Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
As fetal echocardiography has advanced over the past several decades, our understanding of the natural history of congenital heart disease in utero has progressed. For select lesions that evolve in utero and lead to significant morbidity and/or mortality by the time of birth, relatively simple anatomic modifications may alter the natural history and improve prognosis. This understanding, along with improvements in interventional obstetric and catheterization techniques, has led to minimally invasive percutaneous fetal cardiac interventions. Since the first report of fetal cardiac intervention by Maxwell et al. in London in 1991, the field has developed considerably. However, fetal cardiac intervention is not curative and represents the first step toward a management strategy that must be pursued in the postnatal setting.
This chapter focuses on three forms of structural congenital heart disease for which fetal cardiac intervention is performed: severe aortic stenosis (AS) with evolving hypoplastic left heart syndrome (HLHS); pulmonary atresia with intact ventricular septum (PA/IVS) and evolving hypoplastic right heart syndrome (HRHS); and established HLHS with intact or highly restrictive atrial septum (IAS). Fetal aortic and pulmonary valvuloplasty are performed for severe AS with evolving HLHS and PA/IVS with HRHS, respectively, to promote a biventricular circulation. The goal is to prevent the life-long morbidity and mortality that accompanies staged reconstruction for functionally univentricular heart (see also Chapters 71 and 73 ). In contrast, fetal atrial septoplasty (perforation/balloon dilation), often with atrial septal stent placement, is performed for established HLHS with IAS or highly restrictive atrial septum to improve survival of an otherwise nearly lethal disease. Staged reconstruction remains necessary for such patients postnatally.
