Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The pericardium can be conceptualized as a “balloon” with the heart being a fist pushed into it. The visceral pericardium adheres to the heart itself and is separated from the parietal pericardium by a space, the pericardial cavity. The entire structure is housed in the fibrous pericardium. The serosal space normally holds a small collection of approximately 50 mL of fluid that is transudative with low protein content. It also contains prostaglandins that modulate cardiac reflexes and coronary tone. The fluid within the pericardial space is in dynamic equilibrium with the blood serum. Because there are many smaller sinuses and recesses in the pericardial space (around the atria, the superior vena cava [SVC], the great vessels, the pulmonary veins [PVs], and the inferior vena cava [IVC]), 250 mL is the approximate limit of fluid; the normal pericardial reserve volume is exceeded with more than this amount, and the intrapericardial pressure rises.
The fibrous pericardium is essentially made of collagen and short elastic fibrils. Superiorly, it is continuous with the adventitia of the great vessels and attaches to the central tendon of the diaphragm inferiorly. Anteriorly, it attaches to the sternum via ligaments. Laterally, it is contiguous with the parietal lung pleura, and posteriorly, it is contiguous with the bronchi, esophagus, and descending aorta. The phrenic nerves and pericardiophrenic vessels track between the fibrous pericardium and mediastinal pleura anteriorly. Arterial supply is via branches of the internal mammary arteries and descending aorta. Venous drainage is into the superior intercostal veins and internal thoracic veins to the innominate vein.
The serosal pericardium is characterized by the surfaces of the “balloon” and is made of a single layer of mesothelium that covers the heart as epicardium and that lines the fibrous pericardium to form the parietal pericardial structure. Beneath the visceral pericardium and the myocardium are variable amounts of adipose tissue that are particularly prominent in the atrioventricular (AV) and interventricular grooves, and the acute border of the right ventricle (RV). The normal thickness of the pericardium is approximately 1.5 mm, depending upon the imaging method used to assess it.
The pericardium provides a thin tissue barrier between the heart and the surrounding structures; it exerts constant pressure on the heart, which affects the thin structures (the atria and the RV) more than the thicker walled left ventricle (LV). Resting diastolic pressures within the heart are directly affected by this pericardial constraint, and the restraint differs among the chambers. For instance, pericardial removal results in greater dilatation of the RV than of the LV. Pericardial influence on the thinner walled RV and right atrium (RA) accounts for approximately 50% of their normal diastolic pressures.
Normal intrapericardial pressures range from −6 to −3 mm Hg, which directly reflects the intrapleural pressures. The pressure differential between the pericardium and the cardiac chambers (transmural pressure) is approximately 3 mm Hg. The pericardium is much stiffer than cardiac muscle, and once the pericardial reserve volume is exceeded, the pressure–volume curve of the normal pericardium rises steeply. The pericardium has little effect on ventricular systole; however, interactions between the right- and left-sided cardiac chambers are enhanced by the pericardium because atrial and ventricular septal movements are independent of pericardial constraint.
Intracardiac pressures reflect the contraction and relaxation of individual cardiac structures and the changes imparted to them by the pleural and pericardial pressures ( Fig. 57.1 ). Changes in pleural or pericardial pressure primarily affect the intracardiac diastolic pressure. With inspiration, the intrapleural pressures drop and the abdominal cavity pressure increases. Blood flow to the right side of the heart increases, whereas blood return to the left side of the heart decreases slightly. The fall in the intrapleural pressures also causes an increase in the transmural aortic root pressure, which slightly increases impedance to the LV ejection. The reverse occurs during expiration. In the normal setting, the respiratory changes are reflected in the intrapericardial and intracardiac pressures, with inspiration lowering the measured RA pressures and the systolic RV pressure more than the left-sided heart pressures.
