Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Spindle cell tumors in children and adolescents encompass a wide range of benign, intermediate, and malignant neoplasms, with the predominant phenotypic category being fibroblastic-myofibroblastic tumors. The most frequent spindle cell sarcomas of childhood include spindle cell and embryonal rhabdomyosarcoma (RMS), malignant peripheral nerve sheath tumor, synovial sarcoma, leiomyosarcoma, and various fibroblastic-myofibroblastic sarcomas. The majority of the nonrhabdomyosarcomatous spindle cell sarcomas occur more frequently in adults. In this chapter, the focus is on the fibroblastic-myofibroblastic tumors that occur predominantly in children and adolescents, and on several other spindle cell neoplasms that occur principally in younger patients. Round cell tumors are discussed in Chapter 8 .
When confronted with a spindle cell tumor in a child or adolescent, the considerations for the pathologist include the following :
Appropriate handling and triage of the fresh specimen to optimize diagnosis. For example, acquisition of fresh tissue for cytogenetic analysis, frozen tissue for molecular diagnostic tests, and a sample preserved for ultrastructural analysis may be useful. This is in addition to conventional light microscopy and immunohistochemistry on formalin-fixed, paraffin-embedded tissue.
Careful consideration of differential diagnosis. The range of spindle cell tumors includes reactive or pseudosarcomatous proliferations, as well as benign, intermediate, and malignant neoplasms. The pathologic diagnosis is critical for clinical management of these lesions, and morphologic overlap can present a challenge in classification.
Ancillary diagnostic techniques. Immunohistochemistry is an important diagnostic adjunct, especially for phenotypic classification, but does not generally allow a distinction between benign and malignant neoplasms within a specific group. Further evaluation with cytogenetic or molecular diagnostic tests can, in some cases, facilitate a specific diagnosis.
Mesenchymal tumors with fibroblastic and myofibroblastic components are an important group of neoplasms in childhood and adolescence. They account for approximately 12% of soft tissue tumors in the first two decades of life. The histologic similarities, differences in biologic potential, and clinical and molecular variations in this interesting group of lesions create diagnostic challenges. Nonetheless, precise classification is essential for treatment, prognosis, and, in some instances, genetic counseling ( Table 4.1 ). Histologically benign lesions are generally classified as fibromas or fibromatoses and malignant lesions as various types of sarcoma. In recent years, the concept of intermediate or “borderline” fibroblastic-myofibroblastic tumors has been refined for lesions with a tendency for local recurrence or very rare metastases, which may not be predictable on clinical or morphologic grounds. Many of these neoplasms of intermediate biologic potential have a predilection for children and young adults.
| Type of Lesion | Genetic Properties |
|---|---|
| Benign Lesions | |
| Nodular fasciitis | t(7;22)(p13;q13) with MYH9-USP6 gene fusion |
| Cranial fasciitis | Insufficient data |
| Other fasciitis and myositis variants | Insufficient data |
| Fibromas | |
| Gardner fibroma | APC mutation in patient |
| Cardiac fibroma | del(9)(q22) or somatic copy number losses involving PTCH1 gene |
| Fibromatoses | |
| Desmoid fibromatosis | Trisomies 8 and 20, APC or CTNNB1 mutation, del(5)(q) |
| Infantile myofibromatosis | Mutations in PDGFRB , NDRG4 , NOTCH3 |
| Fibromatosis colli | Insufficient data |
| Infantile digital fibroma | Insufficient data |
| Fibrous hamartoma of infancy | EGFR mutations |
| Calcifying aponeurotic fibroma | FN1-EGF gene fusion |
| Lipofibromatosis | Insufficient data |
| Juvenile nasopharyngeal fibroma | APC or CTNNB1 mutations |
| Hyaline fibromatosis | ANTXR2 (CMG2) mutation |
| Superficial fibromatosis (plantar, palmar) | Autosomal dominant |
| Intermediate Neoplasms | |
| Inflammatory myofibroblastic tumor | ALK , ROS1 , PDGFRB , RET , or NTRK3 gene rearrangements |
| Infantile fibrosarcoma | t(12;15)(p13;q25) with ETV6-NTRK3 gene fusion; LMNA-NTRK1 gene fusion; trisomies 8, 11, 17, 20 |
| Primitive myxoid mesenchymal tumor of infancy | BCOR internal tandem duplications |
| Sarcomas | |
| Low-grade fibromyxoid sarcoma | FUS-CREB3L2 , FUS-CREB3L1 , or EWSR1-CREB3L1 gene fusion |
| Myofibrosarcoma | Insufficient data |
| Infantile rhabdomyofibrosarcoma | Insufficient data |
A summary of fibroblastic-myofibroblastic tumors in the first two decades of life is shown in Table 4.2 .
| Diagnosis | Number | Percent |
|---|---|---|
| Desmoid fibromatosis | 151 | 29 |
| Infantile myofibromatosis | 60 | 12 |
| Fibromatosis colli | 49 | 10 |
| Infantile digital fibroma | 18 | 3 |
| Fibrous hamartoma of infancy | 43 | 8 |
| Calcifying aponeurotic fibroma | 11 | 2 |
| Juvenile nasopharyngeal fibroma | 13 | 3 |
| Superficial (plantar and palmar) fibromatosis | 47 | 9 |
| Hyaline fibromatosis | 1 | <1 |
| Fibromatosis, not otherwise classified a | 89 | 17 |
| Fibrosarcoma | 33 | 6 |
a Category includes congenital keloid and visceral and skeletal fibromatoses that were not otherwise designated.
