Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The approach to perioperative fluid therapy has changed markedly over the last 40 years. This has followed the change in approach by surgeons recognizing that by reducing primary injury and blood loss during surgery the physiological impact for the patient is reduced, resulting in reduction in complications and improved outcomes. The establishment of minimal invasive surgery as a reproducible way of performing complex colorectal resections developed at the same time as the introduction of enhanced recovery pathways in parts of Europe. The synergism between both approaches has led to rapid uncomplicated recovery in a field where only 20 years ago the length of stay was an average of 10–14 days and mortality approached 4%. These figures are now closer to 2–6 days and less than 2% mortality. Ultrashort lengths of stay have also been reported. These guiding principles have now transcended all surgical specialties with published guidelines and national adoption of enhanced recovery pathways in most surgical specialties in many countries. (See https://www.ahrq.gov/hai/tools/enhanced-recovery/index.html ; https://www.gov.uk/government/publications/enhanced-recovery-partnership-programme .)
When open surgery and high blood loss were common, a lot of work around fluid therapy concentrated on maintaining central intravascular volume using normal saline or colloid solutions. This led to a lot of research and debate around the risk/benefit of which type of solution to use during the perioperative period. The medical community has gone full circle using dextrose or normal saline infusions, crystalloids versus colloids, the use of albumin and early use of coagulation factors in blood loss resuscitation.
Modern fluid therapy is just a single piece of the strategy for hemodynamic optimization, which involves manipulating stroke volume and cardiac output, hematocrit, oxygen delivery, and maintenance of arterial tone and venous capacitance and mean arterial pressure (MAP) ( Fig. 16.1 ).
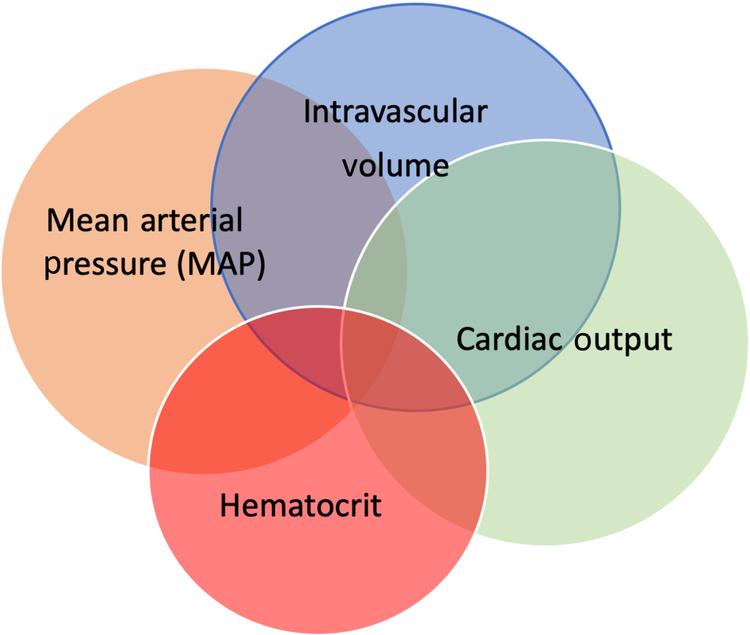
This new concept of Goal Directed Hemodynamic Therapy (GDHT) is focused on maintaining body homeostasis for as much of the perioperative time as possible. Preoperative hemoglobin optimization, avoiding preoperative dehydration, optimizing intravascular volume, cardiac output and MAP using balanced intravenous fluids and low dose vasopressors during physiological derangement and then rapidly restoring normal enteral intake is now a key strategy of modern surgical care.