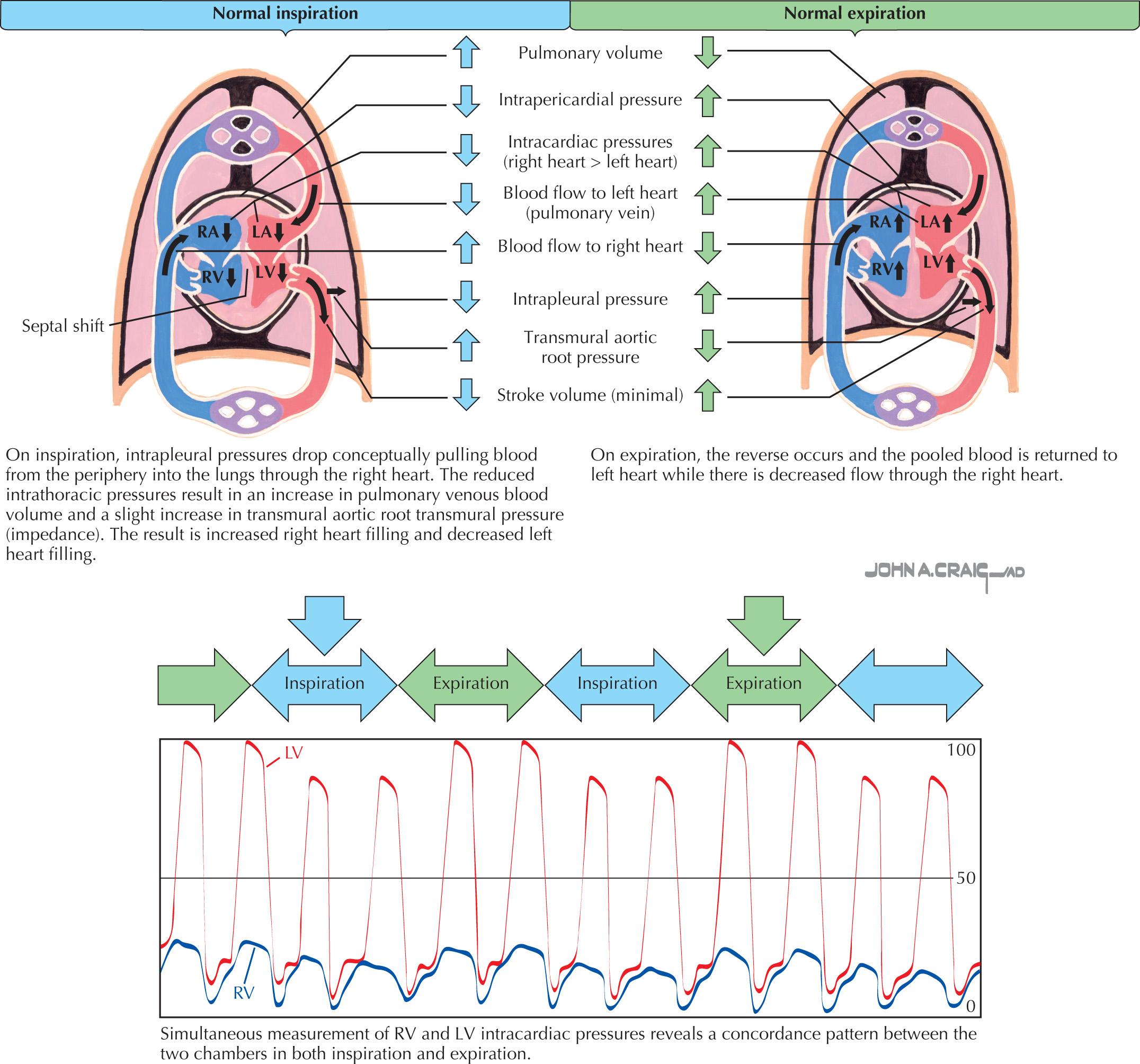
The slight reduction in LV filling and the slightly increased impedance to LV ejection with inspiration normally produce a modest decline in the LV stroke volume and slightly lower systolic aortic pulse pressures with inspiration. Marked swings in the intrapleural pressures from negative during inspiration to positive during expiration (which occur in asthma or severe chronic obstructive lung disease) exaggerate these changes in LV filling and may produce a paradoxical pulse (>10 mm Hg decline in the aortic systolic pressure with inspiration) purely from the associated pleural pressure swings. Such a paradoxical pulse related to marked swings in the intrapleural pressure must be differentiated from a similar phenomenon due to pericardial tamponade.
