used (0.1 ms) is effective in stimulating motor fibers, but a longer-duration pulse (0.3 ms) will also stimulate sensory fibers, a useful feature if a pure sensory nerve is being sought.
Ultrasound imaging allows direct visualization of peripheral nerves, the block needle tip, and local anesthetic distribution. This imaging modality has proven highly useful for guiding targeted drug injections and catheter placement. This section describes the general principles of ultrasound imaging for regional blocks.
Ultrasound is sound with a frequency above the audible range (>20,000 cycles per second). The frequencies used in clinical imaging are within the range of 1 to 20 MHz. High-frequency ultrasound beams are well collimated and therefore can provide high resolution. For most regional blocks, the highest frequency is selected that adequately penetrates the depth of field. Sound waves reflected at the interface of two tissues with different acoustic impedances generate echoes. Ambient lighting has a large effect on visual discrimination; therefore dim lighting without glare is especially useful for imaging low-contrast targets such as peripheral nerves.
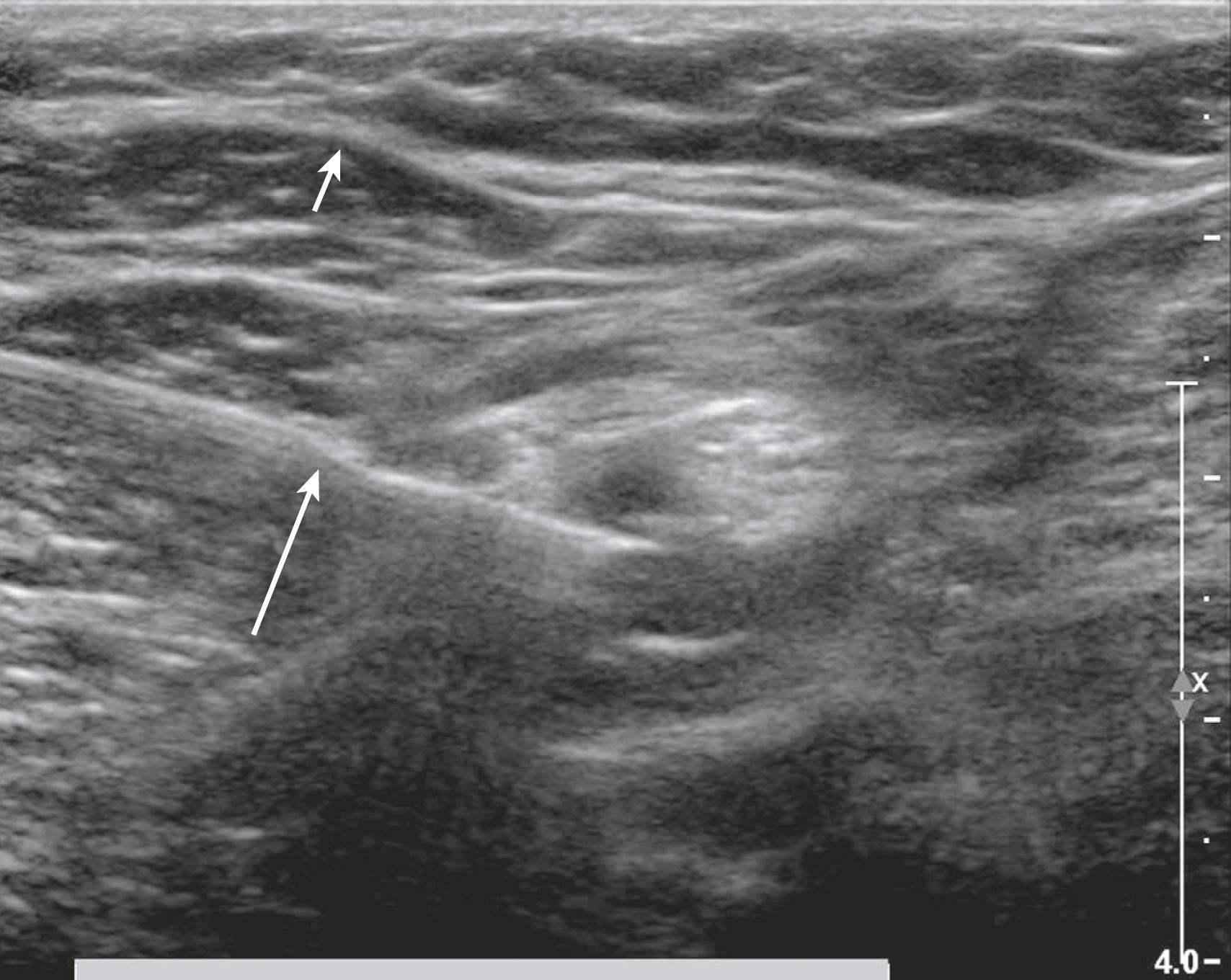
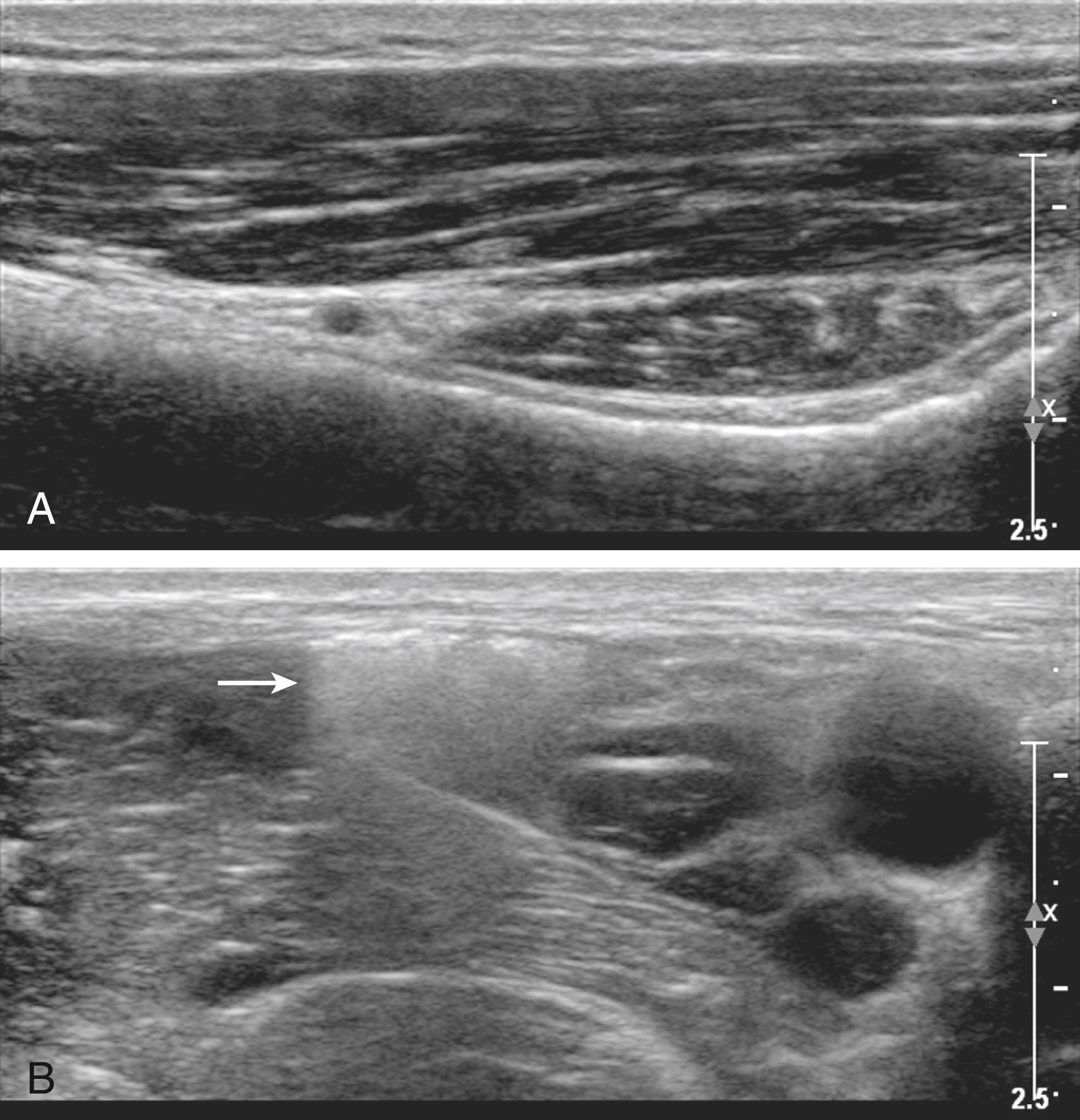
Ultrasound imaging is predicated on several common assumptions. First, the speed of sound through soft tissue is 1540 m/s, meaning 13 μs elapse for each centimeter of soft tissue traversed back and forth for the total fly-back time of received echoes. This assumption allows interconversion of time and distance for echo ranging. Local heterogeneities in soft tissue can cause artifactual bending of the block needle on ultrasound scans, known as the bayonet artifact ( Fig. 46.1 ). Bayonet artifacts are commonly observed during the lateral in-plane approach to popliteal block (see Sciatic Nerve Blocks in the Popliteal Fossa) because more adipose tissue is present over the nerves near the posterior midline of the leg (adipose tissue has a slower speed of sound than the adjacent muscle). The speed of sound artifacts relate both to time-of-flight considerations and to refraction that occurs at the interface of tissues with different speeds of sound.
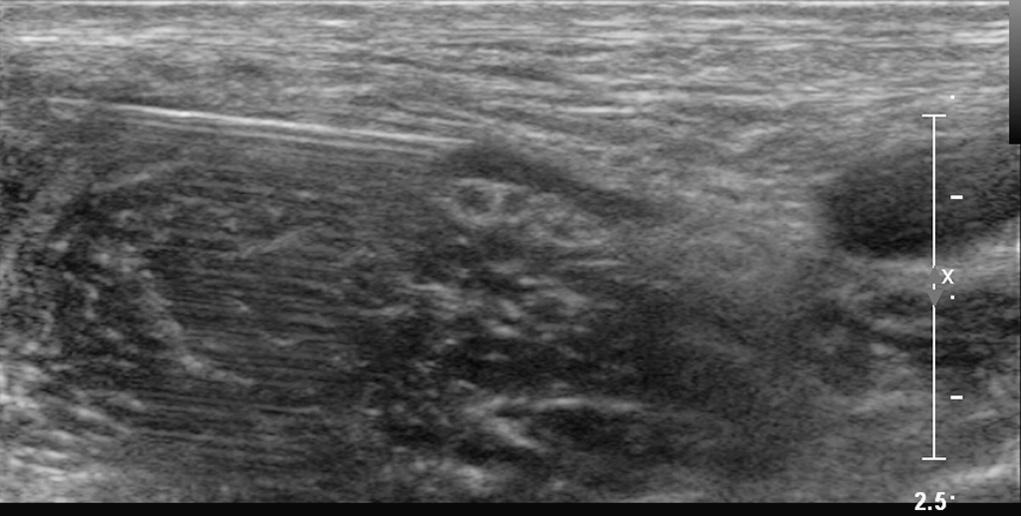
Second, ultrasound waves are assumed to take a straight path to and from tissue. When this does not occur, reverberation artifacts are displayed deep to the reflector. Reverberation artifacts are commonly observed from the block needle shaft at shallow angles of insertion because sound waves bounce back and forth between the walls of the needle before returning to the transducer ( Fig. 46.2 ). Comet tail artifact is another type of reverberation artifact and helps identify strong reflectors such as the pleura during supraclavicular and intercostal blocks. At low receiver gain, the comet tail is seen as a tapering series of discrete echo bands just deep to a strongly reflecting structure. The spacing between the bands represents the distance between the anterior and posterior walls of the object. Internal reverberations (arising from within the object) cause the comet tail artifact, most intensely observed when the anatomic object is perpendicular to the beam. Comet tail artifact from the pleura relates to lung water content, because small collections of lung water lined by the strongly reflecting pleura can allow the sound beam to enter and then return at varying times to the transducer ( Fig. 46.3 ).