Enhanced Recovery Pathways have simplified the approach to modern fluid therapy because the time a patient stays on an intravenous fluid infusion is reduced because patients will have early return of enteral fluid intake and enteral food according to the type of surgery. Even patients undergoing bowel resection will have an early diet of protein drinks within 24 hours of surgery, which has a profound effect on the metabolic response to surgical injury. The principles of fluid therapy within Enhanced Recovery Pathways are outlined in Fig. 16.2 . Key principles are to avoid fluid shifts, individualizing fluid, hematocrit, and MAP, then rapid normalization to enteral intake postoperatively. Patients should arrive hydrated prior to induction of anesthesia. Oral fluid loading and avoidance of bowel preparation if not needed are key strategies. There are many stressors that modify perioperative fluid requirements outlined in Fig. 16.3 . The surgeon has a major role in minimizing injury and blood loss, which directly affect fluid shifts and activate the inflammatory response. Although blood loss and fluid shifts during surgery are well recognized factors contributing to blood volume, the effects of drugs, anesthetic agents, intermittent positive pressure ventilation, pneumoperitoneum, and position of the patient all affect venous and arterial tone with a resulting effect on effective intravascular volume. When considering the dynamic interaction of all these factors, it becomes apparent that in order to maintain optimal intravascular volume at all times there needs to be constant clinical vigilance by the anesthesiologist to maintain optimal intravascular volume, venous and arterial tone to maintain MAP. Postoperatively the patient should return to a normal oral fluid intake as soon as possible, which avoids the iatrogenic intravenous fluid and sodium loading. Tolerance to oliguria is also key because it is a normal response to surgical injury with 0.3 mL/kg per hour averaged over 2–4 hours a good starting point to assess adequacy of renal function; however, extra diligence is necessary in higher risk patients.
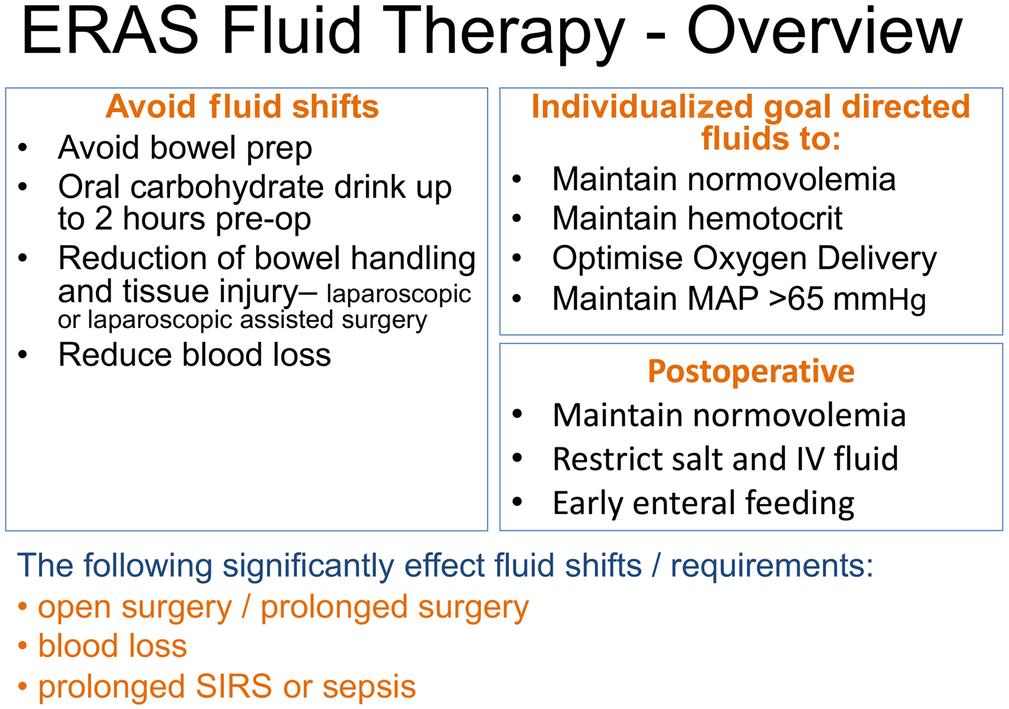
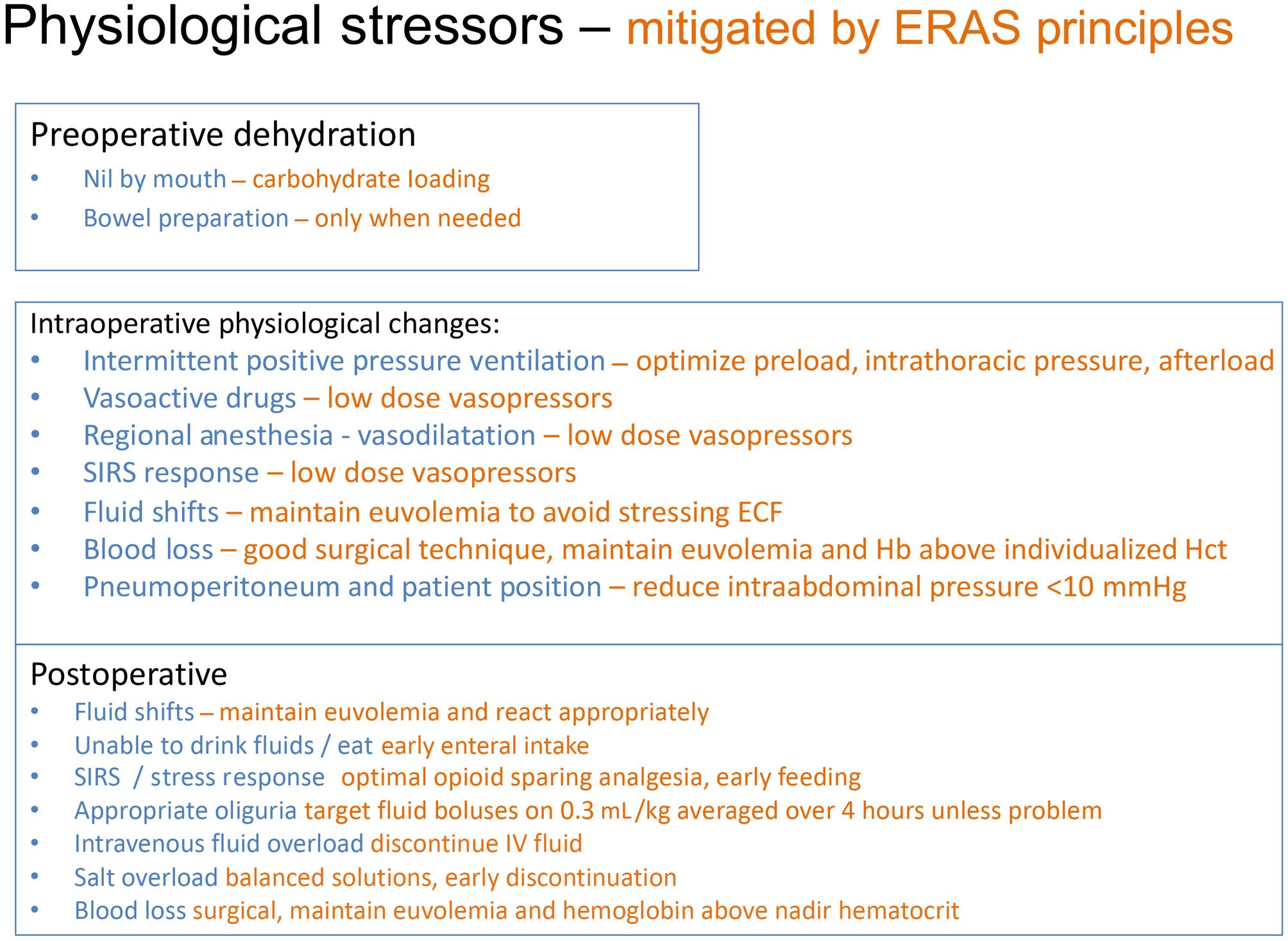
Enhanced Recovery Pathways also address many of the issues facing patients during the perioperative period.