The normal atrial and ventricular waveforms are shown in Fig. 57.2 . With atrial contraction, the atrial pressures rise ( a wave). With the onset of ventricular contraction, the AV valves bulge toward the atria, and a small c wave results (the c wave is evident on hemodynamic tracings but usually is not visible to the examiner observing the jugular venous pulsations). As ventricular contraction continues, the AV annulus is pulled into the ventricular cavity, and the atria undergo diastole, enlarging the atria and decreasing the atrial pressure (represented by the x descent). Passive filling of the atria during ventricular systole produces a slow rise in the atrial pressure (the v wave) until the AV valves reopen at the peak of the v wave; the pressure then abruptly falls as the ventricles begin active relaxation. Passive filling of the ventricles then follows until atrial contraction recurs, and the cycle repeats. Ventricular diastole can be conceptually divided into an initial active phase (a brief period when the ventricle fills about halfway) and a later, passive filling phase. The nadir, or lowest, diastolic pressure during ventricular diastole occurs during the early active relaxation phase (suction effect).
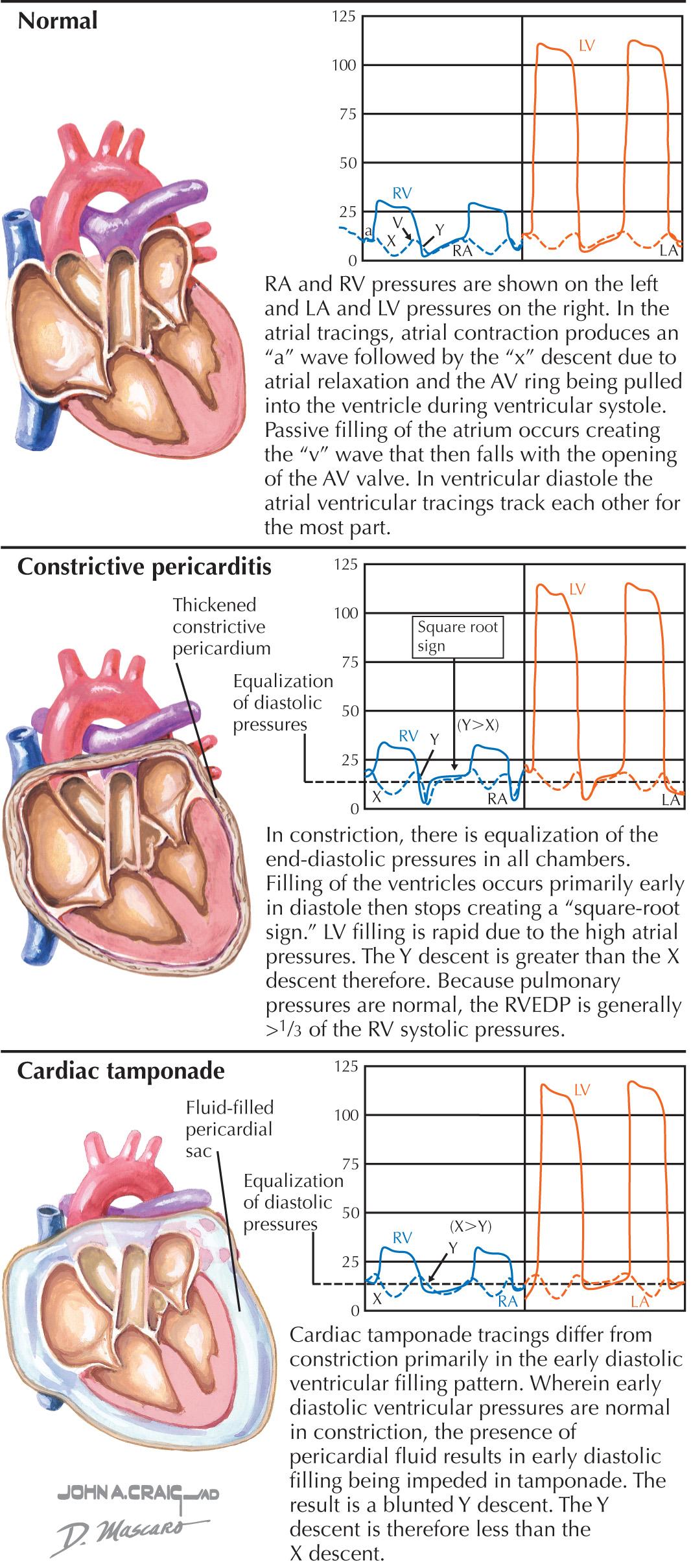
Constrictive pericarditis and pericardial tamponade alter the normal intracardiac pressures in distinctive ways. Some of the hemodynamic abnormalities, such as ventricular interdependence, are seen in both processes, whereas other findings, such as the magnitude of the y descent, are unique to each (see Fig. 57.2 ).