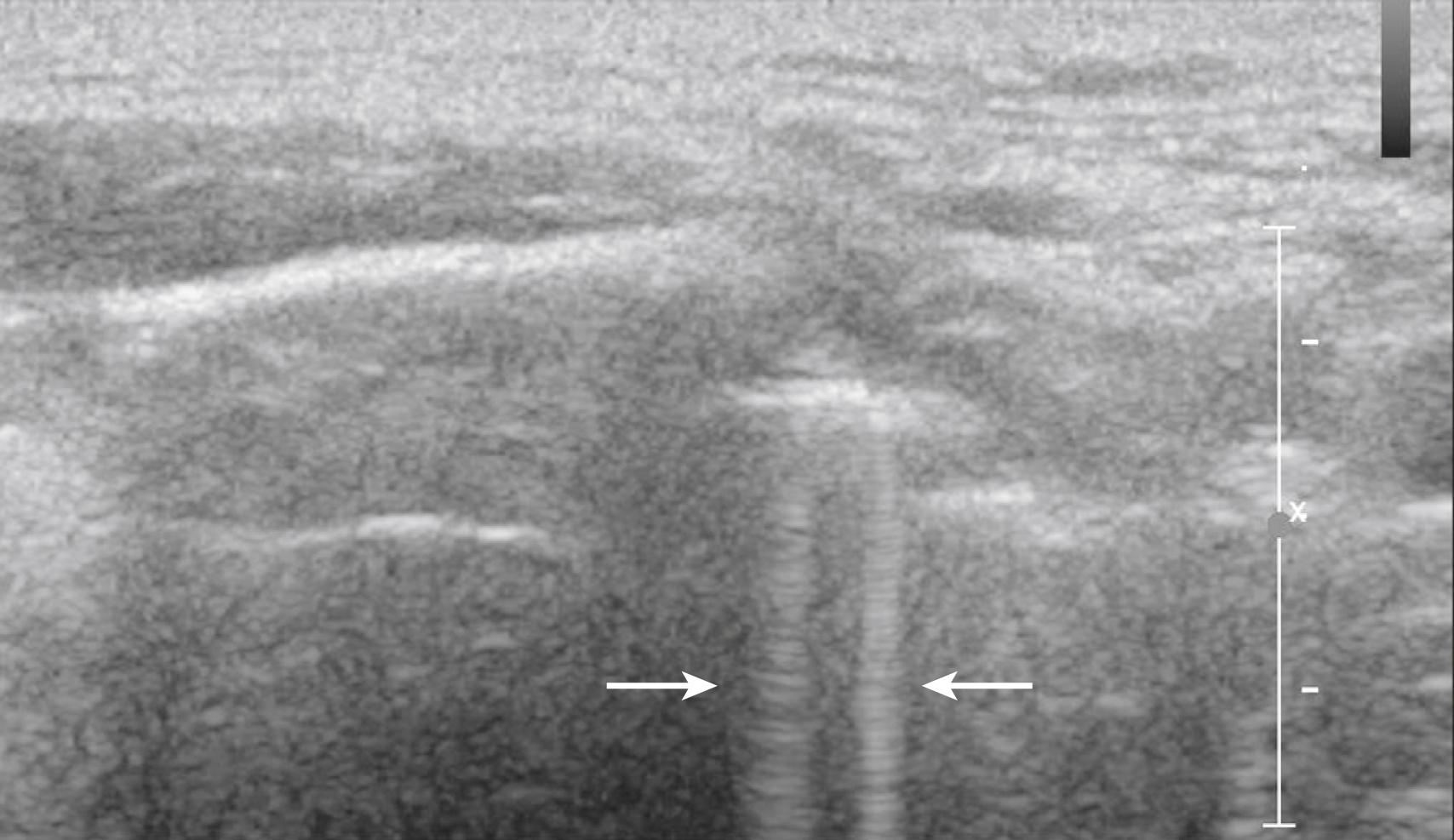
Third, all reflectors are assumed to be on the central ray of the transducer beam. When this assumption is not true, out-of-plane artifacts are observed (slice thickness artifacts). Definitive proof of out-of-plane artifacts requires multiple views, which are recommended when such ambiguities arise.
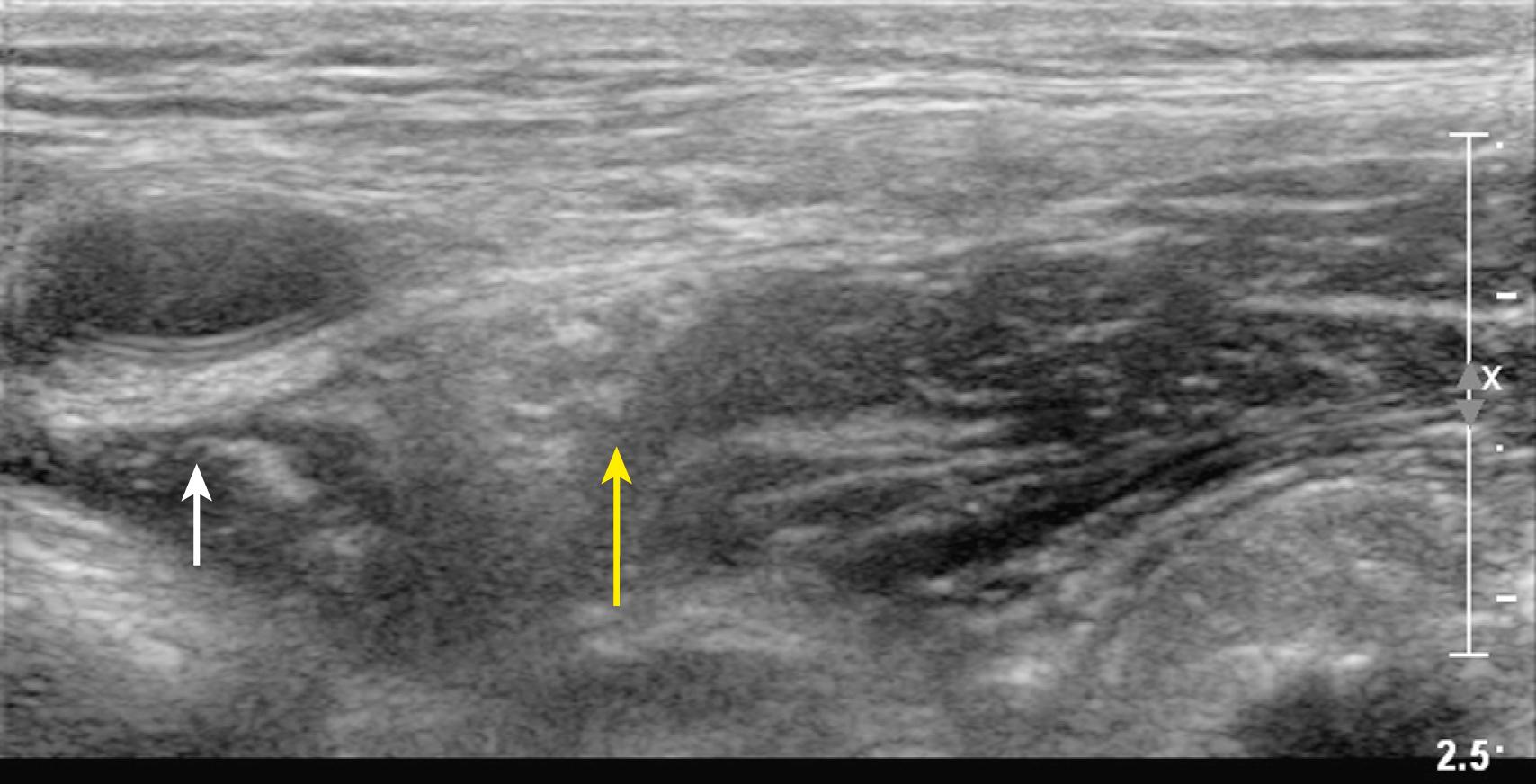
Unlike adjacent soft tissue, most biologic fluids do not significantly attenuate the sound beam and therefore cause acoustic enhancement (sometimes referred to as posterior acoustic enhancement or increased through-transmission). Acoustic enhancement artifacts deep to blood vessels can be erroneously interpreted as peripheral nerves ( Fig. 46.4 ). For example, acoustic enhancement deep to the second part of the axillary artery in the axilla can be mistaken for the radial nerve. In the infraclavicular region, acoustic enhancement deep to the axillary artery can be mistaken for the posterior cord of the brachial plexus (and similarly, for the femoral artery and the femoral nerve in the inguinal region).
Acoustic shadowing occurs deep to strong reflecting structures, such as the cortical surface of mature bone ( Fig. 46.5 ). Acoustic shadows from refraction (also termed refractile shadowing or lateral edge shadowing) are often observed deep to the edges of blood vessels when the vessels are imaged in the short-axis view. Refractive edge shadows can be seen from the carotid artery during stellate ganglion block or from the second part of the axillary artery during infraclavicular block. Refraction artifacts (e.g., refractile shadowing) are less apparent when spatial compound imaging (for further information, see later in this chapter) is used to reduce angle-dependent artifacts.
Ultrasound transducers consist of piezoelectric crystals that emit and receive high-frequency sound waves by interconverting electrical and mechanical energy. Transducer selection is important to the success of ultrasound-guided regional anesthesia procedures. High-frequency sound waves provide the best resolution but will not penetrate far into tissue. The frequency range is therefore chosen to be the highest that will allow adequate insonation of the entire depth of field. A low-frequency transducer can be used to image large nerves that lie deep, such as the cords of the brachial plexus that surround the second part of the axillary artery or the proximal sciatic nerve in the gluteal region.
The footprint size (i.e., the length of the active face transducer that contacts the skin) is chosen to provide a broad enough view of the structures of interest. As a general rule, the footprint should be at least as large as the anticipated depth of field. A square or landscape view is better than a keyhole view (i.e., depth greater than footprint) for guidance. As a rule of thumb, for in-plane technique (see Approaches to Regional Block With Ultrasound), every millimeter of the footprint is approximately a millimeter of guidance.
Linear-array transducers generally have a higher scan-line density than curved arrays and therefore produce the best image quality. Images from linear arrays are usually displayed in a rectangular format. When a linear transducer is needed but space at the site of block is limited by anatomic structures such as adjacent bone, a compact linear (hockey stick) transducer that has a smaller footprint can be very useful. Curved arrays provide a broad field of view for a given footprint size and are generally used when space is limited (e.g., infraclavicular region). Curved probes are easier to rock (see Infraclavicular Blocks) and produce images in sector format.
Universal precautions should be used when handling dirty equipment. External surface probes require disinfection between every use and after extended periods of nonuse, per instructions of the manufacturer. Do NOT drop any ultrasound transducer, because the active face of the transducer is especially sensitive to contact with hard surfaces.
One of the essential skills to acquire for regional block with ultrasound is transducer manipulation ( Fig. 46.6 ). For this reason, standardized nomenclature has been established :
Sliding (moving contact) the transducer along the known course of the nerve using a short-axis view often helps.
Tilting (cross-plane, side-to-side) will vary the echo brightness of peripheral nerves. Optimizing this angle is critical to promote nerve visibility.
Compression is often used to confirm venous structures. To improve imaging, compression not only provides better contact, but it also brings the structures closer to the surface of the transducer. Soft tissue is subject to compression; therefore estimates of tissue distances will vary.
Rocking (in-plane, toward, or away from the indicator) is often necessary to improve visibility of the needle and anatomic structures when the working room is limited.
Rotation of the probe will produce true short-axis views rather than oblique or long-axis views.