In a traditional surgical pathway, patients arrive dehydrated because of prolonged NPO status (and in some cases exacerbated by bowel preparation). Patients are thus likely to have splanchnic hypoperfusion after induction of anesthesia and commencement of intermittent positive pressure ventilation. The prolonged use of intravenous fluids postoperatively can lead to salt and fluid overload, pulmonary edema, ileus, and other complications. An ERAS pathway starts with the patient hydrated and a goal directed hemodynamic approach keeps the patient intravascularly normal. Intravenous fluids are reduced and stopped as soon as the patient can take oral intake avoiding the fluid and salt overload leading to edema, ileus and complications. These two pathways are illustrated in Fig. 16.4 .
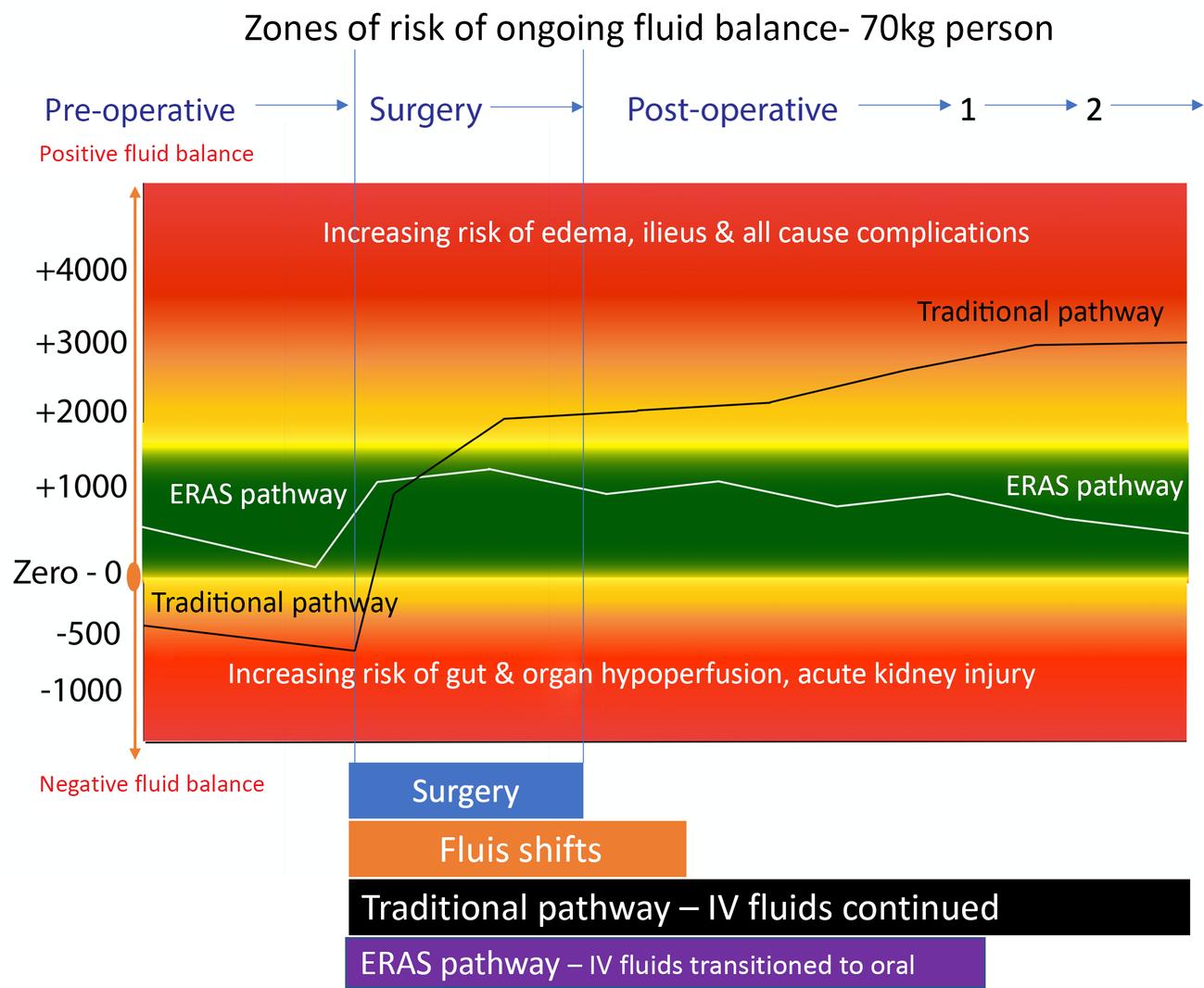
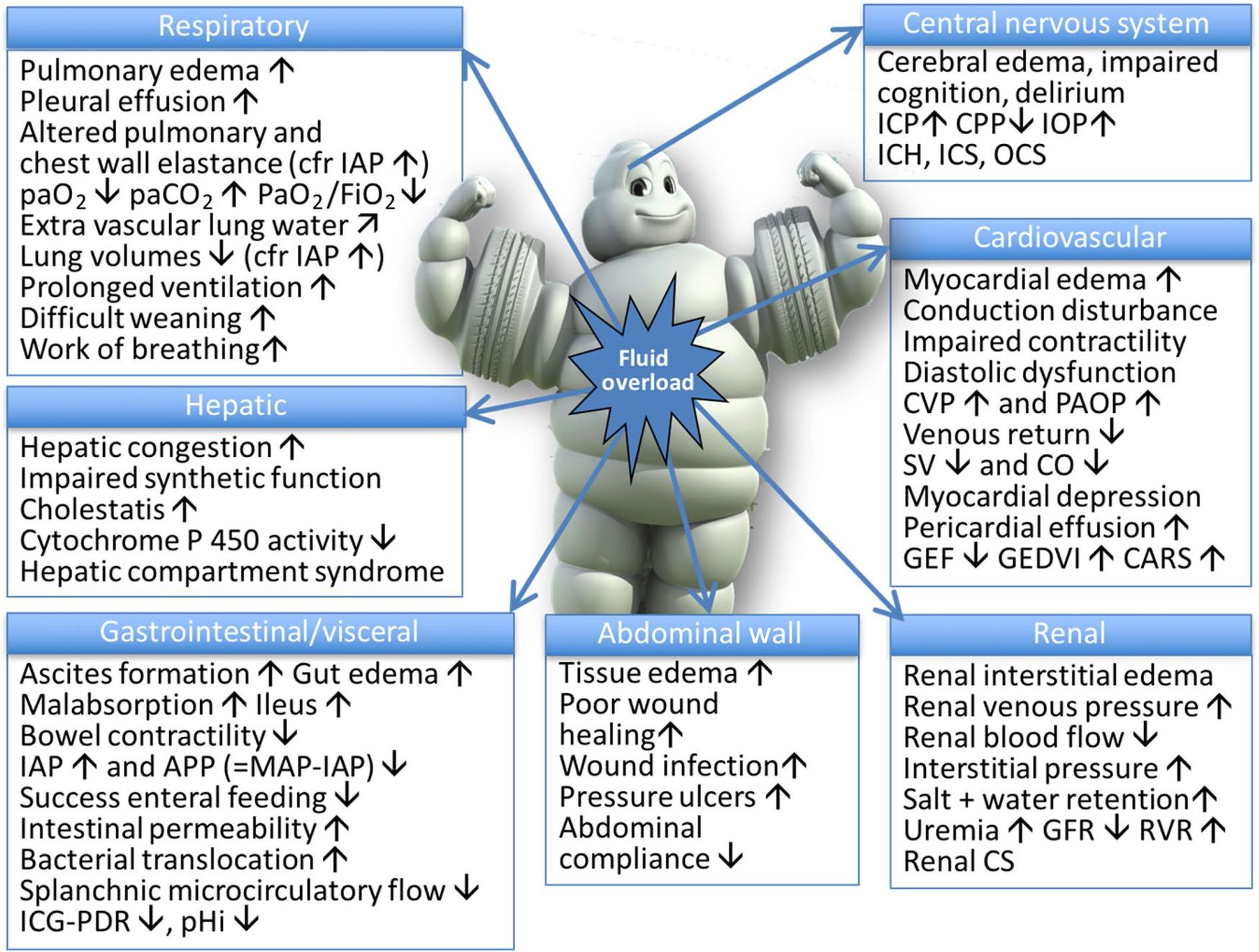
Although it is important to maintain intravascular volume, the indiscriminate use of intravenous fluids leads to positive fluid balance, which is associated with a multitude of downstream complications as summarized in Fig. 16.5 by Malbrain et al. Brandstrup et al. first demonstrated this relationship and the terms restrictive and liberal fluid therapy have been used since then. However, there has been no strict definition of what this actually means in practice and in modern randomized controlled trials (RCTs) such as the ‘Relief Study’, the liberal fluid regime was similar to Brandstrup’s restrictive arm. There are now recognized ‘zones of risk’ in postoperative fluid balance. The Relief Study compared two arms with resultant fluid balance