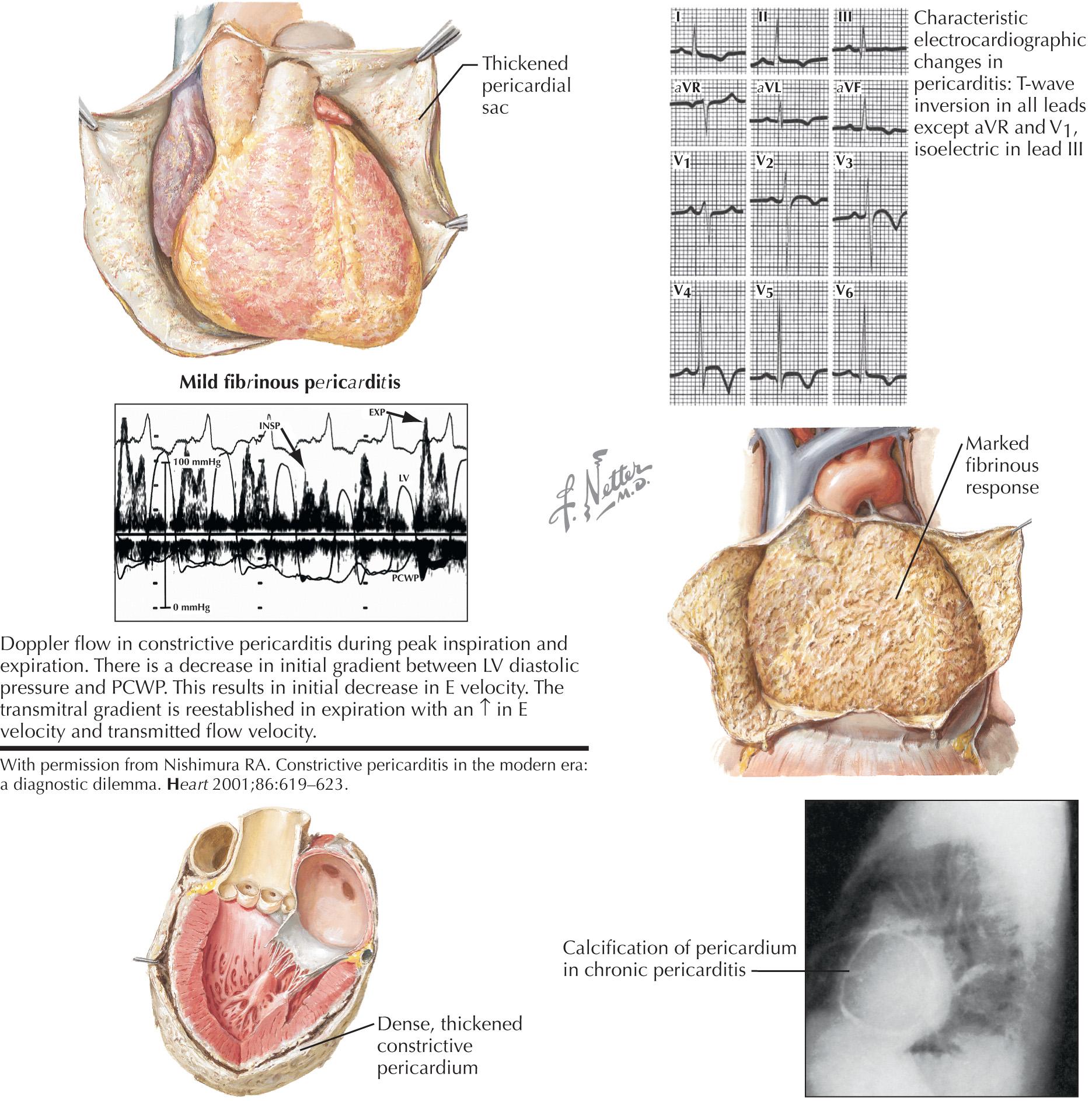
Constrictive pericarditis was recognized at autopsy in the 19th century and was described as a “chronic fibrous callous thickening of the wall of the pericardial sac that is so contracted that the normal diastolic filling of the heart is prevented” ( Fig. 57.3 ). The variable severity of the constrictive process results in a spectrum of hemodynamic changes. Recent guidelines suggest that three separate syndromes should be identified: transient constriction, effusive–constrictive pericarditis, and chronic constrictive pericarditis. Transient constriction occurs at times during the acute phase of pericarditis and resolves within weeks after antiinflammatory therapy. Effusive–constrictive pericarditis is uncommon in developed countries, with tuberculosis being the most common cause. The epicardial layer results in the constriction and is usually not thickened. Effusive–constrictive pericarditis is confirmed when there is remaining evidence of constrictive pericarditis after the associated pericardial effusion is drained. Surgically, it requires sharp dissection of small visceral pericardial fragments until ventricular motion is improved. In chronic constrictive pericarditis, both the visceral and parietal pericardial layers are typically fused.