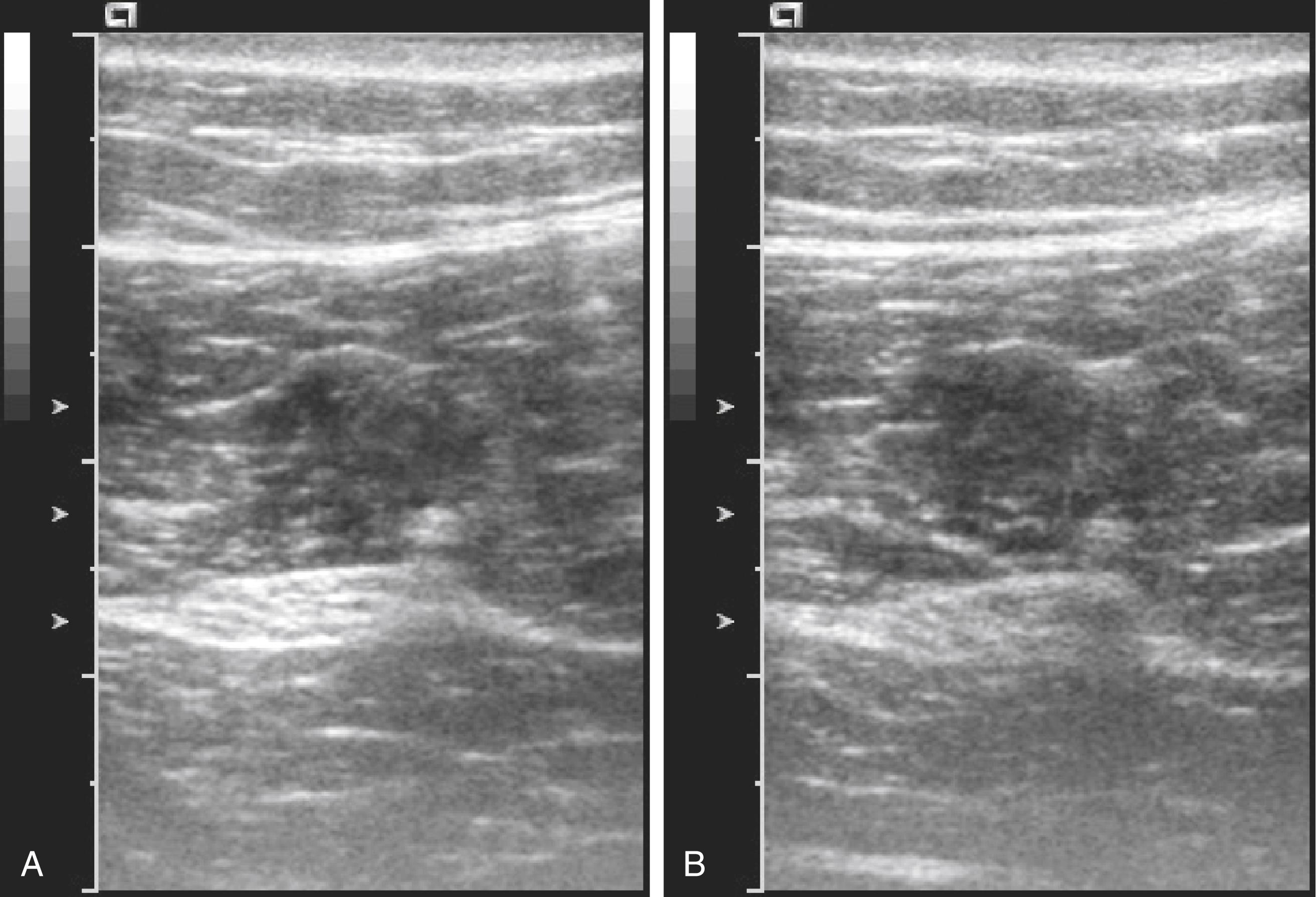
Anisotropy is the change in echogenicity with inclination of the transducer. In general, when objects are obliquely imaged, they appear less echogenic ( Fig. 46.7 ). This relationship is most pronounced for tendons but also occurs for muscle and nerves. Although the term anisotropy was first used to describe changes in received echoes when rocking the transducer with structures viewed in long axis, it has also been used for short-axis views when tilting the transducer. With experience, operators learn to rock and tilt the transducer naturally to fill in the received echoes from peripheral nerves. Sliding and rotating the transducer achieves needle tip localization after optimizing peripheral nerve echoes by tilting.
Spatial compound imaging steers ultrasound beams in different, predetermined angles, typically within approximately 20 degrees from the perpendicular ( Fig. 46.8 ). These multiple lines of insonation are then combined to produce a single composite image. Spatial compound imaging appears to reduce angle-dependent artifacts, anisotropic effects, and acoustic shadows. Another advantage for regional block is that the definition of tissue planes and the detection of nerve borders can be improved. In the systems that have been tested, spatial compound imaging improves needle tip visibility over a limited range of needle insertion angles (<30 degrees). The stray lines of sight (i.e., those that travel off the field underneath the transducer) can be used to form a wider field of view in a trapezoidal format.
A Doppler shift occurs when a wave source and receiver are moving relative to each other, which produces a change in frequency such that the frequencies of the transmitted and reflected sound waves are not the same. When a wave source and receiver are moving toward each other, the observed frequency is greater than the source frequency; and when moving away from each other, the observed frequency is lower. The change in frequency is related to the velocity of moving reflectors and the angle of insonation. In clinical medicine, red blood cells are the primary reflectors that produce Doppler shifts.
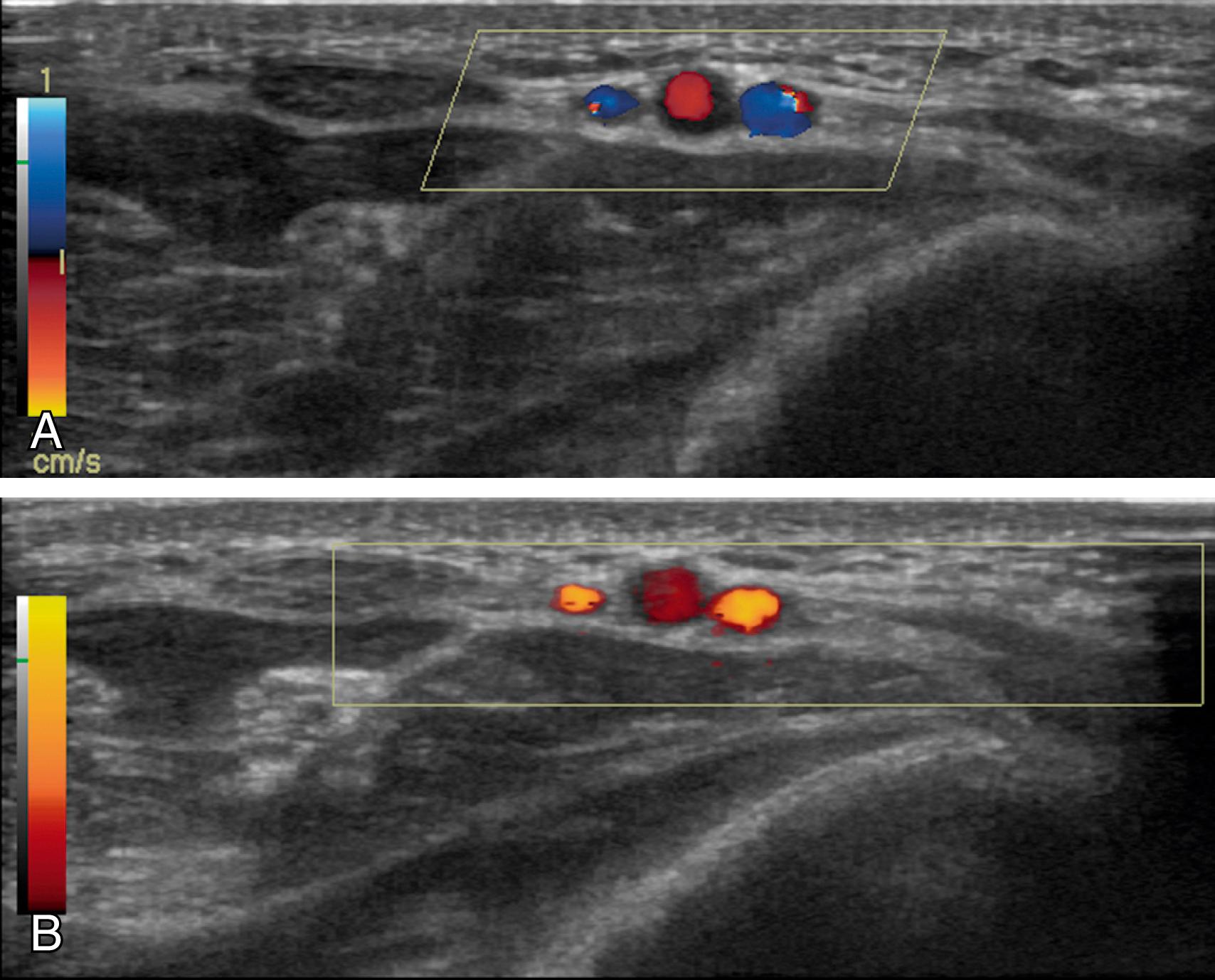
Doppler ultrasound imaging has different modes ( Fig. 46.9 ). Traditional color Doppler encodes mean frequency shifts to provide directional velocity information; that is, conventional blue color indicates flow away from the transducer, whereas red color indicates flow toward the transducer. More recently, a more sensitive Doppler technology has been developed that encodes color based on the integration of the Doppler power spectrum. Power Doppler is less angle dependent and not subject to aliasing. The disadvantages are that no directional information is provided and motion sensitivity (flash artifact) is high. Power Doppler is especially useful for detecting small arteries that accompany nerves ( Box 46.1 ). Power Doppler can detect these small arteries and better delineate the course of tortuous vessels that have unfavorable angles to the ultrasound beam.
A large number of factors influence needle tip visibility in clinical practice. Metal needles are hyperechoic and can cause reverberation artifact. Needle tip visibility is best when the needle path is parallel to the active face of the transducer. Under this condition, the needle is perpendicular to the sound beam; therefore strong specular reflections will be produced; that is, mirror-like reflections will be produced from a smooth surface. As the angle of incidence is increased, the mean brightness will decrease. In this same study, the bevel angles were ground from 10 to 70 degrees but were found to have no effect on the needle tip echo. However, bevel orientation does influence the needle tip echo; visibility is best with the bevel either directly facing or averting the transducer. Because needle diameters are smaller than the scan plane thickness, larger needles are more echogenic than finer ones.
Visualization of needles in echogenic tissue is difficult, particularly in bright adipose tissue. A number of strategies have been proposed to improve needle tip visibility. A low-receiver gain can improve the detection of the needle tip echo. Spatial compound imaging can help identify the needle tip when the needle path is at an angle with respect to the transducer. However, one limitation of this strategy is that only a small triangular section of the field of imaging receives all the lines of sight and is therefore fully compounded. In addition, the range of angles for spatial compound imaging is limited and is usually exceeded by the desired needle insertion path. Rocking back the transducer can improve the angle between the ultrasound beam and needle during in-plane technique (see Approaches to Regional Block with Ultrasound). Most practitioners orient the needle so that the needle bevel faces the transducer.