on POD 1 of 3 mL/kg versus 18 mL/kg POD 1. There was minimal difference but increased risk of AKI in the 3 mL/kg group. Therefore, it is now considered a positive fluid balance of 5–20 mL/kg (1–1.5 L on ideal body weight) is optimal in elective major abdominal surgery whilst avoiding excess of 30–40 mL/kg (2–3 L on ideal body weight).
One of the biggest dogmas in medicine is still nil by mouth from midnight for patients undergoing surgery. The introduction of carbohydrate fluid loading in patients undergoing colorectal surgery within enhanced recovery pathways showed the process of oral fluid loading up to 2 hours before surgery to be safe. This has now been accepted as the new standard in medical practice in all National Anesthesiology Society’s recommendations for oral intake before surgery. Six hours for solid food remains standard because of the risk of aspiration of particulate matter.
Carbohydrate loading was introduced with the concept of avoiding dehydration prior to major surgery as well as giving a carbohydrate load large enough and close enough to surgery to improve insulin sensitivity as the first step of improving the metabolic response to surgery. The effects of carbohydrate loading in abdominal surgical patients are reduction in postoperative insulin resistance, reduced catabolism, and improved muscle mass and strength.
The type of carbohydrates used in solution are maltodextrins, which do not cause a delay in gastric emptying. Carbohydrate loading is normally given the night before and the morning of surgery; the night before dose is 800 mL in volume containing 100 g of