In constrictive pericarditis, the diastolic pressures in the atria are elevated due to the restriction of ventricular diastolic inflow. As opposed to a restrictive myocardial process, myocardial relaxation is typically normal. In constriction, the elevated atrial pressures and the normal early LV filling result in a rapid decrease in atrial pressure after the AV valves open and are responsible for the rapid y descent (see Fig. 57.2 ). However, the constraint imposed by the pericardium as the ventricle rapidly fills results in the sudden halting of this rapid early flow and an abrupt rise in diastolic pressure, which produces the “square root sign” or “dip and plateau” in the pressure tracings. The x descent is generally minimally affected; thus, the atrial y descent is greater than the x descent in constrictive pericardial disease. RV systolic and pulmonary systolic pressures are usually normal or only mildly elevated, with the result that the RV end-diastolic pressure (EDP) tends to be greater than one-third of the RV systolic pressure. Because the ventricles are confined by the pericardium at the end of ventricular diastole, the RV and LV EDPs equalize.
The normal respiratory changes in cardiac flow are also altered in constriction. With inspiration, the lower intrathoracic pressure is transmitted to the PVs but not to the left atrium (LA) due to the rigid pericardium; this reduces the gradient between the two and reduces the inflow into the LA and the LV. However, the normal increased flow to the right heart with inspiration still occurs from the IVC to the RA because the abdomen is not exposed to the lower intrathoracic pressures. However, flow in the exposed SVC to the RA fails to increase for the same reason as flow to the LA. Therefore the normal inspiratory fall in the RA pressures may not occur, and the pressure in the RA may not fall or may even rise (Kussmaul sign). This is thought of as the inspiratory “pulling” of blood into the lung through a stiff rigid vessel (the RA and the RV). Kussmaul sign is not specific for pericardial constriction because it can also be observed in acute or chronic RV failure, RV infarction, RV volume overload, and restrictive cardiomyopathy. In these latter conditions, the constrictive physiology is due more to RV volume overload (reaching the limit of RV chamber capacity) than to constriction from the pericardium.
Because the atrial and ventricular septa are unaffected by the pericardial process, changes in atrial and ventricular filling on the right side of the heart can affect left-sided filling (ventricular interdependence). Demonstration of ventricular interdependence is generally accepted as a fundamental requirement for diagnosing constrictive pericarditis. In constriction, as the negative intrapleural pressure draws blood through the RV with inspiration, the increase in RV filling into the confined RV results in an increase in RV systolic pressure, whereas a normal decrease in LV systolic pressure occurs. This phenomenon is illustrated in Fig. 57.4 and is referred to ventricular discordance. For further quantification, normally with inspiration, the area within the RV pressure tracing should fall in a compensatory manner compared with that of the simultaneous LV pressure tracing area. During inspiration in constriction, the RV receives more flow than the LV, and the RV pressure tracing area increases, whereas the LV pressure tracing area decreases. The simultaneous LV/RV pressure tracing area ratio thus becomes smaller with inspiration compared with the LV/RV ratio with expiration. When this inspiratory ratio is divided into the expiratory ratio, a number >1 would therefore be expected if constriction is present. When the Mayo Clinic found this expiratory ratio/inspiratory ratio (index) to be >1.1, their report revealed that the sensitivity was 97%, with a specificity of 100%, a positive predictive value of 100%, and a negative predictive value of 95% for constrictive pericarditis.