Patient selection for fetal cardiac intervention technical aspects of the procedures, and postnatal outcomes and management are discussed. Maternal considerations of fetal cardiac intervention have been central to the development of the field, with no major adverse events reported to date. Although maternal issues remain paramount to consider as new therapies evolve, they are beyond the scope of this chapter.
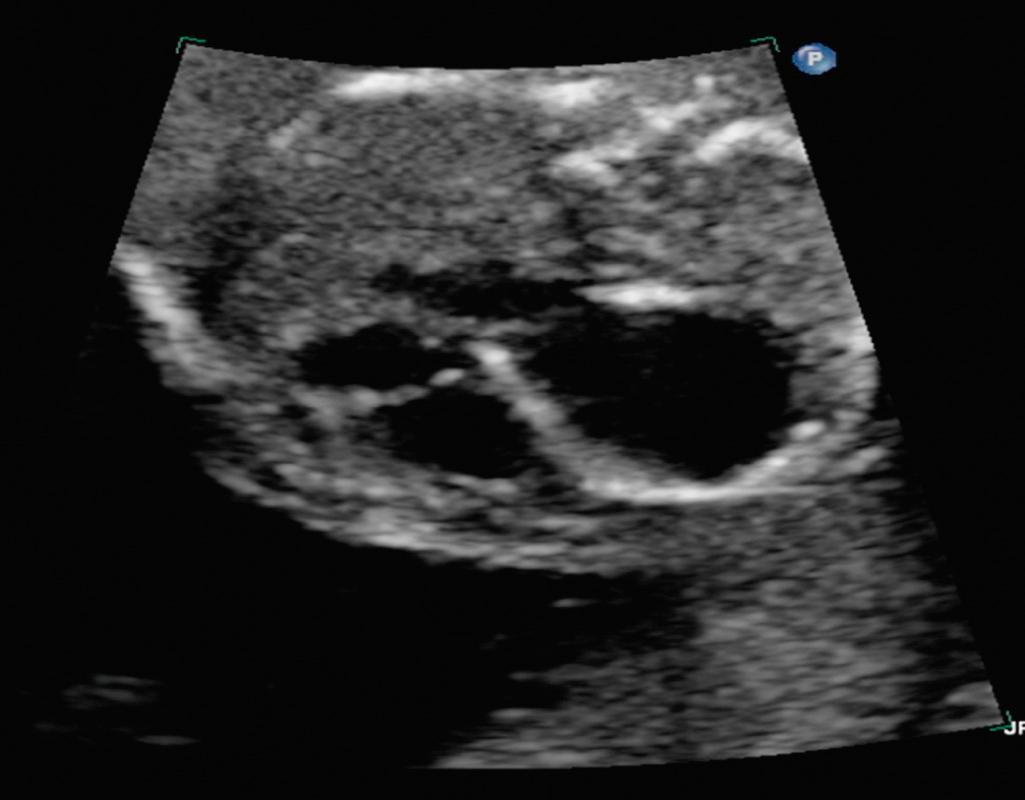
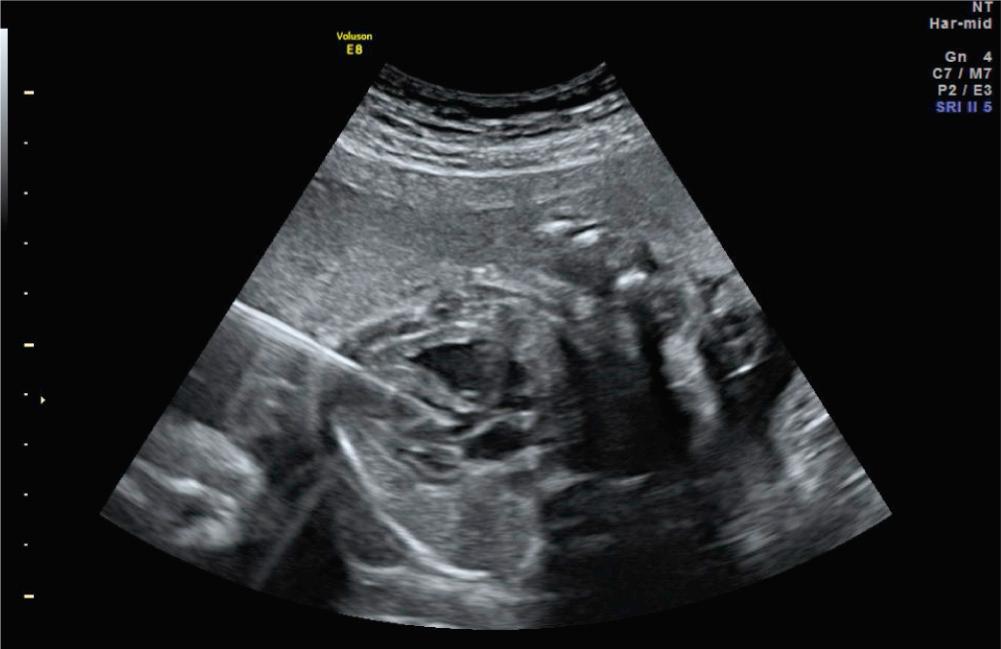
Fetal aortic valvuloplasty for severe midgestation AS with evolving HLHS is the most commonly performed fetal cardiac intervention. Although early surgical survival has improved for infants with HLHS, staged univentricular palliation to a Fontan circulation carries significant life-long morbidity and mortality. A subset of patients with HLHS have a common pathophysiologic etiology in utero; namely, severe valvar AS with left ventricular (LV) dilation and dysfunction. Natural history studies have demonstrated that severe AS in the midgestation fetus initially leads to LV dilation as the ventricle attempts to overcome significant afterload ( Fig. 10.1 ). As gestation progresses, the LV becomes dysfunctional and ultimately growth arrest of left-sided structures ensues. By the time of birth, the left side of the heart is incapable of supporting the systemic circulation, resulting in HLHS.
The first attempt at fetal aortic valvuloplasty was reported by Maxwell et al. in 1991. Over the next decade, multiple centers attempted to perform the procedure; however, due to selection of severe cases in the third trimester and technical difficulties, the experience was largely unsuccessful. In 2000, investigators at Boston Children's Hospital and Brigham and Women's Hospital embarked on a program to perform fetal aortic valvuloplasty among second trimester fetuses that met specific physiologic criteria of fetal AS with evolving HLHS. With technical modifications to the procedure, greater success was able to be achieved. The experience has since been replicated at other centers worldwide.