carbohydrates. The dose of the night before ensures the patient is well hydrated and the extracellular fluid volume is normalized. In our modern society, patients are often dehydrated because of their lifestyle, caffeine intake, and alcohol intake, therefore the night before dose aims to make sure the patient is properly hydrated prior to surgery. The morning dose is 400 mL in volume with 50 g of carbohydrate—enough to improve insulin sensitivity. The benefits of carbohydrate loading are less evidence-based in patients who have early return of gut function such as elective orthopedic surgery. This is probably because the patients do not have bowel manipulation and have early predictable postoperative feeding. Therefore in patients undergoing nonabdominal surgery, the key goal is to ensure the patient is not dehydrated prior to induction of anesthesia. Proprietary oral carbohydrate drinks come as either cartons or powder to be mixed with water.
Purposes of oral carbohydrate drinks:
To avoid dehydration prior to surgery and improve patient well-being
To allow safe general anesthesia without the increased risk of aspiration of gastric contents; the solution is composed of maltodextrins, which empty readily and predictably from the stomach.
To give a large enough carbohydrate load (50 g) to allow the patient to be in a metabolically fed state prior to surgery (800 mL; 100 g night before, 400 mL; 50 g morning of surgery). The optimal metabolic effect occurs when given 2–4 hours before surgery.
Improved compliance with patients drinking a suitable volume of liquid to have the desired physiological effect.