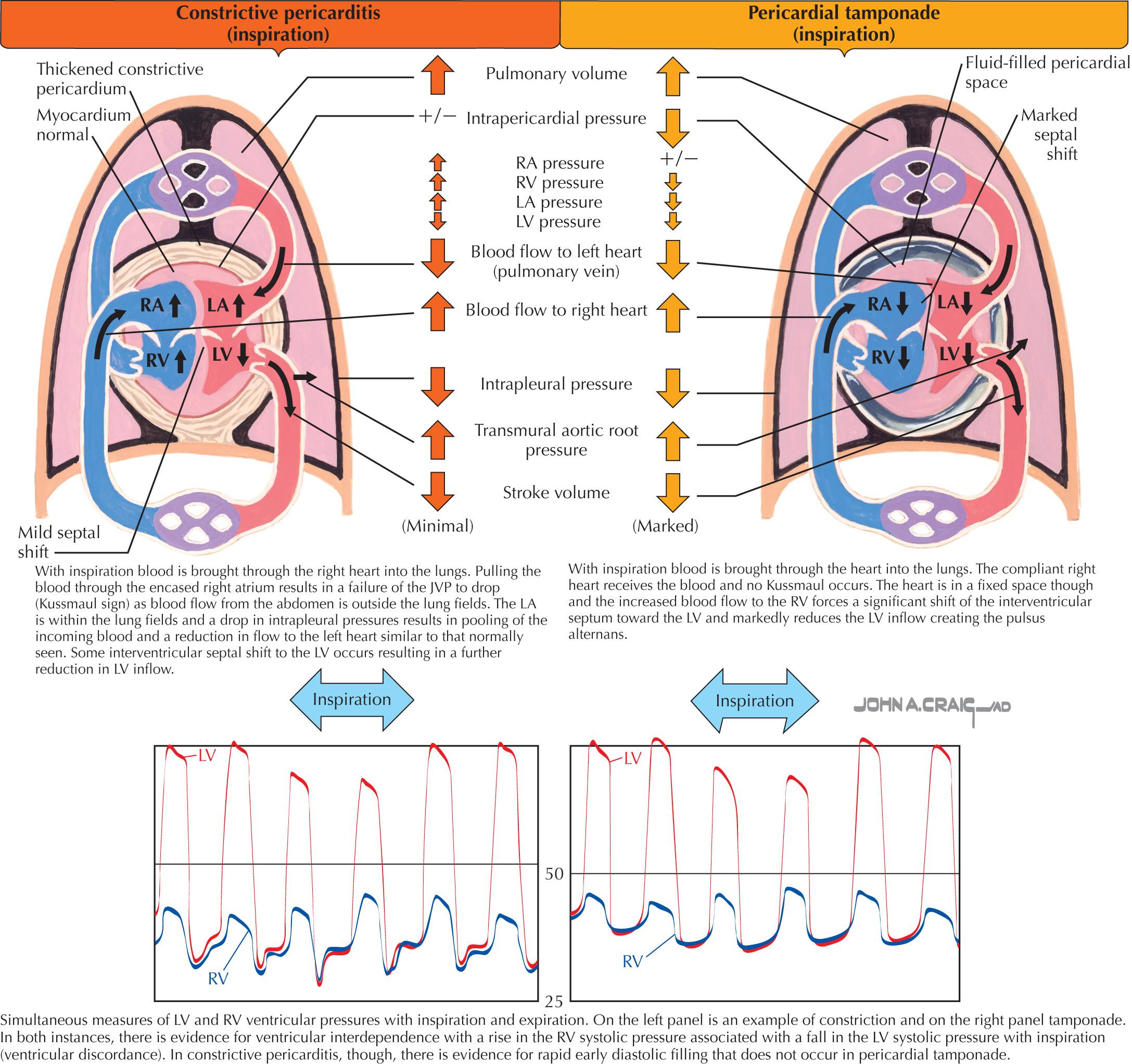
Another hemodynamic observation considered reduced LA filling with inspiration in a patient with constriction by noting a lower pulmonary capillary wedge pressure to LV diastolic gradient in inspiration and a greater pulmonary capillary wedge pressure/LV diastolic gradient in expiration (see Fig. 57.3 ).