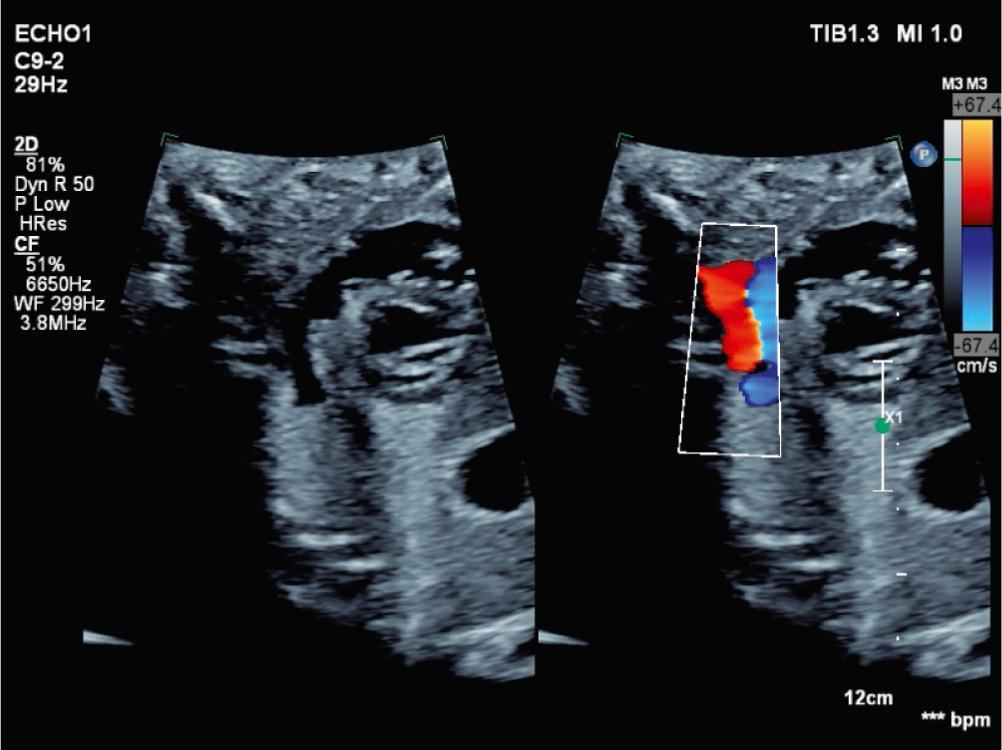
Makikallio and colleagues outlined specific pathophysiologic features of fetuses with severe midgestation AS that reliably predicted evolution to HLHS. These features included LV systolic dysfunction, retrograde flow in the transverse aortic arch ( Fig. 10.2 ), monophasic mitral inflow, and left-to-right flow across the foramen ovale, which have since been validated in a distinct cohort. The goal of fetal intervention for this disease is to relieve the severe AS that triggers these hemodynamic alterations in the second trimester, thereby avoiding evolution to HLHS and enabling a biventricular circulation postnatally.
When selecting candidates for fetal aortic valvuloplasty, the first consideration is whether the fetus has evidence of severe valvar AS and pathophysiologic features that are highly suggestive of evolution to HLHS. These features are the hemodynamic findings outlined previously, namely, LV systolic dysfunction, retrograde flow in the transverse aortic arch, monophasic mitral inflow, and left-to-right flow across the foramen ovale. This is particularly important because a small subset of patients with midgestation AS may not present with these features and may achieve a biventricular circulation with postnatal aortic valvuloplasty alone.