Nodular fasciitis is a pseudosarcomatous fibroblastic-myofibroblastic proliferation (see also Chapter 3 ). Both the clinical and pathologic diagnoses present pitfalls in recognition because nodular fasciitis simulates a sarcoma, with its rapid growth and high cellularity. In some cases, atypia and mitotic activity further contribute to the difficulty in distinguishing nodular fasciitis from higher-grade neoplasms.
Up to 20% of cases occur in children and adolescents, with a slight male predominance. Most are solitary. All areas of the body can be affected, including the head and neck, trunk, and extremities. In young patients, nodular fasciitis is more frequent in the second decade of life and usually occurs after 5 years of age, although infants and young children are sometimes affected. The rapidly growing, sometimes tender, or painful nodule can occur in subcutaneous or deep soft tissue, including skeletal muscle and fascia. However, approximately 80% are subcutaneous. Unusual sites include upper respiratory tract and oral cavity submucosa and joint spaces.
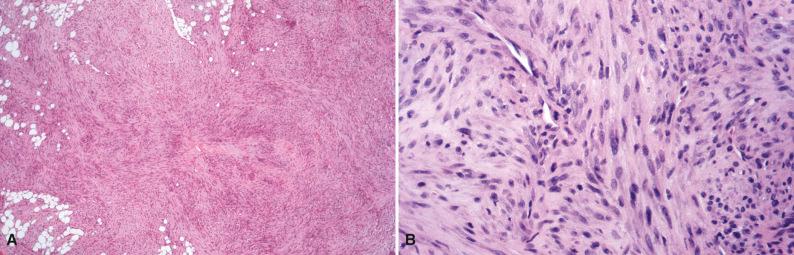
Grossly, nodular fasciitis appears as a round or ovoid, unencapsulated variegated soft tissue mass with a rubbery or myxoid cut surface. Histologically, the cellular phase of nodular fasciitis consists of plump, spindled myofibroblastic and ganglion-like mesenchymal cells in whorls, interlacing fascicles, and sheets ( Fig. 4.1A ). Clefts or clear slits occur between cells, and the background varies from myxoid or mucoid to collagenous (see Fig. 4.1B ). Microcysts with mucoid material in the lumina can be present (see Fig. 4.1C ). Sparse chronic inflammation, extravasated erythrocytes, feathery areas of spindle cells in the center of the lesion with a “tissue culture” pattern, and marginal fat necrosis are often seen. In earlier stages, the pattern is predominantly myxoid. Well-developed lesions display osteoclast-like multinucleated giant cells intermingled with spindled and ganglion-like cells (see Fig. 4.1D ). In later stages, keloidal collagen and a more fibrous appearance predominate. Histologic variations include cartilaginous metaplasia, calcification, and heterotopic ossification.
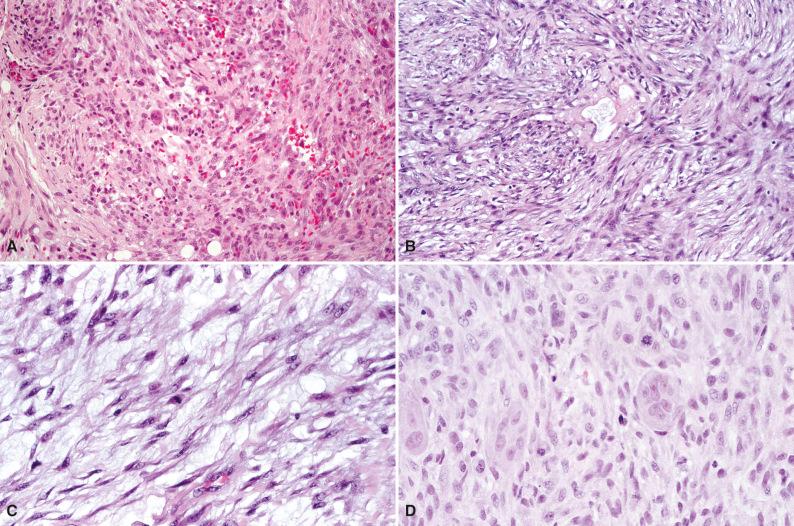
As is the case for most fibroblastic/myofibroblastic lesions, immunohistochemistry plays a minor role in the diagnosis of nodular fasciitis. The fibroblasts and myofibroblasts demonstrate reactivity for vimentin, smooth muscle actin, muscle-specific actin, and calponin. Focal CD68 reactivity is present in histiocytes and osteoclast-like giant cells. Nodular fasciitis is nonreactive for h-caldesmon. Low to moderate proliferative activity is seen with a Ki67 stain and other proliferative markers. Ultrastructural examination reveals myofibroblastic differentiation.
Nodular fasciitis is a clonal lesion. Recent studies have demonstrated an MYH9-USP6 gene fusion in nodular fasciitis. Nearly all cases examined harbor a USP6 rearrangement, which in 65% of cases is fused to MYH9. The gene expression signature of nodular fasciitis is distinct from desmoid-type fibromatosis.