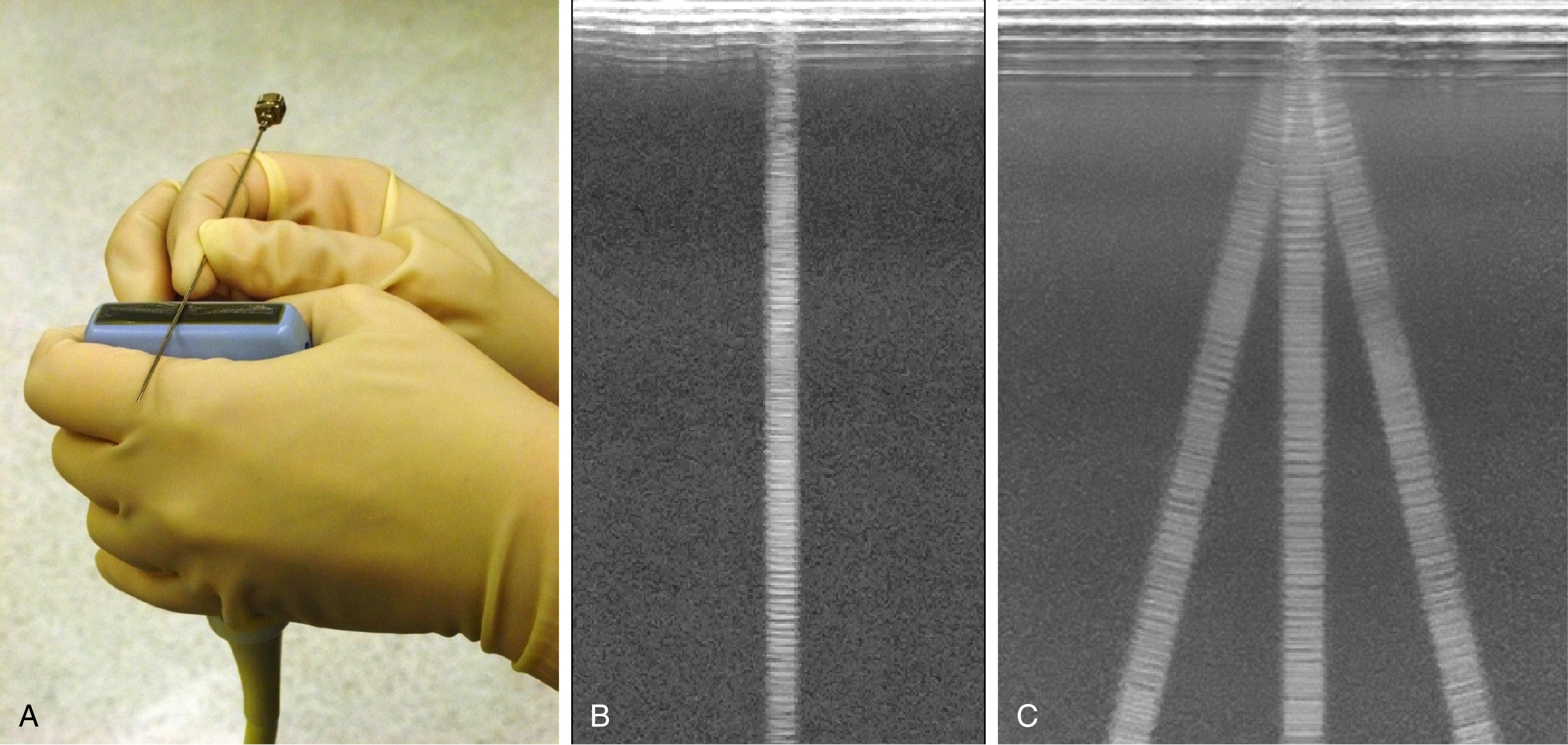
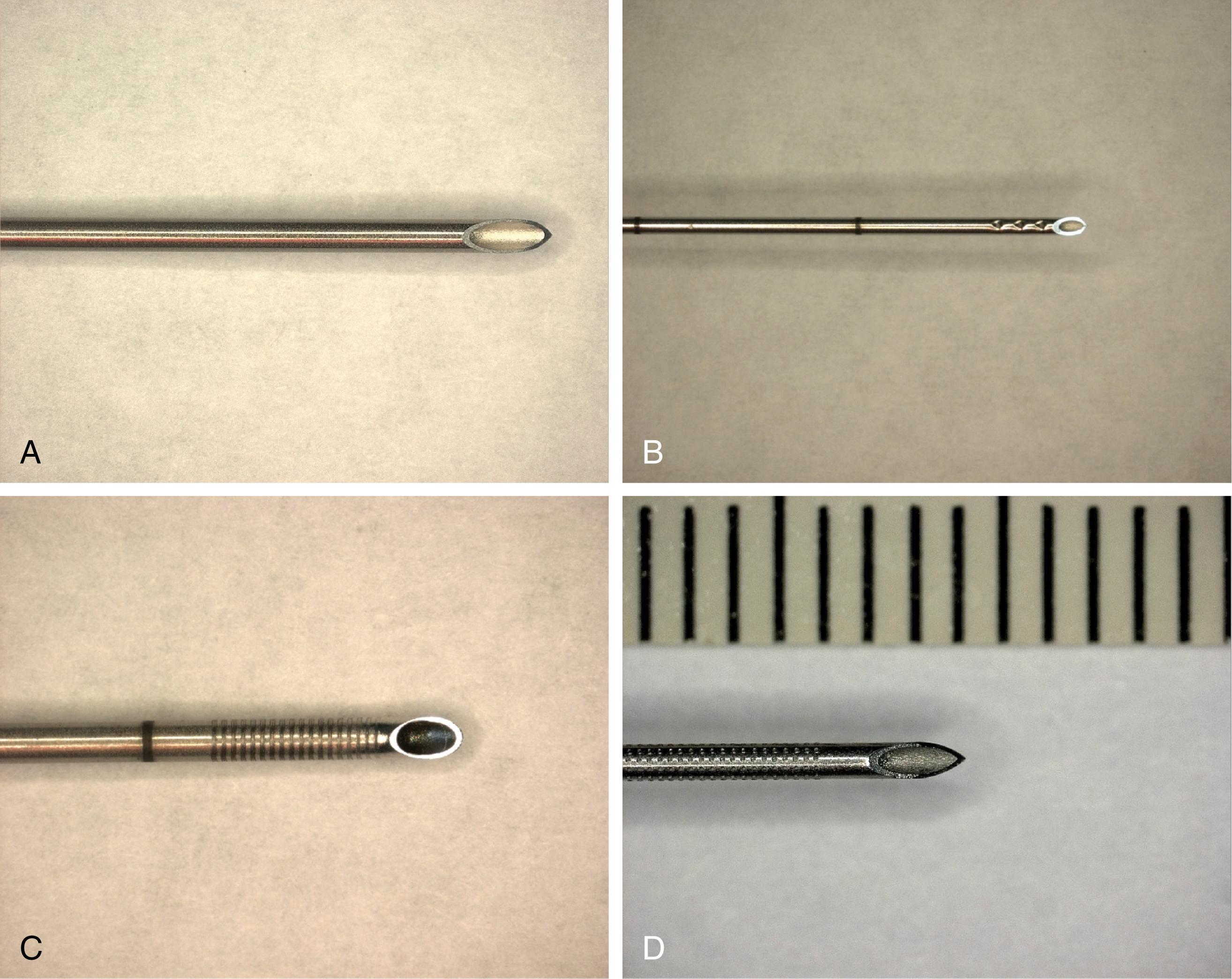
Among needles originally developed for use in regional anesthesia, Hustead bevels tended to be more visible than side port needles that lack cutting bevels. Needles with echogenic modifications are now commercially marketed for peripheral nerve blocks. One engineering strategy has been to texture the needle surface so that echoes return to the transducer source, regardless of the angle of insonation ( Fig. 46.10 ). One potential limitation of these needle designs is the finite size of the needle texturing. Low-frequency transducers produce longer wavelengths that may be too large to reflect strongly back from the textured surface of the needle.
Peripheral nerves can be directly detected with high-resolution ultrasound imaging. The fascicular echotexture is the most distinguishing feature of nerves (honeycomb architecture) ( Fig. 46.11 ). More central nerves, such as the cervical ventral rami, have fewer fascicles and can appear monofascicular on ultrasound scans. Ultrasound frequencies of 10 MHz or higher are required to distinguish tendons from nerves based on echotexture alone. One of the most powerful techniques to identify nerve fascicles is to slide a broad linear transducer over the known course of a peripheral nerve with the nerve viewed in short axis (transverse cross section).
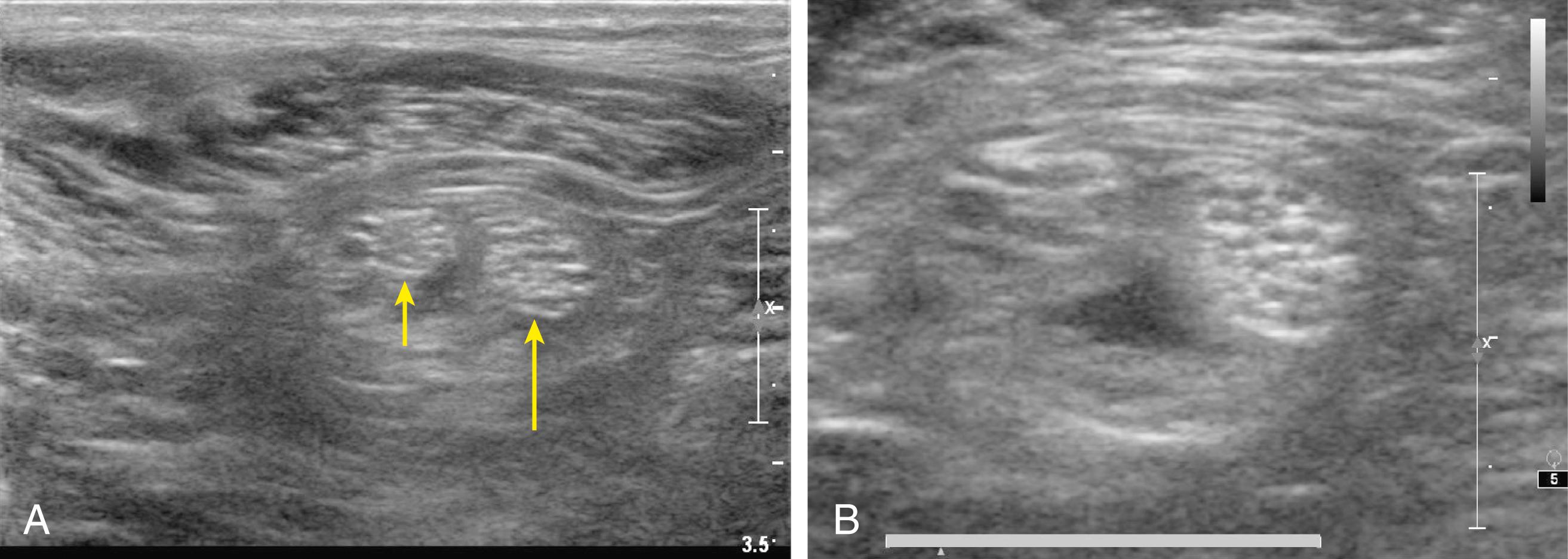
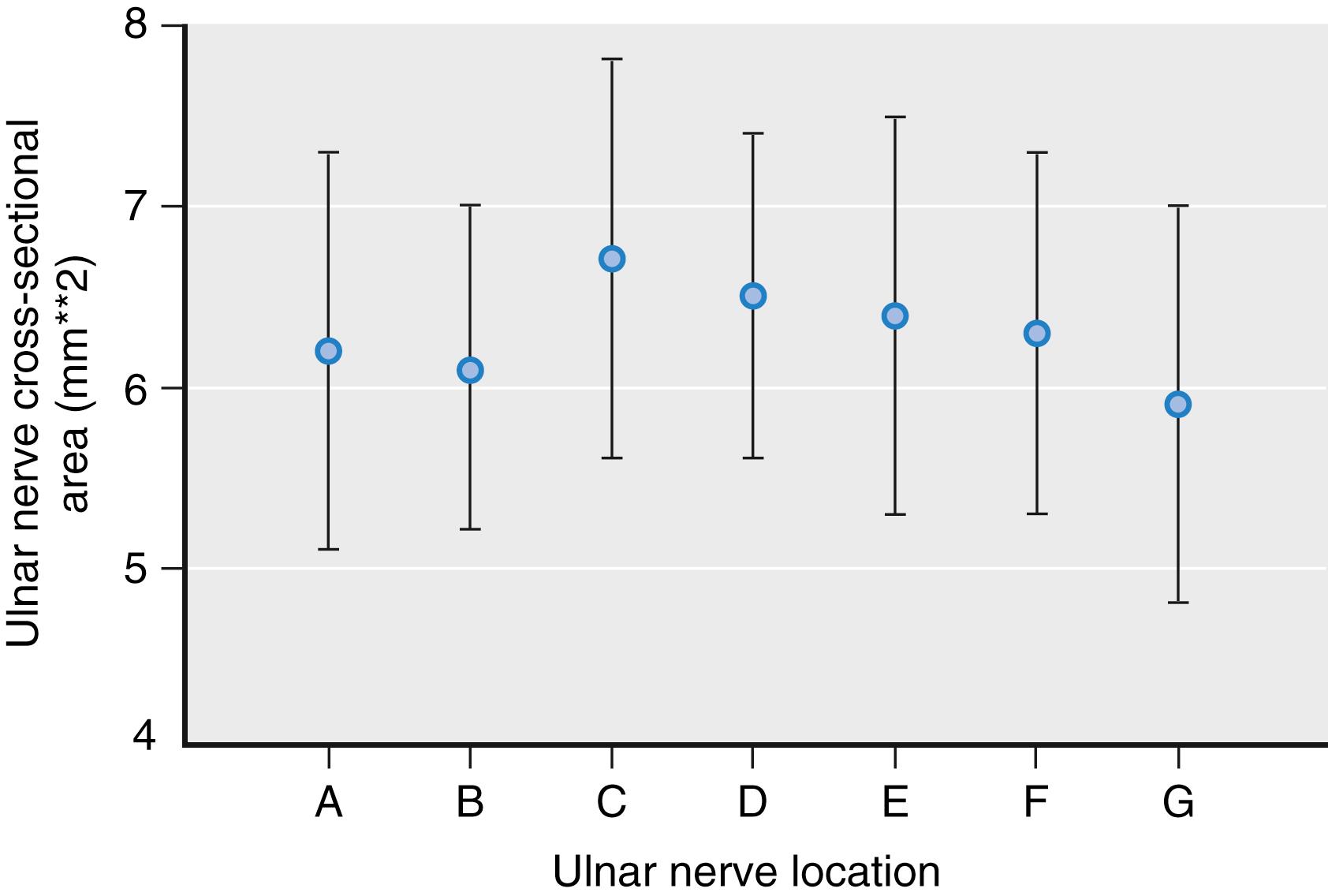
Nerves can be round, oval, or triangular in shape. Although nerve shape can change along the nerve path, the cross-sectional nerve area is relatively constant in the absence of major branching ( Fig. 46.12 ). Peripheral nerves are pathologically enlarged either by entrapment or in certain neuromuscular disorders, such as Charcot-Marie-Tooth, type 1A, disease ( Fig. 46.13 ). Some evidence suggests that patients with diabetic neuropathy also have enlarged peripheral nerves.