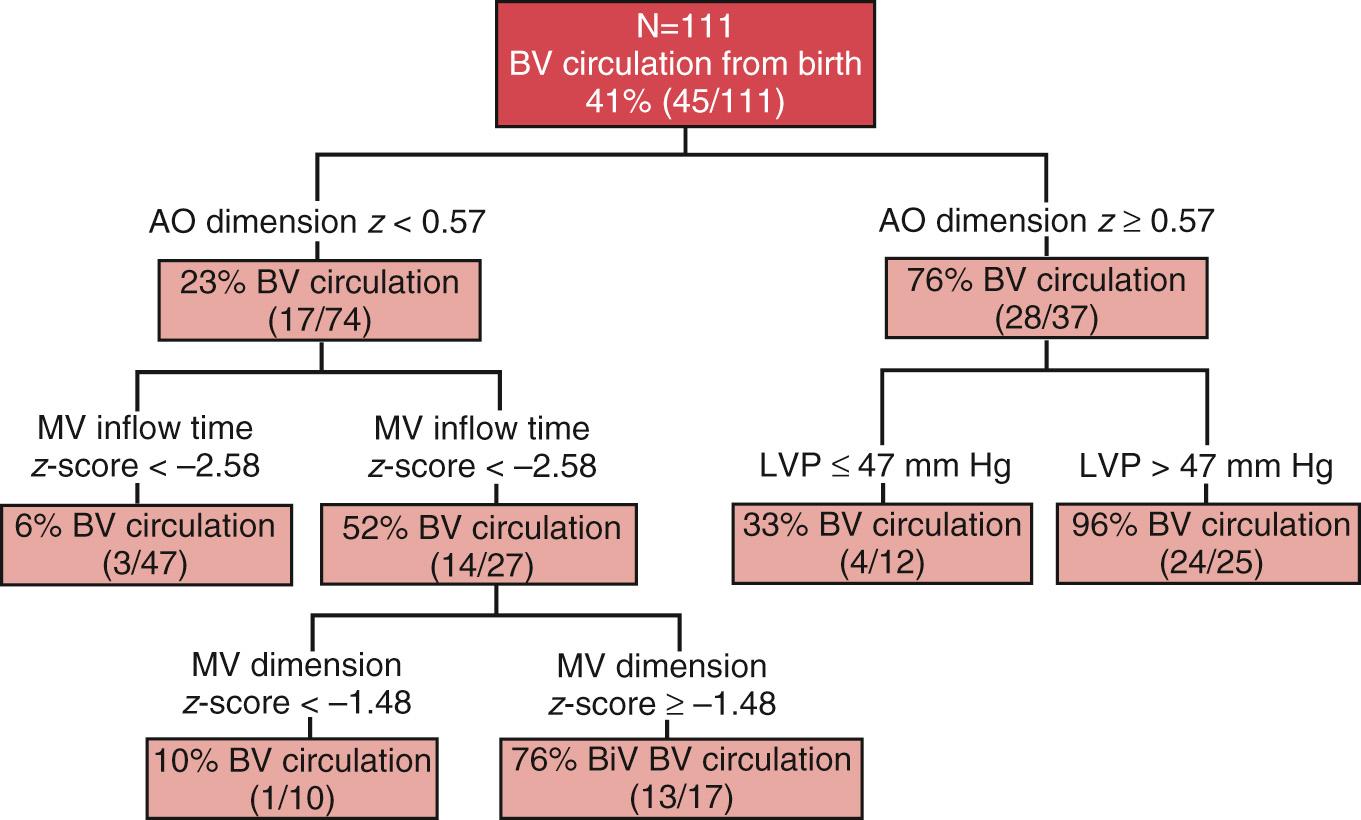
The second consideration is whether the left heart is salvageable. Some fetuses present with disease that is too far advanced. For example, if the LV is already hypoplastic at the time of presentation, then recovery is unlikely. Based on fetal aortic valvuloplasty performed in 70 fetuses, McElhinney et al. devised a scoring system to help predict which patients would be likely to have a biventricular circulation postnatally. The scoring system included LV long-axis z -score greater than 0, LV short-axis z -score greater than 0, aortic annulus z -score greater than −3.5, mitral valve annulus z -score greater than −2, and AS (or mitral regurgitation) maximum systolic gradient ≥20 mm Hg. The presence of four or more of these features had 100% sensitivity and 38% positive predictive value for identifying patients with a biventricular outcome. In addition, certain patients may present with a normal or dilated LV, but extensive scar tissue, or endocardial fibroelastosis is present. Endocardial fibroelastosis is associated with abnormal LV geometry and more severe diastolic dysfunction, which also limits the ability to salvage the LV. For this reason, the Boston group recently reanalyzed their experience of 123 fetuses. In addition to the size of left-heart structures (particularly the ascending aorta and mitral valve) and the estimate of LV pressure, the mitral valve inflow time, which is a marker of diastolic dysfunction, was retained in the model to estimate likelihood of biventricular outcome from birth ( Fig. 10.3 ).