Pericardial constriction may be subtle, whereas significant constriction presents as primarily right-sided heart failure with normal LV systolic function and clear lungs. A history of antecedent pericarditis, pericarditis induced by drug use, uremia, cardiac surgery, or thoracic radiation (which may also be a contributing factor in restriction) may be a clue. There is usually evidence of venous congestion, pedal edema, ascites (often out of proportion to peripheral edema), fatigue, dyspnea, and low cardiac output. Tachycardia occurs to compensate. Atrial arrhythmias are common. Jugular venous distention is universal, and a positive Kussmaul response is expected. Sharp, rapid x and y descents can be seen in the jugular venous pulsations at bedside by careful observation. Because the jugular veins may be so distended that they are invisible when the patient is reclining, patients should be examined in an upright position. To time the pulse waveforms, the opposite carotid pulse should be felt—the x descent in the jugular venous pulse (JVP) occurs during ventricular systole (the positive carotid impulse). The rapid y descent should then be observed immediately after the carotid impulse. The JVP thus looks like two prominent positive waves at the beginning of the carotid impulse and at the end of the carotid impulse. This is sometimes described as a “W” in the JVP. Precordial palpation may be normal, or the apex may even retract with systole. The rapid filling of the ventricles may produce a loud filling sound (pericardial knock) on auscultation, although this is less common now than in the past. The liver is often enlarged, and ascites is often the prominent examination feature. A paradoxical pulse is not usually demonstrated unless associated lung disease or concurrent pericardial tamponade exists.
As discussed in Chapter 56 , although they are often helpful, the ECG and chest radiograph should not be relied on to diagnose either pericardial constriction or pericardial tamponade, or to distinguish the two. In pericardial constriction, the ECG is frequently abnormal, with occasional low voltage and abnormal T waves being common (see Fig. 57.3 ). Interatrial block demonstrated by a wide P wave is common. An RV strain pattern may present, together with right-axis deviation. In chronic pericardial constriction, myocardial calcification and fibrosis can affect coronary perfusion and the conduction system. Stress tests in patients with pericardial constriction can produce a false-positive result, with ECG changes due to ischemia caused by myocardial calcification and fibrosis rather than typical coronary artery disease. Atrial arrhythmias, especially fibrillation, are common. The chest x-ray reveals a normal size heart with clear lung fields. Pericardial calcification (that spares the LV apex) may be present (see Fig. 57.3 ).