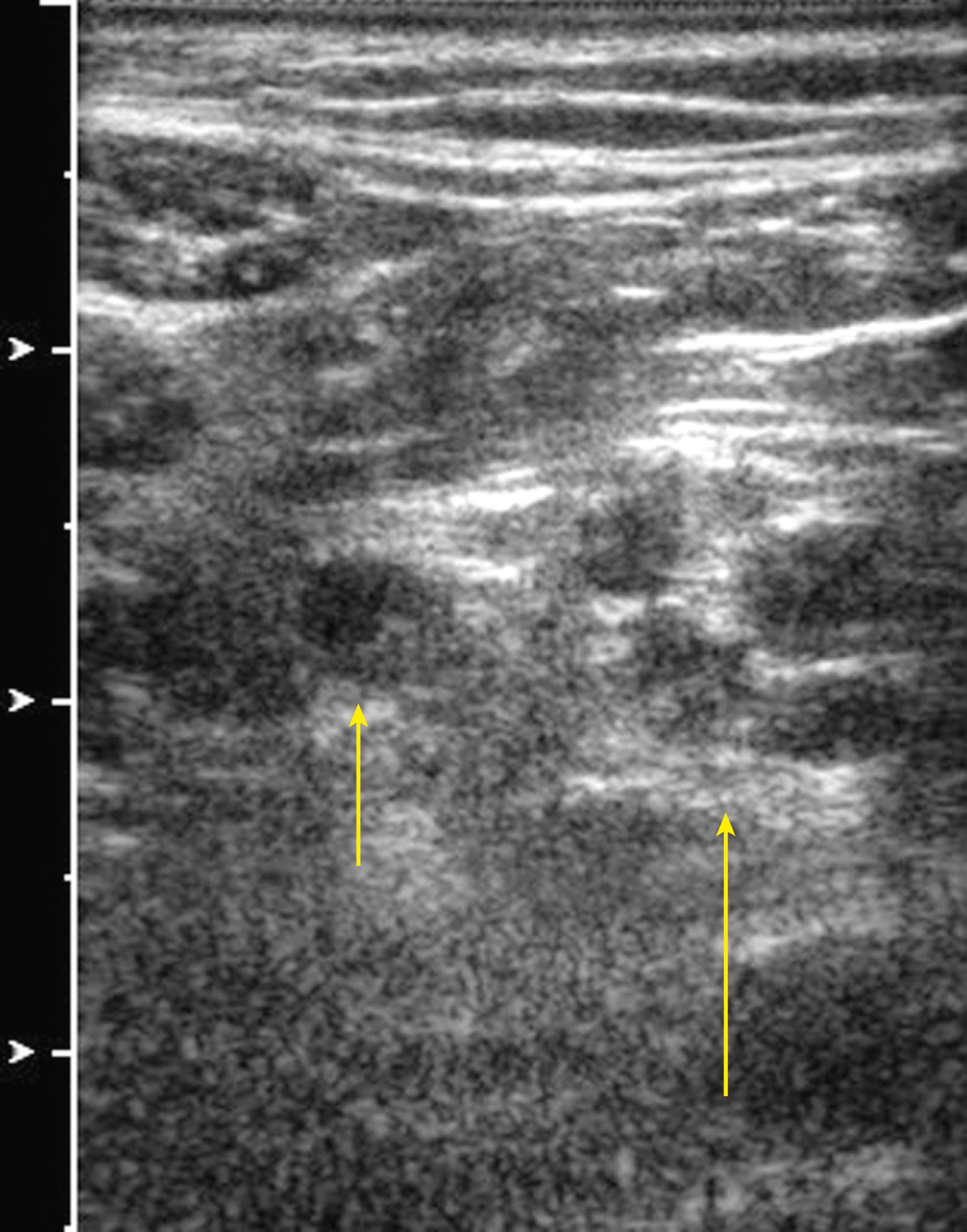
Although direct nerve imaging has led to a phenomenal increase in ultrasound-guided regional anesthesia, the identification of other nearby anatomic structures, such as the fascia and other connective tissue, is also critical in this endeavor. These layers permit favorable distribution of local anesthetic, making nerve contact with the block needle unnecessary.
Many approaches to regional blocks with ultrasound are available ( Table 46.1 ). Peripheral nerves are usually viewed in short axis rather than long axis. The needle can approach within the plane of imaging (in-plane technique) or cross the plane of imaging as an echogenic dot (out-of-plane technique). For some regional blocks, offline markings (skin markings before needle insertion) are used instead of online imaging (i.e., imaging during needle insertion and injection). Most studies have suggested that adequate visualization and correct identification of the relevant structures (e.g., peripheral nerve, needle tip, local anesthetic, adjacent anatomic structures) is more important than the approach, per se, for outcomes after regional blocks. Nevertheless, consistent practice patterns are developing among institutions and illustrate the underlying principles.
| Approach | Examples of Regional Block |
|---|---|
| Short-axis view, in-plane | Almost any peripheral nerve block Almost any peripheral catheter placement |
| Short-axis view, out-of-plane | Shallow blocks Interscalene catheter Lateral femoral cutaneous nerve block Femoral nerve catheter placement |
| Long-axis view, in-plane | Proximal fascia iliaca block Proximal obturator block Anterior sciatic block |
| Long-axis view, out-of-plane | Epidural placement (longitudinal paramedian view during midline approach) Transtracheal anesthesia |
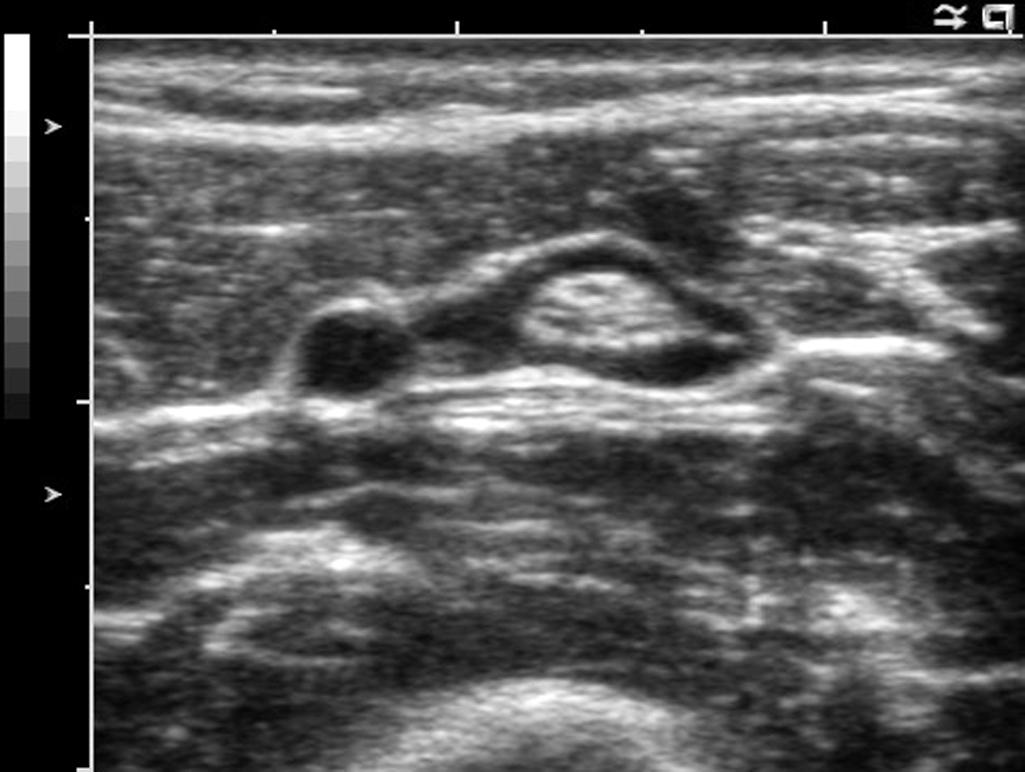
Successful injection for peripheral nerve block has typical characteristics ( Fig. 46.14 ). Injections should distribute around the nerve (clarifying the nerve border), travel along the nerve path and branches, and separate the nerve from common anatomic structures such as adjacent arteries that are wrapped together in common fascia and connective tissue. Because anechoic fluid is typically injected, echoes received from the peripheral nerve will also be enhanced by increased through transmission (but not necessarily a sign of block success).