Finally, and perhaps most importantly, the mother must be a suitable candidate for fetal cardiac intervention. Since the fetus may or may not be of a viable gestational age and carries a significant anomaly, fetal cardiac intervention should not be performed unless there is minimal risk to the mother. All mothers should be thoroughly assessed by maternal-fetal medicine specialists prior to offering fetal cardiac intervention.
Because the safety of the mother is paramount, fetal cardiac intervention should be performed in an obstetric operating room with maternal epidural anesthesia. Once the epidural is placed, the fetal position is determined, and version is often performed to move the fetus into a favorable position. As seen in Fig. 10.4 , the fetus is ideally positioned such that the left chest is anterior and there is a straightforward pathway to the LV outflow tract. Once the fetus is optimally positioned, an intramuscular injection of analgesic (fentanyl), paralytic agent (pancuronium), and atropine is given.
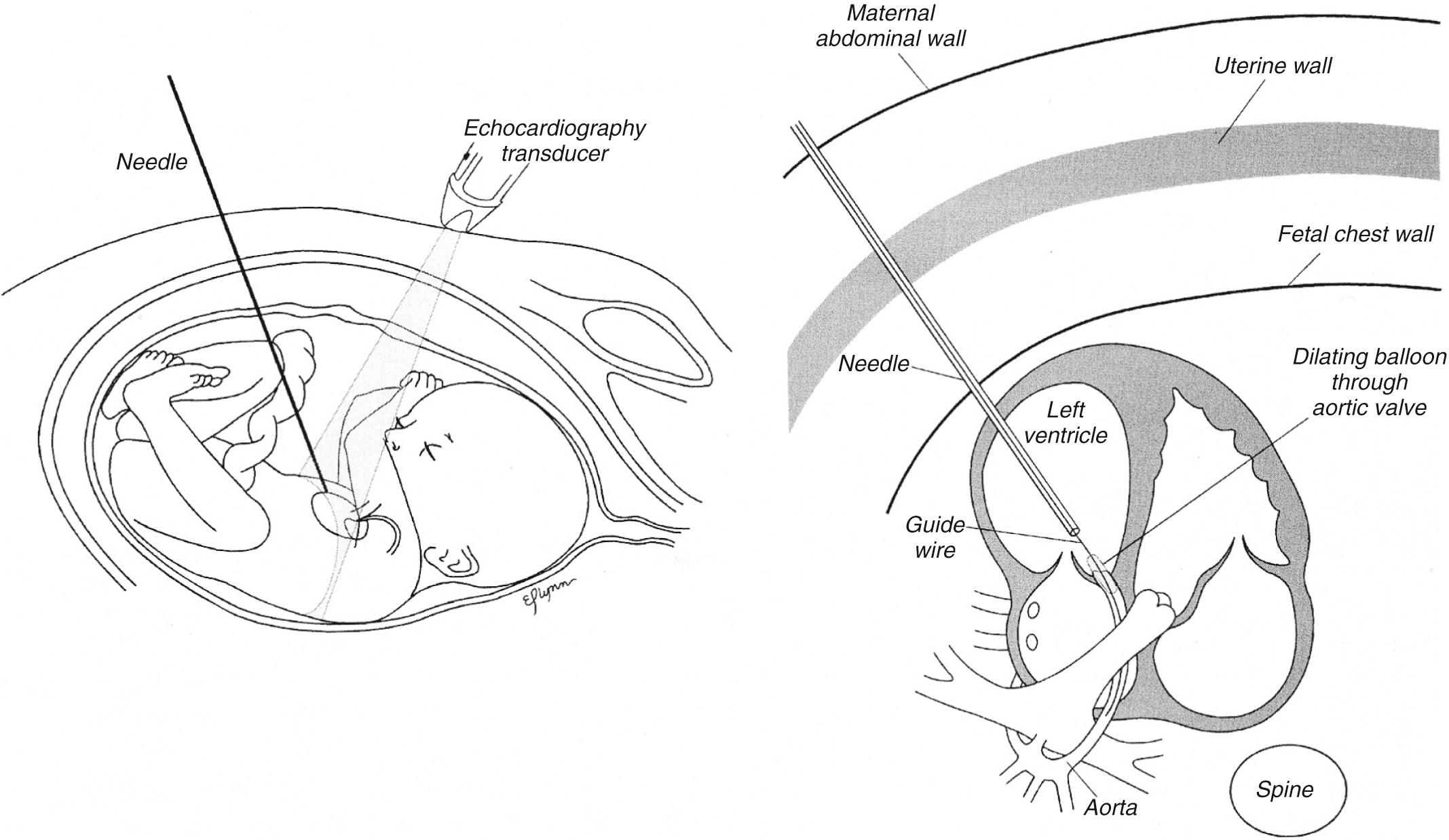
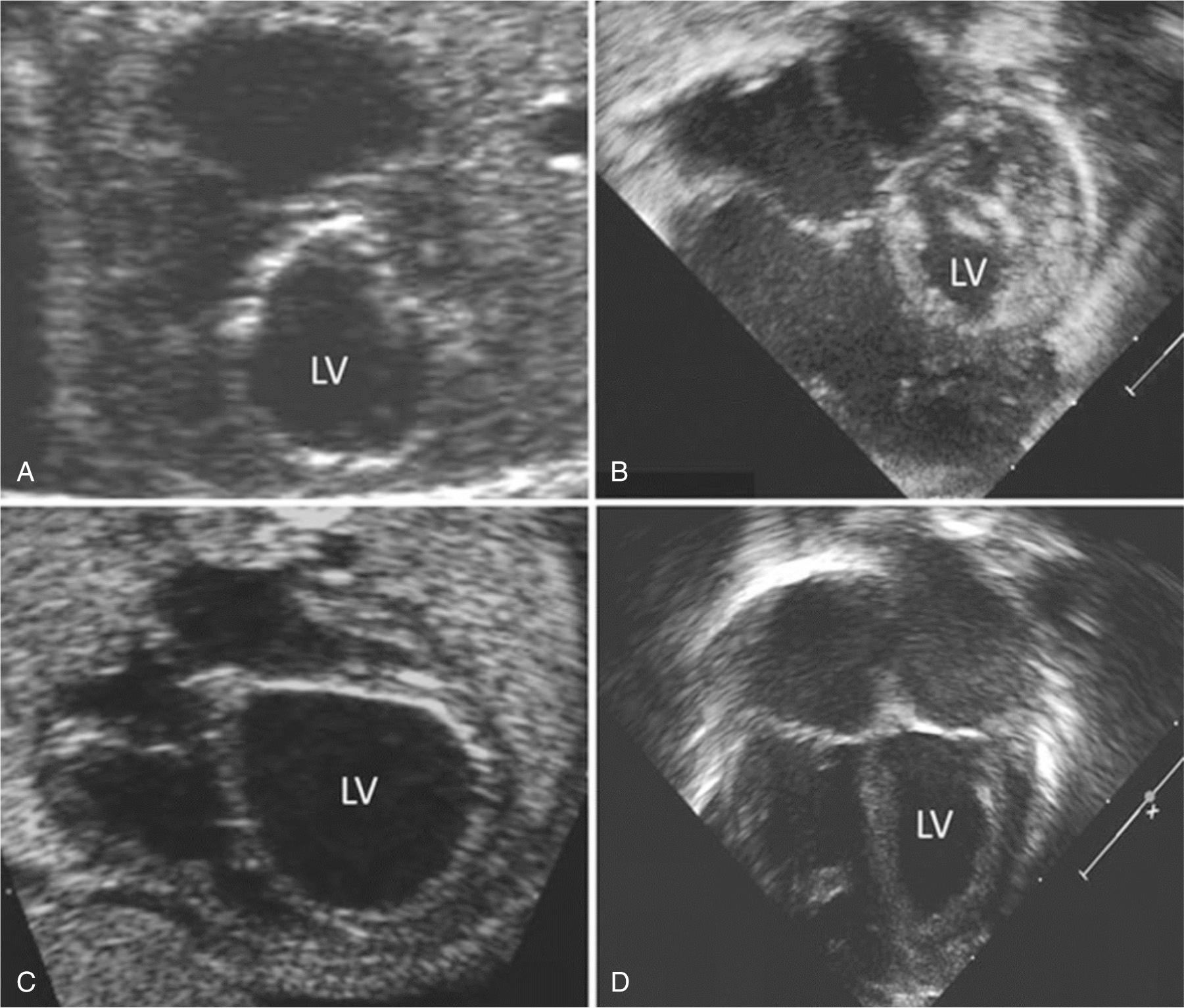
Laparotomy is reserved only for select cases in which optimal fetal positioning is unable be achieved with external version or imaging is limited. Under ultrasound guidance, a 19-gauge cannula and stylet needle are