The differential diagnosis includes desmoid fibromatosis, benign fibrous histiocytoma, infantile myofibromatosis, early fibrodysplasia ossificans progressiva in infancy, low-grade myofibrosarcoma, low-grade malignant peripheral nerve sheath tumor, and low-grade fibromyxoid sarcoma. Desmoid fibromatosis can be a particular challenge in fibrous or late-phase nodular fasciitis, but distinguishing features are aberrant nuclear reactivity for β-catenin; delicate, elongated, thin-walled blood vessels at the edge of spindle cell fascicles; and a mast cell infiltrate. Infantile myofibromatosis lacks the inflammatory infiltrate, mucoid microcysts, extravasation of erythrocytes, and osteoclast-like giant cells of nodular fasciitis. Myofibrosarcoma and other spindle cell sarcomas typically lack the inflammatory infiltrate and zonation pattern of nodular fasciitis, and display more nuclear atypia and pleomorphism.
Nodular fasciitis is a rapidly growing, benign lesion that is usually subcutaneous.
A zonal architectural pattern is typical, and the histologic spectrum ranges from myxoid to cellular or fibrous.
A rearrangement of the USP6 gene is frequent.
Conservative excision is adequate therapy for nodular fasciitis, which generally does not recur. Regression and involution following biopsy or incomplete resection have been observed. Recurrence has been attributed to persistent growth of an incompletely excised lesion.
Cranial fasciitis is similar to nodular fasciitis, except that it is restricted to cranial soft tissue, especially in the temporoparietal region. In contrast to nodular fasciitis, cranial fasciitis typically affects infants and children in the first few years of life. Cranial fasciitis is rare in the spectrum of extracranial subcutaneous scalp and skull masses in young children. Like nodular fasciitis, the rapidly growing mass can lead to a clinical concern for malignancy.
Cranial fasciitis involves the subcutaneous and deep soft tissue of the head, with bone involvement reported in 80% to 90% of cases. Children younger than 2 years of age are most often affected, and there is a male predominance. Intracranial extension, rapid growth, and a radiologically aggressive appearance simulate higher-grade lesions.
Grossly, cranial fasciitis has a median diameter of 2.5 cm with a range of 1.5 to 9 cm. The gray rubbery mass is usually ovoid and well circumscribed, although occasional examples are multinodular. Histologically, spindled and stellate fibroblastic and myofibroblastic cells are loosely arranged in a myxoid background and have a more uniform appearance than nodular fasciitis ( Fig. 4.2A ). Patchy hemorrhage, chronic inflammation, occasional osteoclast-like multinucleated giant cells, calcification, osseous metaplasia, and ganglion-like cells are variable. The mitotic rate varies, with up to 10 mitoses per 10 high-power fields. A vague storiform pattern or abundant collagen is sometimes observed (see Fig. 4.2B ).
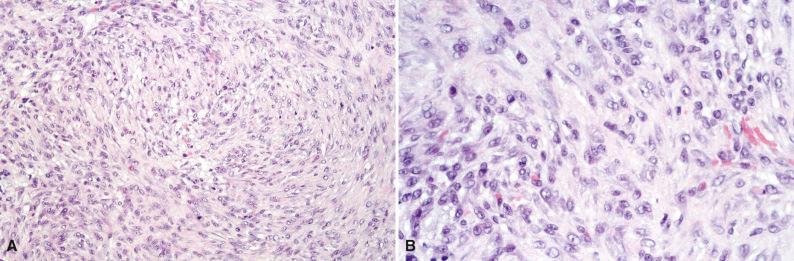
Similar to nodular fasciitis, immunohistochemical analysis reveals reactivity for vimentin and smooth muscle actin.
The differential diagnosis of cranial fasciitis is similar to nodular fasciitis, except that cranial fasciitis occurs earlier in childhood, is more restricted in site, and has a more uniform histologic appearance than nodular fasciitis. Differential diagnostic considerations include infantile fibrosarcoma, fibromatoses such as desmoid fibromatosis and infantile myofibromatosis, and low-grade myofibrosarcoma of the head and neck. Infantile fibrosarcoma is larger and more cellular with more nuclear atypia, and can have a hemangiopericytoma-like growth pattern. Infantile myofibromatosis generally lacks inflammation, giant cells, and a prominent myxoid background. Low-grade myofibrosarcoma displays more cellular atypia, lacks inflammation and multinucleated cells, and has an aggressive appearance on imaging studies.
Conservative surgical excision is the preferred treatment for cranial fasciitis. Recurrence is rare. Similar to nodular fasciitis, cranial fasciitis may involute spontaneously.
Gardner fibroma is a distinctive hypocellular, prominently collagenized growth that is frequently an early manifestation of familial adenomatous polyposis (FAP; Gardner syndrome). It can be associated with concurrent or subsequent development of desmoid-type fibromatosis.
Gardner fibroma is soft, poorly demarcated, and slow-growing. It occurs in superficial or deep soft tissue in the paraspinal region, trunk, abdomen, head and neck, or extremities. Approximately 70% of patients with Gardner fibroma have a history of FAP, and more than 10% have a family history of desmoid-type fibromatosis or soft tissue tumors. Although the age at diagnosis ranges from infancy to adulthood, nearly 80% of Gardner fibromas are recognized in the first decade of life. The clinicopathologic features are summarized in Table 4.3 .
| Feature | Specifics |
|---|---|
| Age at presentation (range, infancy to fourth decade) | |
| First year | 29% |
| First decade | 78% |
| Second decade | 15% |
| After second decade | 7% |
| Male-to-female ratio | 1.3 : 1 |
| Sites | |
| Back and paraspinal tissue | 61% |
| Head and neck | 14% |
| Extremities | 14% |
| Chest and abdomen | 11% |
| Genetics | |
| FAP in patient or family | 69% |
| Family history of soft tissue tumor | 4% |
| Concurrent or subsequent desmoids | 19% |
Grossly, the plaque-like rubbery mass has an infiltrative appearance and a white cut surface speckled with yellow. Histologically, the bland hypocellular proliferation consists of haphazardly arranged sheets of coarse collagen fibers separated by clear cracks with intervening small bland spindle cells, small blood vessels, and a sparse mast cell infiltrate ( Fig. 4.3 ). There is no fascicular or bundling architectural pattern. The collagenized proliferation entraps adjacent tissues such as adipose tissue, peripheral nerves, and blood vessels. Gardner fibroma may merge into an adjacent desmoid fibromatosis, which is distinguished by a more compact proliferation of spindle cells arranged in fascicles with more prominent cellularity.