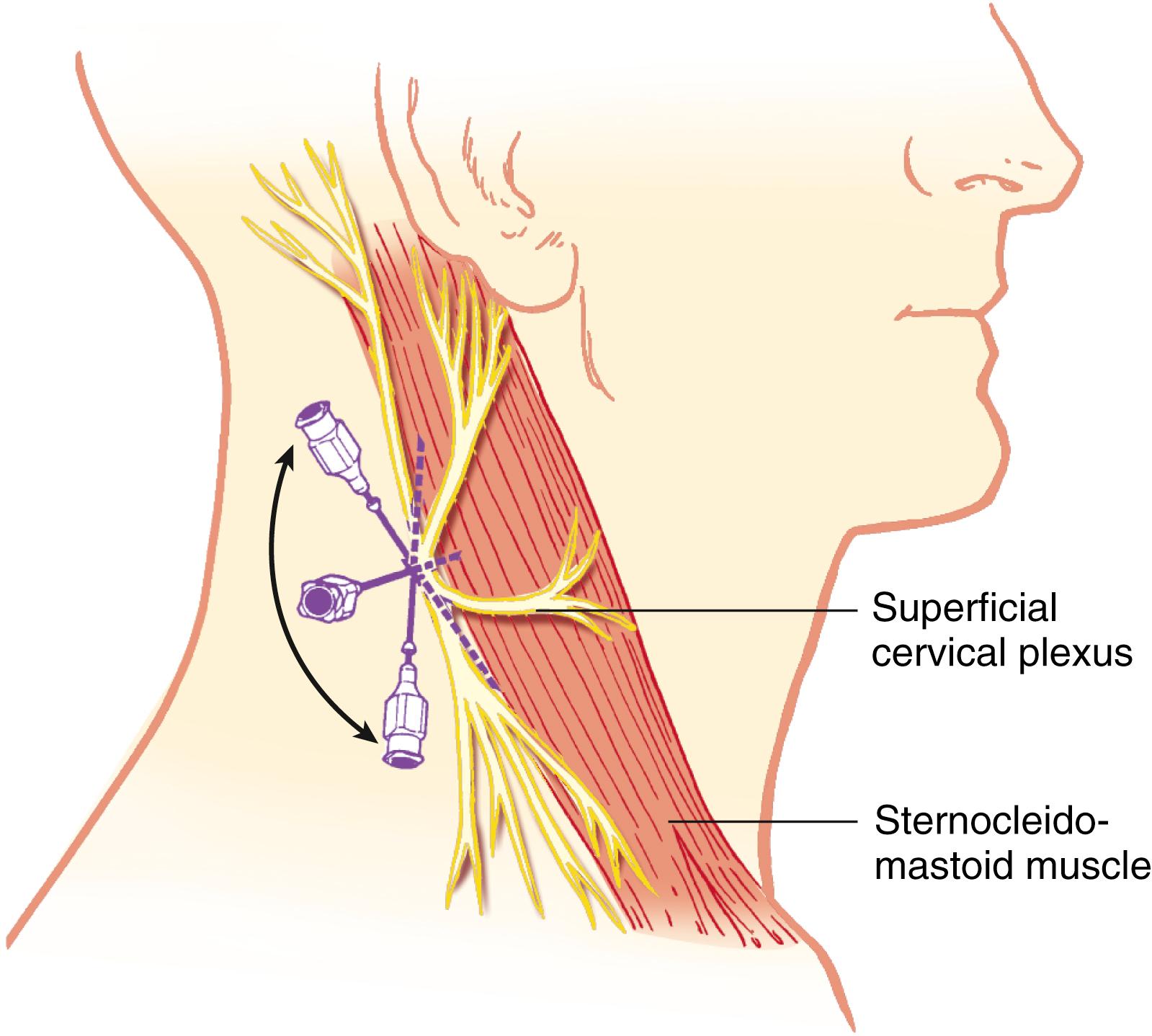
The cervical plexus is derived from the C1, C2, C3, and C4 spinal nerves and supplies branches to the prevertebral muscles, strap muscles of the neck, and phrenic nerve. The deep cervical plexus supplies the musculature of the neck. The superficial cervical plexus provides cutaneous sensation of the skin between the trigeminal innervation of the face and the T2 dermatome of the trunk.
Blocks of the cervical plexus are easy to perform and provide anesthesia for surgical procedures in the distribution of C2 to C4, including lymph node dissections, plastic surgery repairs, and carotid endarterectomy. The ability to continuously monitor the awake patient’s neurologic status is an advantage of this anesthetic technique for the latter procedure and has resulted in an upsurge in the popularity of this technique. Bilateral blocks can be used for tracheostomy and thyroidectomy. A variety of approaches to cervical plexus block have been described, including some guided by ultrasound imaging. ,
The superficial cervical plexus is blocked at the midpoint of the posterior border of the sternocleidomastoid muscle. A skin wheal is made at this point, and a 22-gauge, 4-cm needle is advanced, injecting 5 mL of solution along the posterior border and medial surface of the sternocleidomastoid muscle ( Fig. 46.15 ). It is possible to block the accessory nerve with this injection, resulting in temporary ipsilateral trapezius muscle paralysis. Deep cervical plexus blocks also are possible but have been associated with a higher incidence of respiratory complications.
The brachial plexus is derived from the anterior primary rami of the fifth, sixth, seventh, and eighth cervical nerves and the first thoracic nerve, with variable contributions from the fourth cervical and second thoracic nerves. After leaving their intervertebral foramina, these nerves course anterolaterally and inferiorly to lie between the anterior and middle scalene muscles, which arise from the anterior and posterior tubercles of the cervical vertebra, respectively. The anterior scalene muscle passes caudally and laterally to insert into the scalene tubercle of the first rib; the middle scalene muscle inserts on the first rib posterior to the subclavian artery, which passes between these two scalene muscles along the subclavian groove. The prevertebral fascia invests the anterior and middle scalene muscles, fusing laterally to enclose the brachial plexus in a fascial sheath.
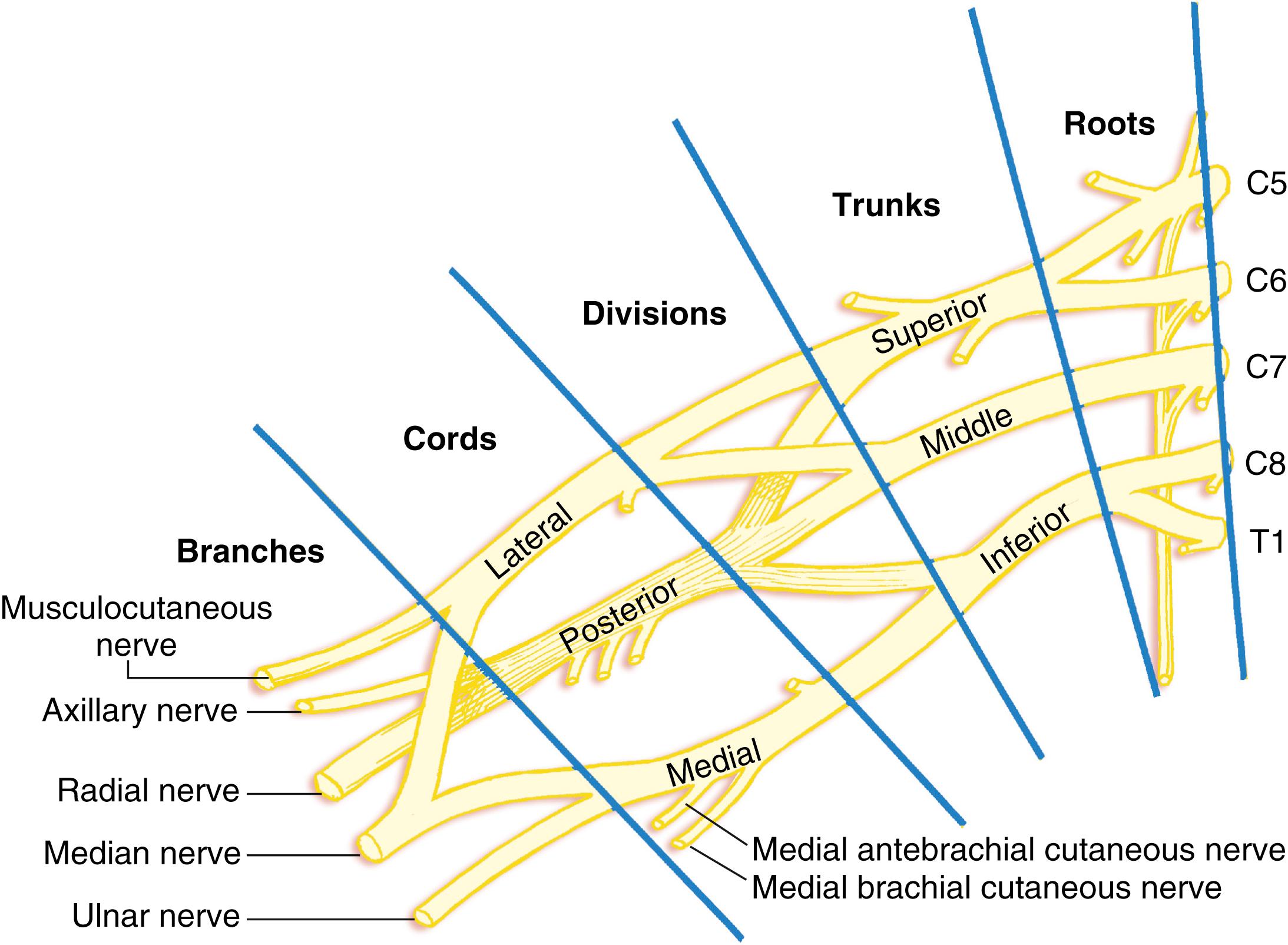
Between the scalene muscles, these nerve roots unite to form three trunks, which emerge from the interscalene space to lie cephaloposterior to the subclavian artery as it courses along the upper surface of the first rib. The superior (C5 and C6), middle (C7), and inferior (C8 and T1) trunks are arranged accordingly and are not in a strict horizontal formation, as often depicted. At the lateral edge of the first rib, each trunk forms anterior and posterior divisions that pass posterior to the midportion of the clavicle to enter the axilla. Within the axilla, these divisions form the lateral, posterior, and medial cords, named for their relationship with the second part of the axillary artery. The superior divisions from the superior and middle trunks form the lateral cord, the inferior divisions from all three trunks form the posterior cord, and the anterior division of the inferior trunk continues as the medial cord.
At the lateral border of the pectoralis minor, the three cords divide into the peripheral nerves of the upper extremity. The lateral cord gives rise to the lateral head of the median nerve and the musculocutaneous nerve; the medial cord gives rise to the medial head of the median nerve, as well as the ulnar, the medial antebrachial, and the medial brachial cutaneous nerves; and the posterior cord divides into the axillary and radial nerves ( Fig. 46.16 ).
Aside from the branches from the cords that form the peripheral nerves as described, several branches arise from the roots of the brachial plexus providing motor innervation to the rhomboid muscles (C5), the subclavian muscles (C5 and C6), and the serratus anterior muscle (C5, C6, and C7). The suprascapular nerve arises from C5 and C6, supplies the muscles of the dorsal aspect of the scapula, and makes a significant contribution to the sensory supply of the shoulder joint.
Sensory distributions of the cervical roots and the peripheral nerves are shown in Fig. 46.17 .