advanced through the maternal abdomen, uterine wall, and fetal chest wall, and the LV apex is punctured ( Fig. 10.5 , ). After stylet removal and evidence of blood return confirming intracardiac cannula position, a 0.014-inch guidewire is manipulated across the LV outflow tract into the ascending aorta. A coronary angioplasty balloon is advanced over the wire, positioned across the aortic valve annulus, and inflated, typically at least two times, to 100% to 120% of the size of the aortic annulus ( ).
Technical success is confirmed by color Doppler demonstrating a broader jet of antegrade flow across the aortic valve and/or the presence of aortic regurgitation ( ). After cannula removal, the fetus is monitored for at least 30 minutes in the operating room. The most common complications, which occur in up to 40% of fetuses, are bradycardia, ventricular dysfunction, and hemopericardium. Bradycardia and dysfunction are treated with intracardiac epinephrine and atropine, typically with brisk response. If the hemopericardium is small and hemodynamically insignificant, then no intervention is performed. If the hemopericardium is moderate to large and/or there is associated hemodynamic instability, then pericardiocentesis is performed.
In 2017 the Boston group reported technical success in 101 of 123 patients (83%) who underwent fetal aortic valvuloplasty from 23.9 to 32 weeks. Greater success, up to 94%, was noted in the latter half of the experience, as would be expected after an initial learning curve. An 11% risk of fetal demise (14 of 123) was also reported, mostly from the early experience from 2000 to 2008. Most of these deaths occurred within 24 hours of the procedure. The use of laparotomy has also substantially decreased with greater experience with fetal cardiac intervention. Importantly, no significant maternal morbidity has been noted.
Technically successful fetal aortic valvuloplasty has been shown to alter the hemodynamics ; myocardial performance as determined by both tissue Doppler imaging and strain mechanics ; and growth of left-sided heart structures in utero. In 2014 the Boston group reported the postnatal outcomes of the first 100 patients who underwent fetal aortic valvuloplasty. Most importantly, technically successful intervention resulted in larger left-sided heart structures at the time of birth ( Fig. 10.6 ). Among live-born patients ( n = 88), 31 were managed as biventricular from birth and seven were converted to a biventricular circulation after various stages of single ventricle palliation. Of the technically successful interventions, 45% resulted in a biventricular outcome postnatally.