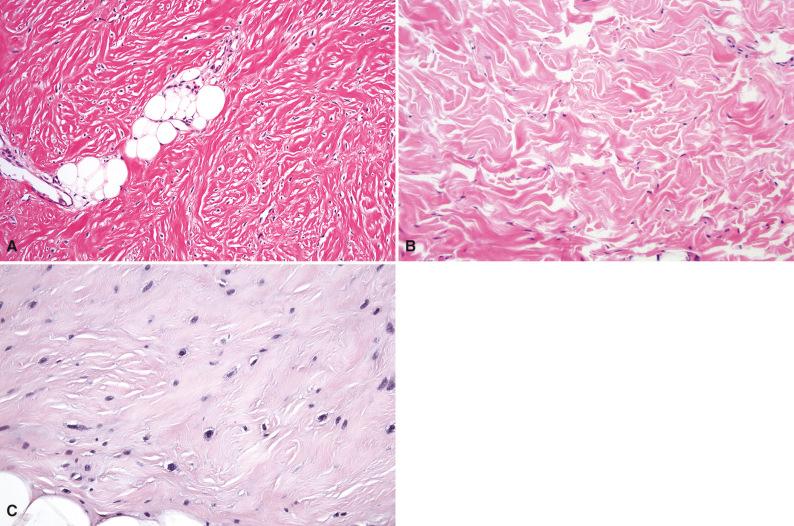
Immunohistochemical analysis of Gardner fibroma usually reveals diffuse reactivity for CD34 and absence of smooth muscle actin within the lesion. Nuclear β-catenin reactivity is variable and can be positive in up to two-thirds of cases ( Fig. 4.4 ), but it may be nonreactive even in patients with an adenomatous polyposis coli ( APC ) mutation. Gardner fibroma overexpresses other proteins in the Wnt and β-catenin pathways, such as cyclin D1 and MYC.
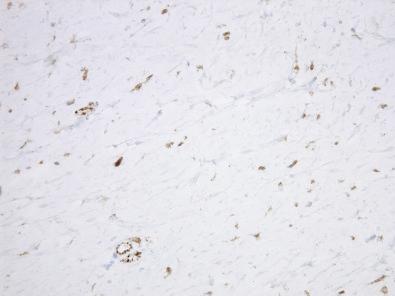
Gardner fibroma is associated with APC mutation.
The differential diagnosis for Gardner fibroma includes other types of fibroma, especially nuchal-type fibroma, and fibromatoses, particularly desmoid fibromatosis with prominent collagen. Table 4.4 compares the pathologic features.
| Feature | Gardner Fibroma | Nuchal Fibroma | Desmoid Fibromatosis |
|---|---|---|---|
| Gross appearance | Rubbery plaque | Poorly demarcated hard mass | Firm, whorled nodule |
| Microscopic features | Patternless | Lobules, criss-crossing areas, neuroma-like areas | Fascicles |
| Hypocellular | Hypocellular | Moderately cellular | |
| Hypovascular | Hypovascular | Distinctive vessels | |
| Matted collagen | Thick collagen bundles | Fibrillary or keloidal collagen | |
| No mitoses Mast cells |
No mitoses Lymphocytes |
Variable mitoses Mast cells |
|
| Clonality | Unknown | Unknown | Yes |
| Immunohistochemistry | CD34 β-catenin (nuclear) |
Unknown | Smooth muscle actin β-catenin (nuclear) |
Gardner fibroma is a benign lesion with two important caveats. First, it can be the sentinel event in a child for the diagnosis of FAP, Gardner syndrome, familial desmoid-type fibromatosis without other manifestations of FAP, or a new APC mutation. Second, it can be associated with concurrent or subsequent desmoid-type fibromatosis, which has been observed in nearly 20% of cases of Gardner fibroma, although this is probably an underestimate based on limited available follow-up. Children with Gardner fibroma should have ongoing follow-up and evaluation for development of colorectal tumors and desmoid-type fibromatosis, and their families should be screened for FAP.
Gardner fibroma is a poorly demarcated, plaque-like mass that can be an early manifestation of familial adenomatous polyposis/Gardner syndrome and is associated with APC mutation.
Hypocellular sheets of coarse collagen fibers separated by clear cracks infiltrate and entrap adjacent tissues.
Gardner fibroma is a desmoid precursor.
Cardiac tumors are rare in infants and children, and most are benign. After cardiac rhabdomyoma, cardiac fibroma is one of the more common types of this unusual group of lesions.