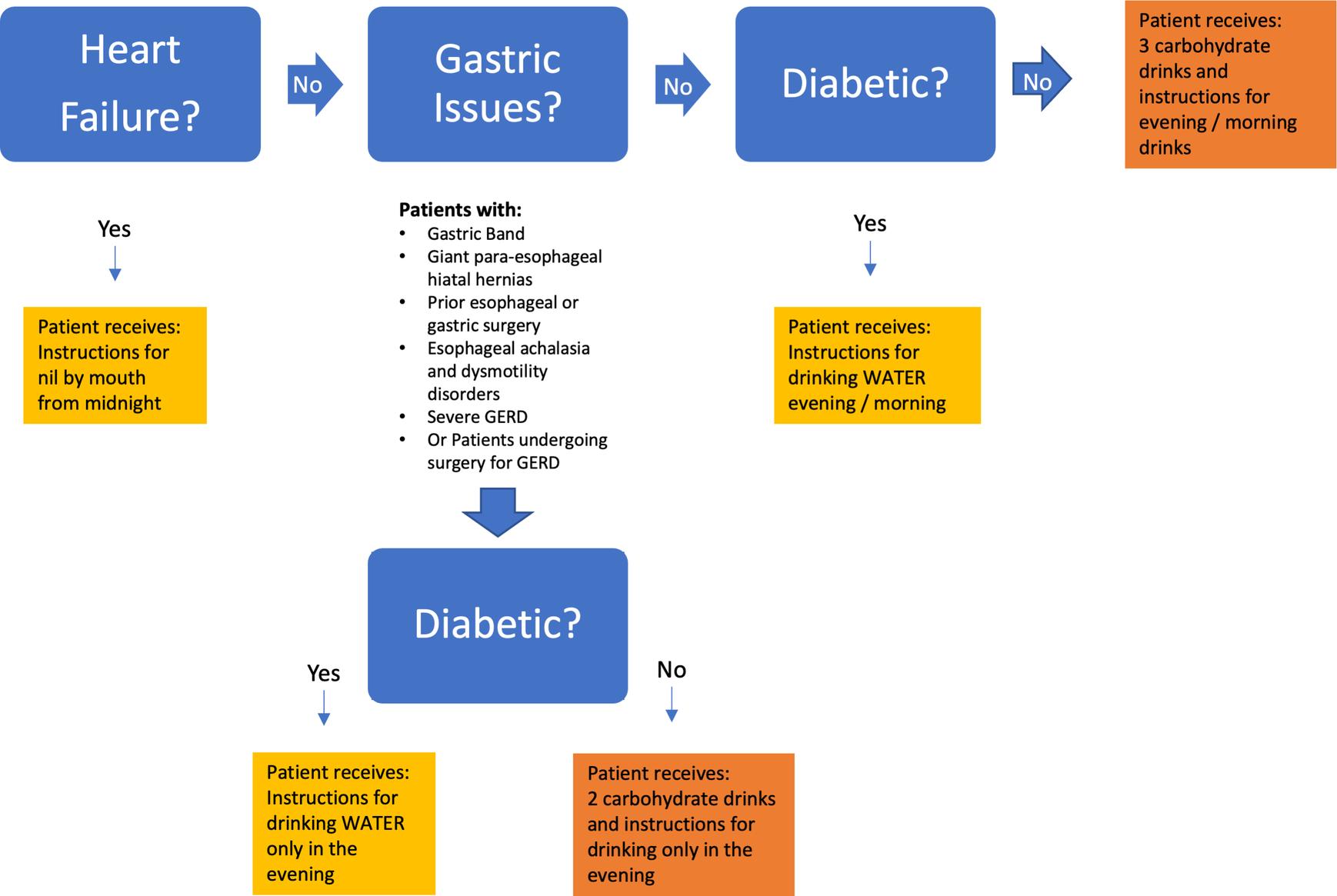
Although the majority of patients can take carbohydrate drinks without any issues, there are certain patient groups who have a high risk of aspiration or have had gastric surgery where there is minimal evidence base. The safe administration of oral carbohydrate drinks according to patient and surgery type is provided in Fig. 16.6 .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here