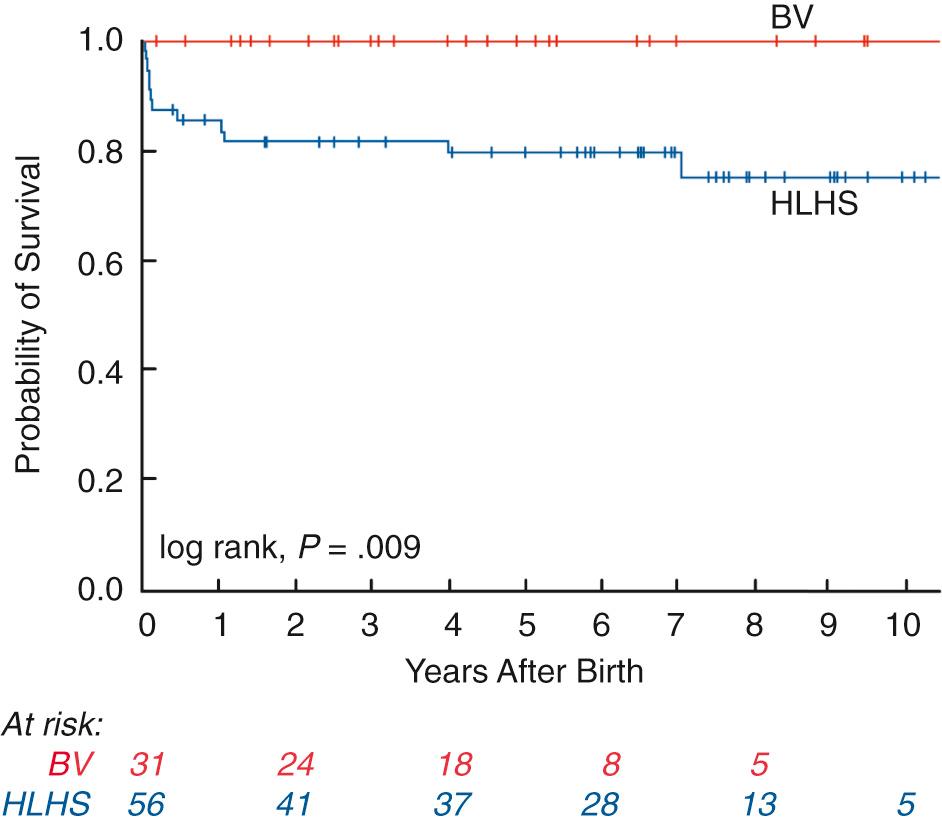
Compared with patients managed as HLHS, freedom from cardiac death was better among patients with a biventricular circulation at midterm follow-up ( Fig. 10.7 ). However, biventricular patients had substantial cardiac morbidity, often owing to residual AS or borderline left heart structures. Nearly all patients required postnatal cardiac catheterization and/or surgery. Valve replacements were among the most common procedures performed, and 18% of patients required both aortic and mitral valve replacements at midterm follow-up. Resection of endocardial fibroelastosis was also performed at the time of cardiac surgery for other indications in 86% of patients. Not surprisingly, in addition to the borderline size of the left heart structures, diastolic dysfunction and pathologic LV remodeling may complicate postnatal management. The borderline left heart structures and diastolic dysfunction resulted in approximately one-third of this population demonstrating pulmonary hypertension, typically in the mild range, at latest follow-up.
As with other patients with borderline left ventricles, there is no precise formula that will dictate management in the neonatal period. However, because the etiologic lesion in this subgroup of patients appears to be the aortic valve, an attempt at postnatal aortic valvuloplasty is reasonable to consider if there is residual obstruction. If there is concern regarding the development of severe aortic regurgitation then a surgical valvotomy may be preferred, depending on institutional preference. Subsequent hemodynamics will provide critical data as to whether the LV may support the systemic circulation. If the left heart remains inadequate, Emani and colleagues have demonstrated that staged LV recruitment, involving aortic and mitral valvuloplasties, resection of endocardial fibroelastosis, and restriction of the atrial septum, may preserve a biventricular outcome. For patients who proceed down the single ventricle pathway early in life, conversion to a biventricular circulation is possible with reasonable outcome if the LV end-diastolic pressure is relatively low (<13 mm Hg).
Postnatal management of patients following successful fetal aortic valvuloplasty is complex and heterogeneous, which is often due to differences in myocardial response. Although some patients may require only a postnatal aortic valvuloplasty to achieve a biventricular circulation, others may require initial staged single ventricle palliation with ongoing LV rehabilitation. Long-term follow-up of this population and of patients managed as HLHS is critical to assess which superior management strategy is better over time. Whether it is preferable to have a biventricular circulation with a rehabilitated LV or to proceed with staged reconstruction with a systemic right ventricle remains to be seen.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here