Cardiac fibroma can be detected antenatally by fetal ultrasound. Initial manifestations in children include unexplained heart failure, arrhythmia, heart murmur, cardiac calcification, or irregular cardiac contours. Most are diagnosed within the first 2 years of life, and nearly all are detected by early adolescence. Males are affected more often than females. Cardiac fibroma can occur anywhere in the heart but most often arises in the left ventricle. The diagnosis can lead to the recognition of the nevoid basal cell carcinoma syndrome (Gorlin syndrome) in the child and the family. In addition, cardiac fibroma can be associated with type 1 neurofibromatosis, tuberous sclerosis, familial myxomas, and bilateral cystic renal dysplasia.
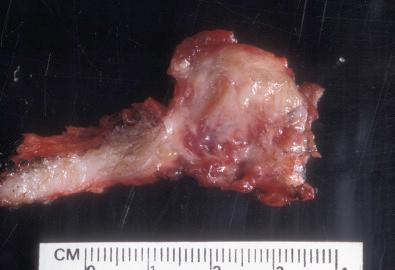
Grossly, cardiac fibroma is a firm, circumscribed, pale tan, intramural, or ventricular cardiac mass ( Fig. 4.5 ). The cut surface displays a firm texture. Satellite nodules may accompany the solitary mass; occasional tumors are multifocal. Histologically, bland fibroblasts intermingle with collagen and elastic fibers ( Fig. 4.6 ). A sparse mast cell infiltrate, prominent vascularity, and variable calcifications may be present. The cellularity ranges from low to moderate.
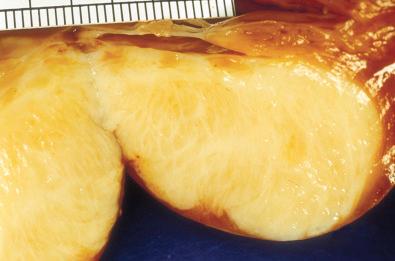
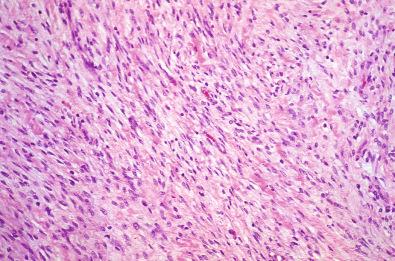
Immunohistochemical analysis reveals diffuse reactivity for vimentin and focal reactivity for smooth muscle actin.
Cardiac fibromas may have a rearrangement, deletion, or somatic copy number losses of the PTCH1 gene at chromosome 9q22.
The differential diagnosis of cardiac fibroma includes other primary cardiac neoplasms such as rhabdomyoma or RMS, cardiac lesions in congenital generalized myofibromatosis (infantile myofibromatosis), and cardiac sarcomas. Cardiac rhabdomyoma is distinguished by the presence of large multivacuolated glycogen-filled cells and “spider” cells. Myofibromatosis is recognized by prominent whorling and an interlacing fascicular architecture with strong reactivity for smooth muscle actin in the clinical setting of congenital generalized myofibromatosis. RMS and other sarcomas are distinguished by a higher-grade appearance with high cellularity, pleomorphism, mitotic activity, and their distinctive immunohistochemical features.
Although cardiac fibroma is histologically benign, its location is potentially lethal. Resection or cardiac transplantation may be effective for cases that are detected early.
Infantile myofibroma or myofibromatosis occurs in three clinicopathologic forms with similar histology but different clinical features and outcome. The three subtypes—solitary, multiple, and congenital generalized myofibromatosis—are summarized in Table 4.5 .
| Type | Sites | Age at Onset | Predominant Sex | Natural History |
|---|---|---|---|---|
| Solitary | Skin, soft tissue | Birth and later | Male | Benign Regression or recurrence |
| Multiple | Skin, soft tissue, bone | Congenital | Female | Benign Regression |
| Generalized | Skin, soft tissue, bone, viscera | Congenital | Male | Progression Death in 73% Regression rare |
The solitary and multiple forms of infantile myofibromatosis involve skin, soft tissue, and bone. In contrast, the generalized form also involves these sites and viscera, including the central nervous system, in varying combinations. Lesions can be numerous. The onset is usually in infancy, with a congenital presentation in up to two-thirds of cases, but may be later in childhood, adolescence, or adulthood. Cutaneous lesions have a purplish nodular or papular appearance ( Fig. 4.7 ). Solitary and multiple myofibromas can undergo spontaneous regression with a gangrenous-appearing shrinkage of the mass. Extensive bone involvement can result in multiple fractures as the initial presentation in infants, and rare cases are associated with fetal death. Familial cases have been reported. Associated malformations are rare.