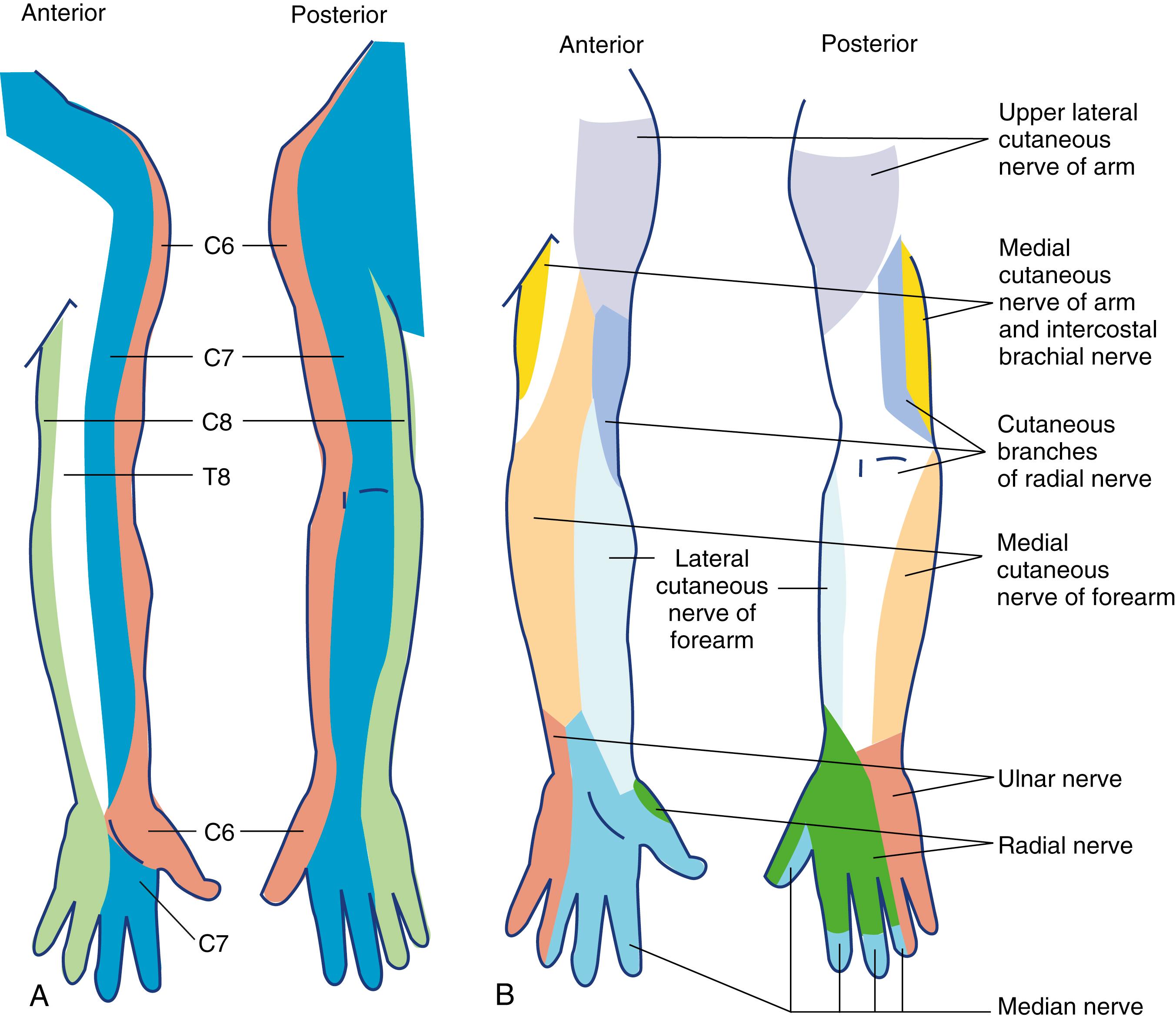
Branches arising from the cervical roots were traditionally blocked with the interscalene approach to the brachial plexus. However, interscalene block has a well-documented risk of concomitant phrenic nerve block. This can result in symptomatic hemi-diaphragmatic paralysis and respiratory compromise, especially among those patients with obesity or moderate to severe obstructive pulmonary disease. Recent evidence suggests diaphragm paresis may be avoidable with more distal “lung-sparing” block techniques that target the terminal nerves supplying the shoulder joint.
By design, brachial plexus blocks above the clavicle (e.g., interscalene and supraclavicular blocks) primarily target local anesthetic placement near the ventral rami, trunks, and divisions. Blocks below the clavicle (e.g., infraclavicular and axillary blocks) primarily target the cords and terminal nerves.
The interscalene block is often chosen for regional anesthesia technique of the shoulder in those patients without significant pulmonary disease. Blockade occurs at the level of the superior and middle trunks of the brachial plexus. Although this approach can be used for forearm and hand surgery, blockade of the inferior trunk (C8 and T1) can be incomplete and may require supplementation of the ulnar nerve for adequate surgical anesthesia in that distribution. Ultrasound guidance for interscalene block reduces the chance of inferior trunk sparing.
Several adjacent anatomic structures can serve as important landmarks for performance of interscalene block. The patient should be in the supine position, with the head turned away from the side to be blocked and the patient’s arm in any comfortable position. The posterior border of the sternocleidomastoid muscle is readily palpated by having the patient briefly lift the head. The interscalene groove can be palpated by rolling the fingers posterolaterally away from this border over the belly of the anterior scalene muscle into the groove ( Fig. 46.18 ). A line is extended laterally from the cricoid cartilage to intersect the interscalene groove, indicating the level of the transverse process of C6. Although the external jugular vein often overlies this point of intersection, it is not a consistent landmark.
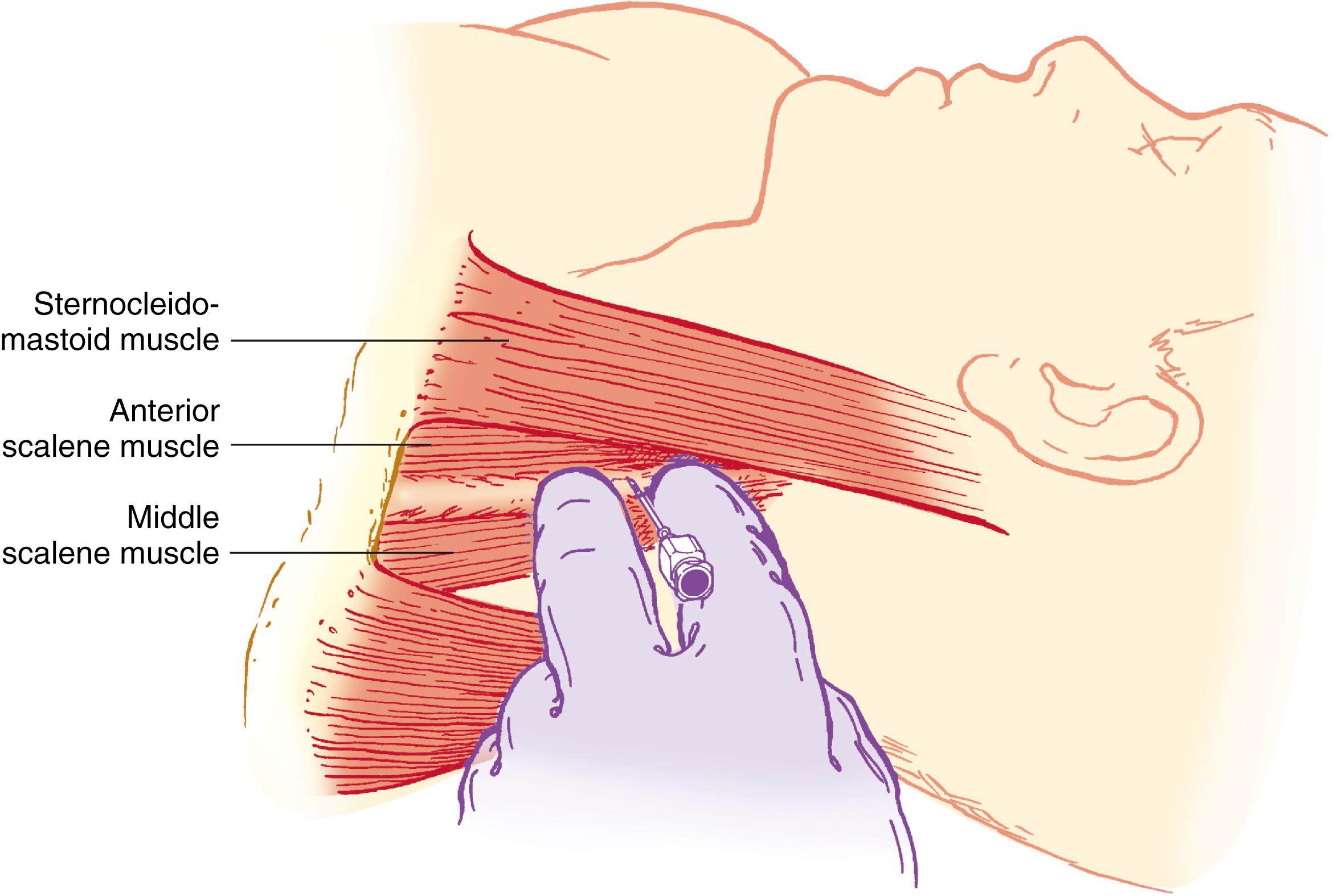
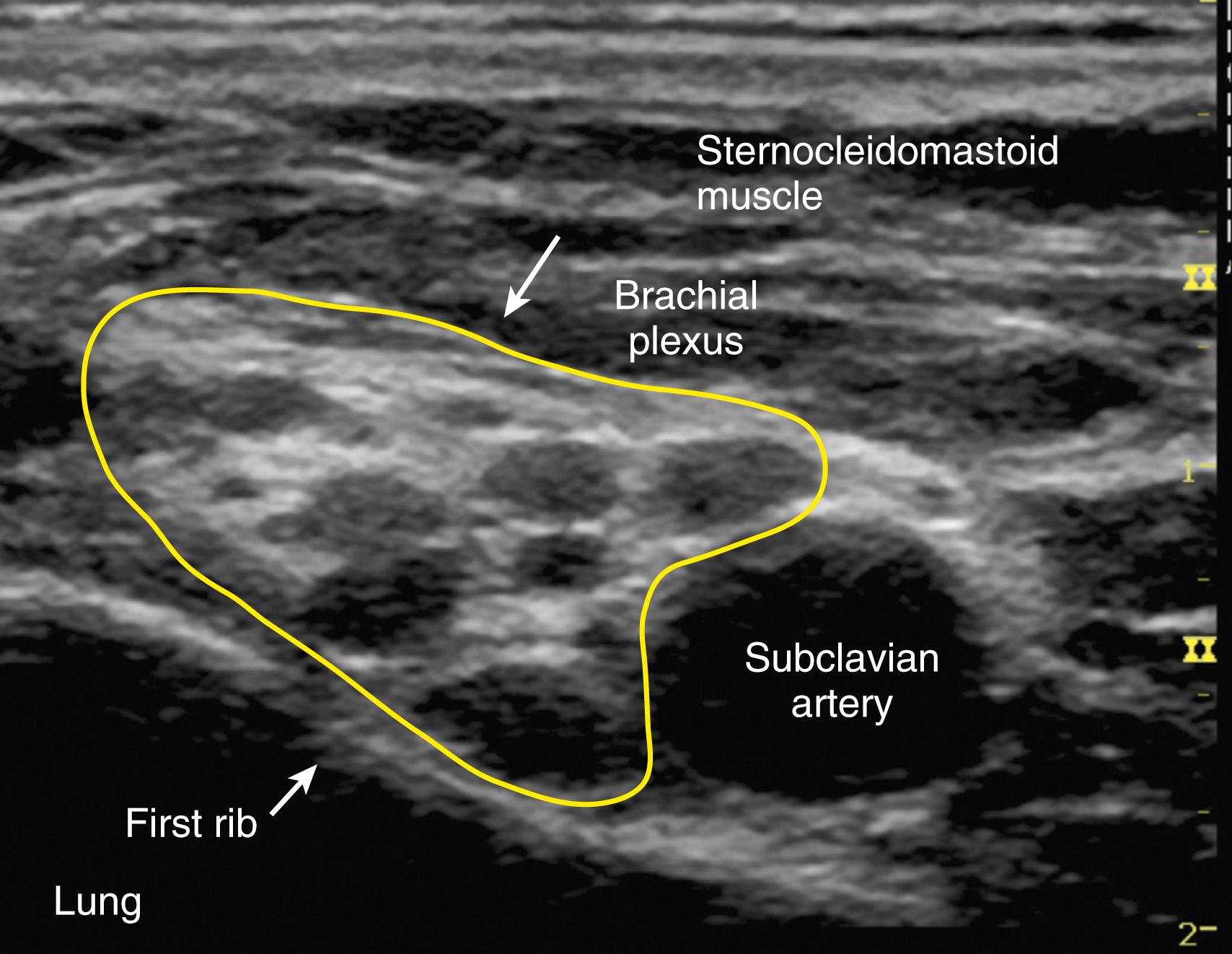
Traditional approaches to the interscalene block include paresthesia or peripheral nerve stimulation technique. However, this block is well suited to the use of ultrasound guidance. It is often easiest to obtain a supraclavicular view of the subclavian artery and brachial plexus ( Fig. 46.19 ) and then trace the plexus up the neck with the ultrasound probe until the plexus trunks are visualized as hypoechoic structures between the anterior and medial scalene muscles (the “stoplight” sign ). The needle can then be advanced with either an in-plane or out-of-plane approach. After negative aspiration, a small test dose is administered, and local anesthetic spread around the brachial plexus confirms appropriate placement of the needle. Volumes as little as 5 mL may be successful and associated with a decreased frequency of diaphragmatic paresis.