Distinguishing features between constrictive pericarditis and restrictive cardiomyopathy can be found in Chapter 31 . Increased pericardial thickness and calcium may be noted on echocardiography but are often difficult to discern. Some correlates of constrictive hemodynamics include diastolic flattening of the LV posterior wall and abrupt posterior motion of the interventricular septum (septal bounce) in early diastole. LV systolic function is generally normal or near-normal. Wall motion abnormalities are seen at times, especially if there is myocardial involvement.
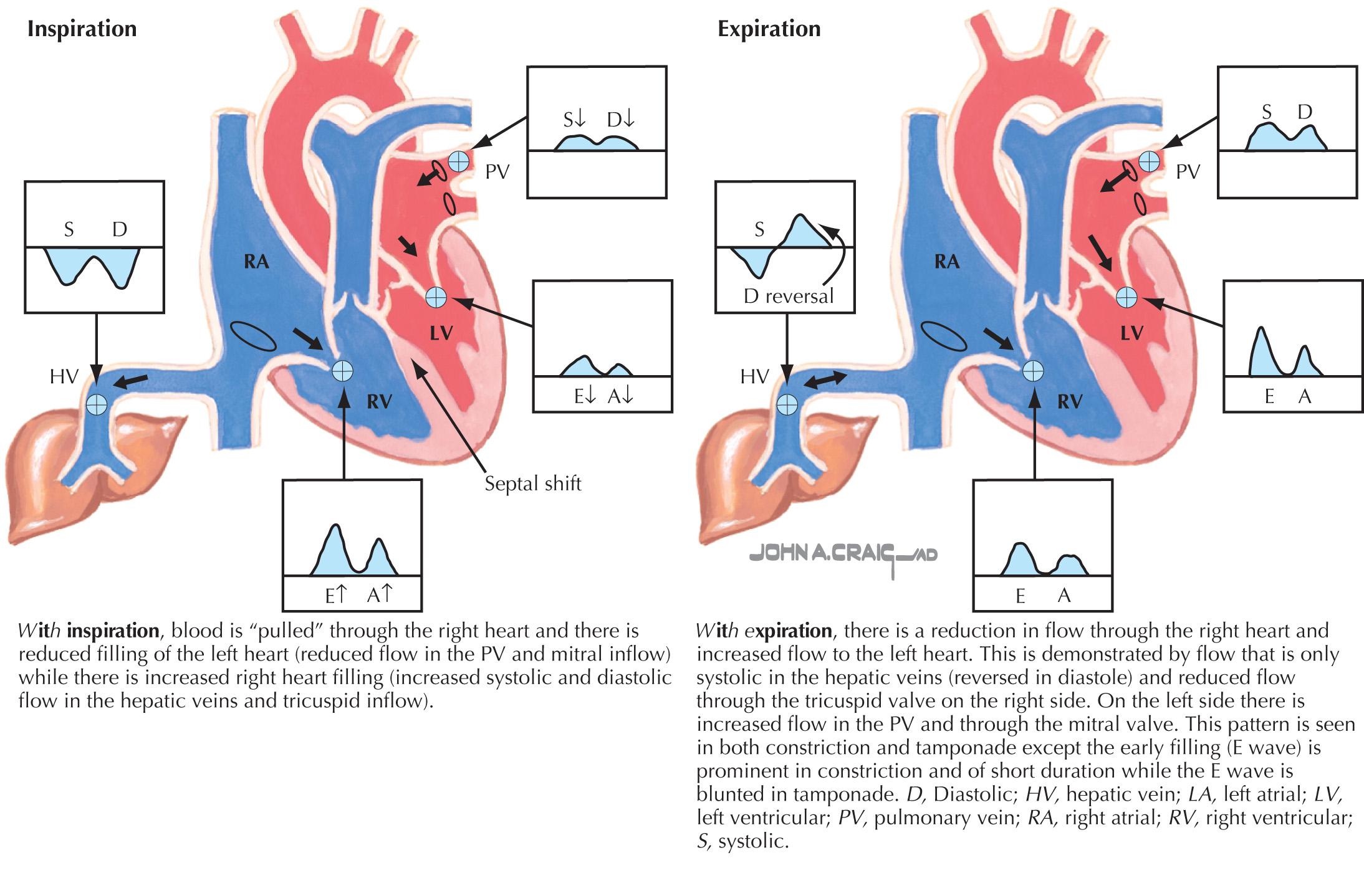
Doppler flow is important in establishing the diagnosis. Because the ventricle fills almost entirely during the first third of diastole, the early filling (E wave) is usually high and has a shortened deceleration time (<160 ms). The atrial filling wave is usually reduced. Normally, with inspiration, the LV minimal pressure and the LA pressures fall equally, and no change is noted in the Doppler mitral inflow velocities. Reflecting the hemodynamics in constriction, the reduced inspiratory LV flow is highlighted by observing a >25% decrease in the peak mitral E-wave inflow velocity in the first beat of inspiration (see Fig. 57.3 ). At the same time, the tricuspid velocity usually increases >40%. The ventricular interdependence can be further documented by examining the hepatic vein (or SVC), the tricuspid and mitral inflows, and the PV inflow patterns with expiration and inspiration ( Fig. 57.5 ). With inspiration , the hepatic systolic (S) and diastolic (D) waves, together with the tricuspid inflow E and A waves, increase, whereas the mitral E and A waves decrease, together with the pulmonary S and D waves. A “w” pattern is seen in the hepatic flow. Up to one in five patients with constriction may not reveal classic interdependence on echo-Doppler due to high LA pressures, and maneuvers to decrease preload (e.g., sitting or head-up tilt) may unmask the changes. Rapid inflow during early diastole is also responsible for the commonly observed septal bounce.
Tissue Doppler echocardiography measures myocardial motion. Because myocardial relaxation is preserved in constrictive pericarditis, the early relaxation observed on tissue Doppler velocity patterns (Ea) is normal. If it is abnormal, then a primary myocardial problem is more likely to be present. For example, if the Ea is >7 cm/s, then that is consistent with constriction, whereas an Ea of <7 cm/s is more indicative of myocardial restriction. With severe constriction, although there is tethering of the lateral mitral annulus to the thickened pericardium, “annulus reversus” may occur when the lateral annular e′ velocity (normally less than the medial annulus e′) becomes greater. This observation has been shown to normalize after pericardiectomy.
A method of speckle tracking of B-mode echoes allows for global assessment of stress and strain (deformation) of the myocardium. When speckle tracking has been performed, constrictive pericarditis appears to have constrained circumferential deformation, whereas restrictive cardiomyopathy exhibits attenuation in the longitudinal direction.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here