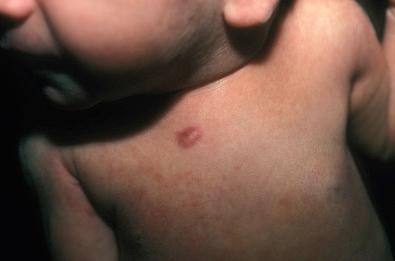
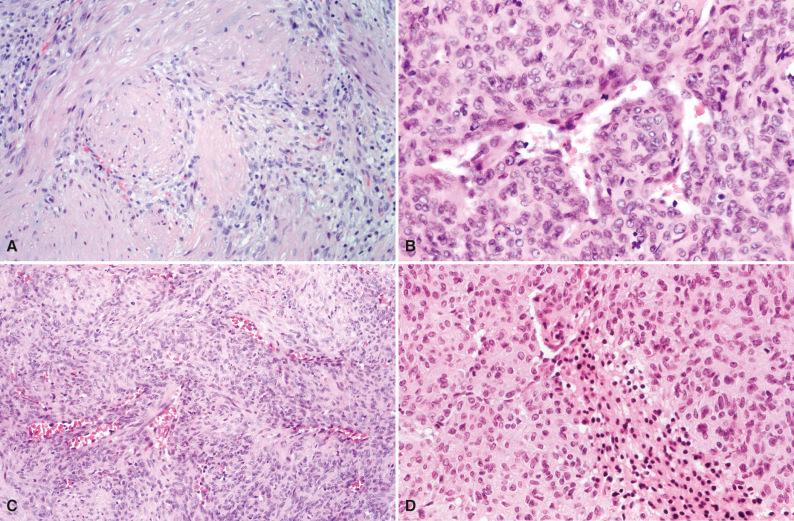
Grossly, solitary myofibroma is a circumscribed nodular mass with a white to tan firm cut surface. Occasional cases have a soft red central area with variable necrosis and calcification ( Fig. 4.8 ). The multiple and generalized myofibromas form deeper nodules with a white or tan firm cut surface and less extensive necrosis and zonation. Microscopically, short interlacing fascicles of spindled myofibroblastic cells are dispersed in a myxoid and collagenized background with moderate cellularity ( Fig. 4.9 ). In sections where adjacent nonlesional tissue is present, peripheral satellite nodules may be seen with a whorled pattern and a focal perivascular and intravascular subendothelial proliferation of immature myofibroblastic and primitive mesenchymal cells. These features suggest a possible origin from perivascular mesenchymal cells and a morphologic continuum with myopericytoma. Cellular atypia is absent. Some cases demonstrate a zonal pattern with central primitive round to polygonal cells associated with delicate irregularly branching blood vessels ( Fig. 4.10A and B ). These areas may merge with more spindled and collagenized whorled myofibroblastic foci (see Fig. 4.10C ). In the past, this variant was called “infantile hemangiopericytoma.” Increased cellularity, loosely cohesive or absent spindled myofibroblastic nodules, infiltrating borders, and perineural invasion or nerve entrapment may be seen. Prominent apoptosis, necrosis, and dystrophic calcification may be encountered, especially in larger myofibromas (see Fig. 4.10D ). Apoptosis has been proposed as a mechanism for spontaneous regression.
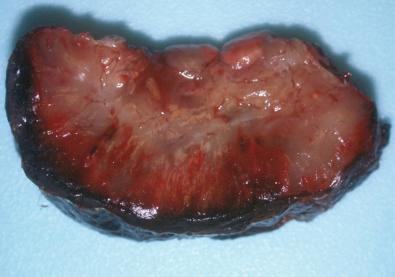
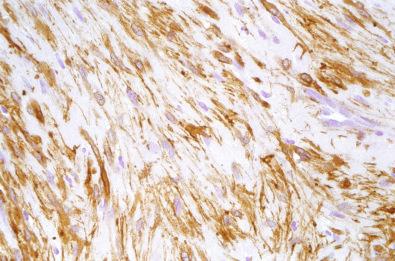
By immunohistochemistry, the spindled and whorled areas show strong reactivity for smooth muscle actin ( Fig. 4.11 ), and the endothelial cells in the hemangiopericytoma-like areas are reactive for CD34.
A single case has been reported with a chromosome 6 deletion. Autosomal dominant and autosomal recessive patterns of inheritance have been reported in some families. Mutations in PDGFRB , NDRG4 , and NOTCH3 have been identified.
The differential diagnosis includes other fibromatoses, nodular fasciitis, solitary fibrous tumor, fibroblastic-myofibroblastic sarcomas, and pericytic neoplasms such as myopericytoma. It is now recognized that infantile hemangiopericytoma is part of the morphologic spectrum of infantile myofibroma, and generally these cases are lumped together as infantile myofibroma/myofibromatosis. Solitary fibrous tumor in adults is a distinct entity that is unrelated to infantile myofibroma, lacks the fascicular and whorled growth pattern, and usually demonstrates CD34 and STAT6 reactivity in the neoplastic cells. Occasional cases of infantile fibrosarcoma closely resemble myofibroma and are sometimes classified as composite fibromatosis because of the inability to reliably distinguish between the two entities on morphologic features alone. In such cases, molecular diagnostic testing for the ETV6-NTRK3 gene rearrangement associated with infantile fibrosarcoma is very useful. Other neoplasms with myofibroblastic features, a hemangiopericytoma-like vascular pattern, and NTRK1 gene fusions can resemble highly cellular myofibromas and composite fibromatosis and are discussed with infantile fibrosarcoma. Other fibromatoses, particularly desmoid fibromatosis, and nodular fasciitis can simulate infantile myofibroma and are distinguished by a combination of clinical and morphologic features. Desmoid fibromatosis has a more uniform architecture with long fascicles and usually exhibits nuclear reactivity for β-catenin. Nodular fasciitis shows a zonal architecture with loose fascicles, variably myxoid stroma, extravasated erythrocytes, and focal osteoclast-like giant cells, and it lacks the whorled nodules and perivascular and subendothelial growth of myofibroma.
Infantile myofibroma/myofibromatosis can be solitary, multiple, or generalized.
The histologic spectrum includes a spindled, myofibroblastic fascicular pattern and a hemangiopericytoma-like pattern.
Mutations in PDGFRB , NDRG4 , or NOTCH3 may be present.
Large, cellular infantile myofibroma mimics infantile fibrosarcoma but lacks the ETV6-NTRK3 gene rearrangement.