At the traditional (C6) level of interscalene block, ipsilateral phrenic nerve block and resultant diaphragmatic paresis are inevitable. This effect probably results from the proximity of the phrenic nerve at this level and may cause subjective symptoms of dyspnea. Respiratory compromise can occur in patients with severe preexisting respiratory disease or contralateral phrenic nerve dysfunction.
Involvement of the vagus, recurrent laryngeal, and cervical sympathetic nerves is rarely significant if unilateral, but the patient experiencing symptoms related to these side effects may require reassurance. The risk of pneumothorax is small when the needle is correctly placed at the C5 or C6 level because of the distance from the dome of the pleura.
Severe hypotension and bradycardia (i.e., Bezold-Jarisch reflex) can occur in awake, sitting patients undergoing shoulder surgery under an interscalene block. The cause is presumed to be stimulation of intracardiac mechanoreceptors by decreased venous return, producing an abrupt withdrawal of sympathetic tone and enhanced parasympathetic output. This effect results in bradycardia, hypotension, and syncope. The frequency is decreased when prophylactic β-adrenergic blockers are administered.
Epidural and intrathecal injections can occur with this block. The proximity of significant neurovascular structures may increase the risk of serious neurologic complications when interscalene block is performed in heavily sedated or anesthetized patients. Accordingly, interscalene blocks are usually placed under light sedation in adult patients.
Indications for supraclavicular blocks include operations on the elbow, forearm, and hand. Blockade occurs at the distal trunk–proximal division level of the brachial plexus. At this point, the brachial plexus is relatively compact, and a small volume of local anesthetic produces rapid onset of reliable blockade.
The patient is placed in supine position, with the head turned away from the side to be blocked. The arm to be anesthetized is adducted against the side of the body. Similar to interscalene block, traditional approaches to the supraclavicular block include paresthesia or peripheral nerve stimulation. Given the widespread use and availability of ultrasound, this block is now more commonly performed with sonographic guidance. This allows the practitioner to visualize the brachial plexus, subclavian artery, pleura, and first rib. The inherent safety of this technique requires continuous visualization of the needle tip and adjacent anatomic structures during needle advancement.
A high-frequency (15-6 MHz) linear transducer is positioned just proximal to the supraclavicular fossa to obtain a supraclavicular view (see Fig. 46.19 ). The brachial plexus trunks and divisions are clustered vertically over the first rib on the lateral side of the subclavian artery. The first rib acts as a medial barrier to the needle reaching the pleural dome and is short, wide, and flat.
The needle can then be advanced under direct ultrasound guidance using an in-plane approach from lateral to medial. The transducer rests near the clavicle so manipulation can be challenging. Thus advanced skills with needle control are required. After negative aspiration, a small test dose is administered, and local anesthetic spread around the brachial plexus confirms appropriate placement of the needle tip. Volumes as low as 15 to 30 mL may be successful.
The prevalence of pneumothorax after supraclavicular block is 0.5% to 6% and diminishes with increased experience. Importantly, although the use of ultrasound may decrease the incidence of pneumothorax, the risk has not been eliminated. When this occurs, the onset of symptoms is usually delayed, and it can take up to 24 hours to develop. Thus routine chest radiography after the block is not justified. The supraclavicular approach is best avoided when the patient is uncooperative or cannot tolerate any degree of respiratory compromise. Other complications include phrenic nerve block (as high as 40%-60%), Horner’s syndrome, and neuropathy. The presence of phrenic or cervical sympathetic nerve block usually requires only reassurance. Although nerve damage can occur, it is uncommon and usually self-limited.
Suprascapular nerve (SSN) block above the clavicle (anterior approach) is a viable alternative to interscalene block for analgesia of the shoulder region. The advantage of this more peripheral approach is that the chance of concomitant phrenic nerve block is significantly reduced. In addition, if the block is semi-selective then other nerves that contribute articular branches to the shoulder joint (e.g., axillary nerve, lateral pectoral nerve) also can be blocked. The anterior approach to SSN block is more shallow (5-10 mm depth) than the more traditional block of the SSN block at the suprascapular notch (20-40 mm depth). Furthermore, the suprascapular notch has variable morphology and in some subjects this landmark is absent. The SSN is the primary sensory innervation of the shoulder joint and is not blocked with approaches to the brachial plexus below the clavicle. Selective low-volume approaches to SSN block above the clavicle also may be useful for pain medicine and rehabilitation.
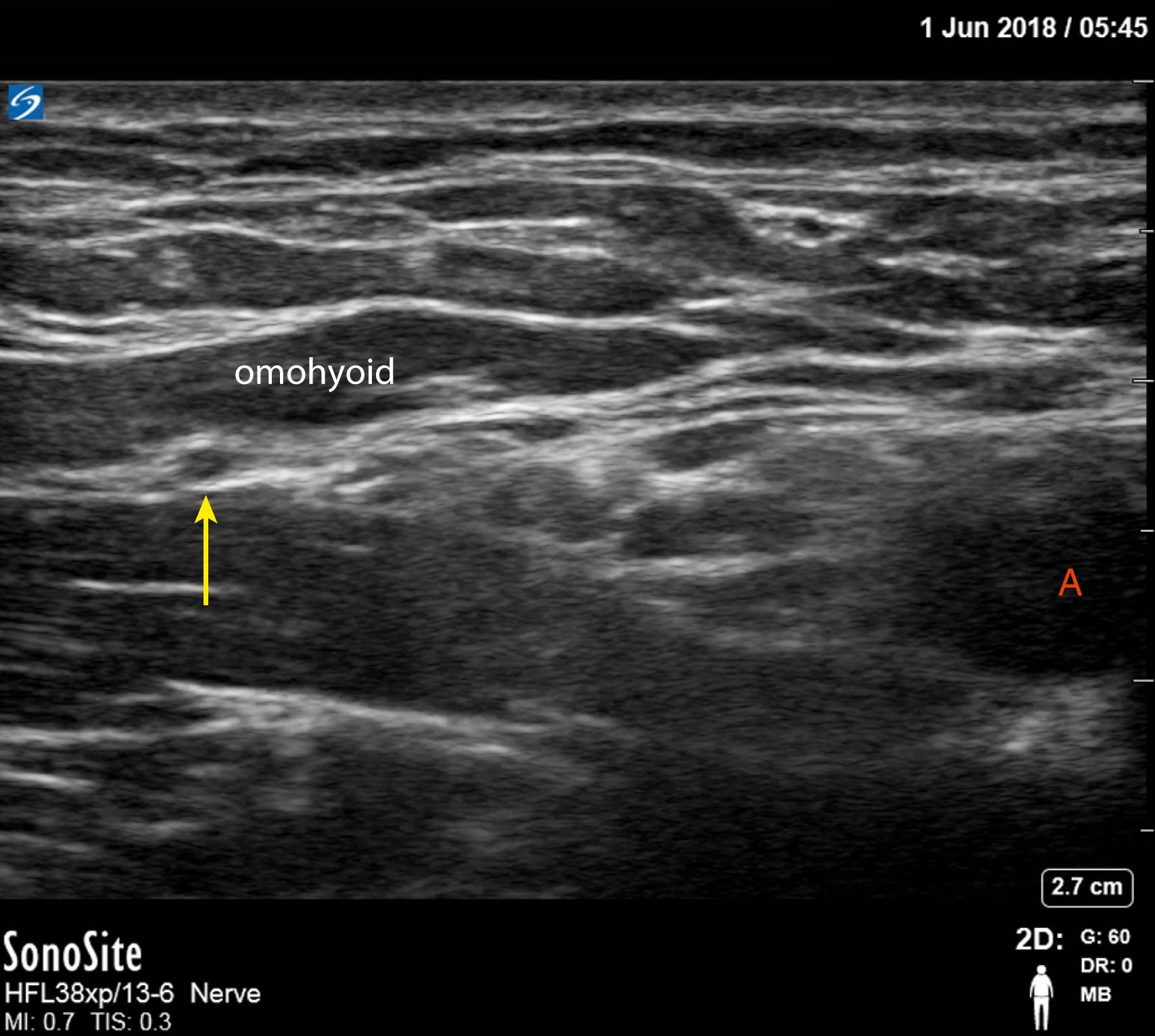
The SSN, a mixed-motor and sensory nerve originating from the superior trunk (C5 and C6 nerve roots and often C4 as well) makes a significant contribution to the sensory supply of the shoulder joint. The SSN root may be accessed from within the posterior cervical triangle of the neck where it passes underneath the omohyoid muscle toward the suprascapular notch. The SSN, unlike the suprascapular vessels that remain superficial, then passes deep to the superior transverse scapular ligament exiting through the scapular foramen into the supraspinous fossa finally providing nerve branches to muscles of the shoulder girdle.
The anterior SSN block is performed in the supine position with head turned to the contralateral side when accessing the nerve within the posterior cervical triangle (similar positioning as the interscalene nerve block). Alternatively, the patient would be in a seated position to access the scapula for a more distal and posterior SSN block. In the seated position, ask the patient to place his/her hand over to the contralateral shoulder (full shoulder adduction) to move the target (nerve) and scapula lateral from the thorax. Ultrasound guidance is the preferred technique, although a landmark-based method with nerve stimulation for neurolocalization is an option.
The anterior SSN block technique has emerged as the preferred lung-sparing block alternative to interscalene nerve block. A high-frequency linear transducer (15-6 MHz) probe is positioned just proximal to the supraclavicular fossa. Under dynamic scanning, the SSN can be visualized as a round hypoechoic structure deep to the inferior belly of the omohyoid muscle and lateral to the superior trunk in the posterior cervical triangle of the neck ( Fig. 46.20 ) Consider tracing the SSN from its origin (nerve root C5) to facilitate identification. The nerve is then traced more posterior-lateral to a distance away from the superior trunk. A 22-gauge, 5-cm needle is most often selected with shallow 2- to 3-cm depths to the target. Through an out-of-plane or in-plane approach approximately 5 to 15 mL of local anesthetic is deposited deep into omohyoid muscle, but shallow to the prevertebral fascia (higher volumes could result in phrenic nerve blockade). Color Doppler use is advised as the superficial cervical artery and the suprascapular artery, also hypoechoic structures, are strong mimickers of the SSN within the posterior cervical triangle. Auyong and colleagues have shown the anterior SSN block technique provides noninferior, yet lung-sparing, analgesia compared to interscalene nerve block without need for additional terminal nerve block supplementation (e.g., axillary or SSN block).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here