The prognosis and treatment of infantile myofibroma/myofibromatosis vary according to the clinicopathologic pattern. Solitary myofibroma is frequently self-limited and can undergo spontaneous regression. In cases that do not regress, excision is effective treatment. Autosomal dominant examples can also regress. Congenital generalized myofibromatosis results in death in up to 70% of patients because of visceral involvement, especially of the lungs, gastrointestinal tract, and genitourinary tract. Limited information suggests that chemotherapy or interferon may be effective in some cases, and rare cases may be self-limited. Multifocal and generalized types require long-term follow-up.
Desmoid-type fibromatosis accounts for up to 60% of fibrous tumors in childhood (see also Chapter 3 ). In several large series, up to 40% of desmoid fibromatoses were diagnosed in the first two decades of life. Information about desmoid fibromatosis from four series of children and young adults is summarized in Table 4.6 . The associations with FAP (Gardner syndrome, with germline APC mutation), sporadic CTNNB1 mutation, and antecedent trauma, surgery, and irradiation are well established. A desmoid in early childhood may be the initial manifestation of an APC mutation in the patient or in the family, and desmoid can occur in the same site as a previous or concurrent Gardner fibroma.
| Feature | Specifics |
|---|---|
| Age | |
| Range | Birth to 28 years |
| Mean | 8 years |
| Congenital | 10% |
| First year of life | 24% |
| Male-to-female ratio | 1.7 : 1 |
| Sites | |
| Head and neck | 30% |
| Trunk | 45% |
| Extremities | 24% |
| Multifocal | 4% |
| Outcome | |
| Recurrence rate | 58% |
| Mortality | 6% |
Desmoid fibromatosis in childhood involves superficial or deep soft tissues. It is often extraabdominal; the most common sites are the head and neck region, extremities, shoulder, trunk, and hip. Unusual locations include the abdomen, retroperitoneum, breast, and spermatic cord. Some children may have multiple desmoids. Most series have demonstrated a male predominance. The median age for desmoid fibromatosis in the Kiel Pediatric Tumor Registry was 5 years. Up to 30% occur in the first year of life, and congenital examples have been reported.
Although desmoid fibromatosis is occasionally painful, the usual clinical presentation is a slowly growing, hard mass that has been present for weeks to months without tenderness, weight loss, or fever. Desmoids that arise in association with Gardner fibroma may be painful, can grow rapidly, and are firmer and more circumscribed than the adjacent Gardner fibroma. Desmoid fibromatosis is a locally aggressive tumor that can infiltrate adjacent skeletal muscle, tendons, or periosteum; erode bone; or be associated with osteolysis. Magnetic resonance imaging is useful for detection and monitoring.
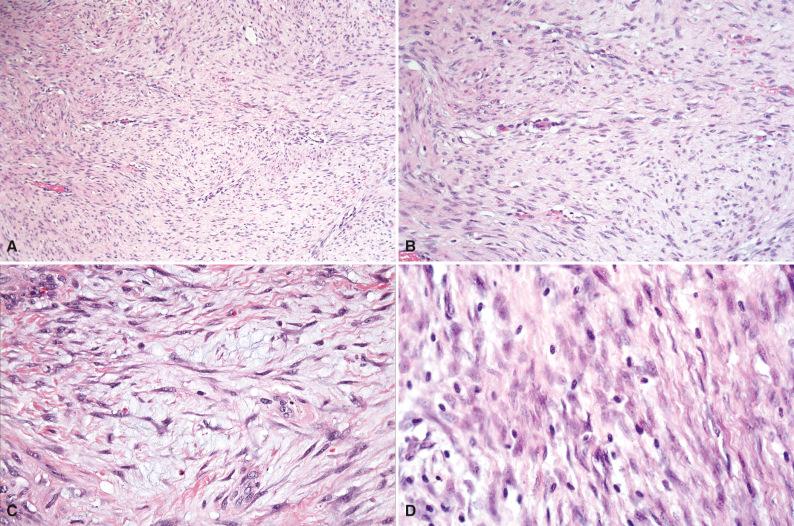
Grossly, the firm gray-white oval or fusiform mass has a whorled or trabeculated cut surface ( Fig. 4.12 ). The diameter averages 6 to 7 cm but can exceed 15 cm. Although the mass may appear to be well circumscribed macroscopically, the margins are often irregular and infiltrative. Histologically, broad sweeping fascicles of spindle cells with an abundantly collagenized background with foci of myxoid change are separated by thin-walled, delicate blood vessels that are aligned along edges of fascicles ( Fig. 4.13A–C ). Cellularity varies from low to high. The tumor cells are slender and relatively uniform, with a spindled configuration and abundant cytoplasm with indistinct cell borders. The nuclei are oval with fine chromatin and occasional small nucleoli and lack atypia or pleomorphism. Morphologic variations include hyalinized or hypocellular areas, dilated staghorn blood vessels, myxoid change, keloidal collagen bundles, and hypercellular foci. Dystrophic calcification is unusual. Mast cells are scattered throughout the tumor (see Fig. 4.13D ) but are more concentrated near blood vessels. A lymphocytic infiltrate may be seen peripherally at the interface between the desmoid and adjacent soft tissue or skeletal muscle. The advancing border infiltrates and entraps muscle fibers or adipose tissue. Atrophic or multinucleated skeletal muscle cells can simulate cellular atypia. Most desmoid tumors have relatively low mitotic rates, but occasional examples may display more than 10 mitoses per 10 high-power fields; this has no prognostic significance. Limited data suggest that foci of myxoid degeneration, abundant plump stellate tumor cells, and a large number of small slit-like blood vessels in the central portions of desmoid may be associated with a higher tendency for recurrence, but in general histology does not correlate with clinical behavior. Recurrent desmoids are histologically identical to the primary tumor. In patients who have had previous surgery, it may be difficult to distinguish between a surgical scar and a recurrent desmoid, or to discern the demarcation between the scar and a desmoid fibromatosis. Occasionally, microscopic foci of a previously unrecognized Gardner fibroma can be seen at the periphery of a desmoid. Radiation therapy does not significantly alter the histomorphology, but the presence of zonal necrosis, hypercellularity, severe nuclear atypia, and increased mitotic activity in a previously irradiated desmoid should prompt consideration of a postradiation sarcoma.
Immunohistochemical studies of desmoid tumors have demonstrated a fibroblastic-myofibroblastic phenotype. Muscle-specific actin, smooth muscle actin ( Fig. 4.14A ), and desmin are expressed in varying proportions of cases. The mast cells in desmoid fibromatosis can be highlighted by mast cell tryptase (see Fig. 4.14B ) and KIT. Immunohistochemistry for β-catenin with a nuclear pattern of positivity is a helpful confirmatory marker of desmoid fibromatosis (see Fig. 4.14C ), but the sensitivity and specificity vary in different studies; overall, approximately 70% of desmoid tumors show nuclear staining for β-catenin. Coexpression of β-catenin and p53 may be associated with a higher risk of recurrence. Estrogen receptor–β expression is frequent in desmoid fibromatosis.
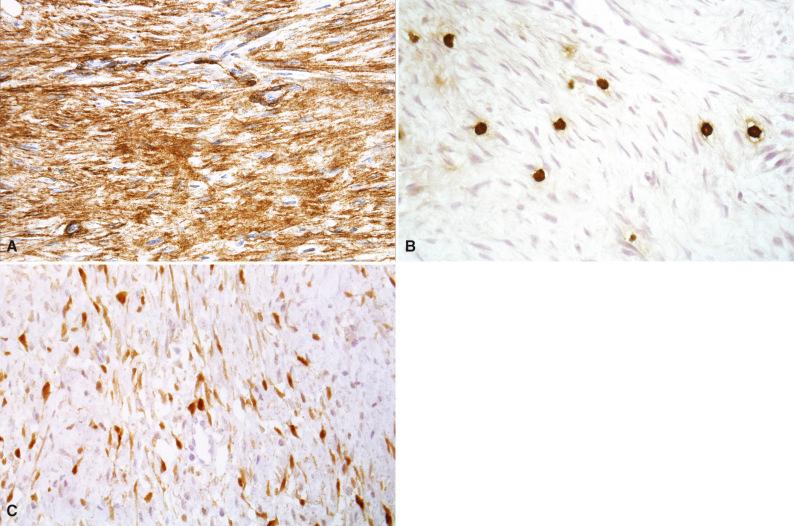
Desmoid fibromatosis is a clonal neoplasm that that can harbor trisomies of chromosomes 8 and 20, 5q deletion, and mutations involving the APC or β-catenin ( CTNNB1 ) genes. Desmoids can be associated with many types of APC mutations, although mutations that are 3' seem to confer higher incidence and greater severity. A significant proportion of sporadic desmoids in children harbor mutations in CTNNB1 , including T41A, S45F, and S45P types. Mutations of AKT1 and BRAF have also been detected in pediatric desmoids.
The differential diagnosis of desmoid fibromatosis includes a wide variety of spindle cell proliferations but especially keloids and hypertrophic scars, nodular fasciitis, Gardner fibroma, other fibromatoses, low-grade fibromyxoid sarcoma, and low-grade myofibrosarcoma. Keloids and hypertrophic scars are usually confined to skin and subcutaneous tissue, which are unusual locations for desmoid tumors, and they lack the fascicular architecture and vascular pattern of desmoids. Gardner fibroma is densely collagenized and contains formless sheets of collagen with indistinct bland spindle cells. Other fibromatoses lack the mast cell infiltrate and vascular pattern of desmoid fibromatosis and lack nuclear β-catenin expression. Low-grade fibromyxoid sarcoma has a well-defined arcading vascular pattern, myxoid zones, and collagen rosettes in some cases; is positive for MUC4 by immunohistochemistry; and has a distinctive t(7;16) translocation with FUS gene rearrangement. Myofibrosarcoma demonstrates greater nuclear atypia and clinical and radiologic evidence of very aggressive growth. Other spindle cell neoplasms, such as inflammatory myofibroblastic tumor (IMT) and spindle cell sarcomas, are distinguished by a combination of histologic, immunohistochemical, cytogenetic, and molecular genetic features.
Desmoid fibromatosis is a relatively frequent fibrous tumor of childhood and can be an early manifestation of familial adenomatous polyposis/Gardner syndrome or a new APC mutation; sporadic desmoids are usually associated with CTNNB1 mutations.
A significant proportion of childhood desmoids occur in the first 5 years of life.
Broad sweeping fascicles of spindle cells are separated by delicate, thin-walled blood vessels and are accompanied by a sparse mast cell infiltrate.
Mitotic activity has no diagnostic or prognostic significance.
Nuclear immunoreactivity for β-catenin is observed in approximately 70% of cases.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here