Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Essentially, three components of the venous system of the leg act in concert: deep veins, superficial veins and perforating-communicating veins. Dysfunction in any of these three systems results in dysfunction of the other two ( Fig. 3.1 ). When the superficial veins are placed under high pressure they dilate and elongate to accommodate an increased blood volume. Their tortuous appearance is termed varicose , derived from the Greek term for ‘grapelike’. This term applies to both the large protruding veins within the superficial subcutaneous tissue and the smaller venectasia, or ‘spider veins’ that occur just beneath the epidermis.
The World Health Organization defines varicose veins as ‘saccular dilation of the veins, which are often tortuous’. This definition specifically excludes any tortuous veins associated with previous thrombophlebitis, arteriovenous connections or with venectasia.
Varicose veins differ from nonvaricose veins in physiologic function. This may occur in one or all of the histologic layers. Endothelial damage can occur in parts of a varicose vein, and has been noted both ultrastructurally and physiologically by a reduction in endothelial-mediated enhancement of norepinephrine (noradrenaline) induced vasoconstriction ( Fig. 3.2 ).
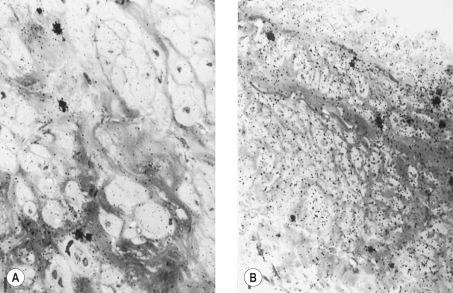
Characterization of the endothelin receptors in varicose veins compared with those in normal veins has shown decreased contraction to endothelin-1 in both varicose and saphenous veins of patients with primary varicosities. It may be that this observation will be associated with a decrease in the number of receptors.
In most investigations alterations have been found in the muscular layer, with varicose veins having a considerable degree of smooth muscle hypertrophy and a 15% increase in muscle content compared with normal veins. This is thought to be a secondary response to venous hypertension. Other investigators have found that smooth muscle cells are capable of phagocytosis and decomposition of collagen fibers. Smooth muscle cells from varicose veins are less differentiated compared with normal veins and demonstrate increased synthetic capacity, greater proliferation and increased migration than smooth muscle cells found in normal veins. Therefore these cells may be part of the cellular basis for collagen breakdown. However, other investigators have noted a decrease in lactate dehydrogenase and creatine kinase activity in varicose versus normal veins and postulate that varicose vein weakness is the result of a thinning or damaged muscular layer. This has been confirmed in a study of aging canine and human veins where a decrease in sympathetic innervation has been correlated with muscular layer thinning. In addition, the protein content of varicose veins, which is predominantly smooth muscle, is reduced. However, one research group has found no significant difference in the quantity of smooth muscle between normal and varicose veins.
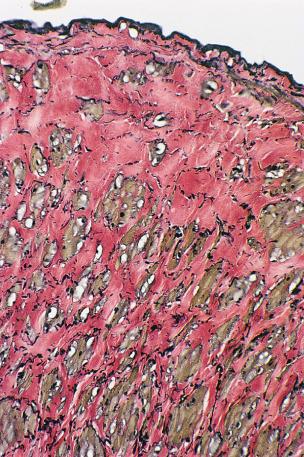
Alterations in the adventitial layer have been noted in varicose veins. Some investigators have found that varicose veins have an extremely dense and compact fibrosis between the intima and adventitia, with a diminished and atrophied elastic network and a disorganized muscular layer ( Fig. 3.3 ). Thickening and fibrillation of individual collagen fibers has also been noted. This translates to a reduced compliance that may lead to poor coaptation of venous valves and increased varicose vein wall stiffness. An in vivo measurement of venous elasticity in patients with normal, ‘high-risk’ and varicose veins confirmed reduced elasticity in both varicose and high-risk veins. In this study, individuals with high-risk veins were defined as having a family history of varicose veins, standing occupations, symptoms of venous disease and Doppler ultrasound reflux.
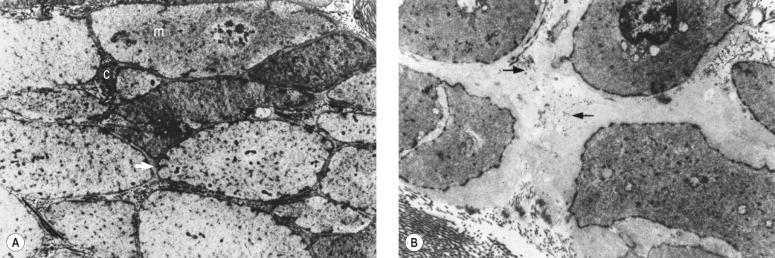
The described loss of tonicity of varicose veins is primarily the result of the loss of coordinated communication between smooth muscle cells. Electron microscopic studies of nonvaricose veins demonstrate the close approximation of smooth muscle cells. When veins become varicose, smooth muscle cells become vacuolated and are separated by collagen. With increasing varicose changes intercellular collagen deposition accumulates and separates the smooth muscle cells, which then atrophy ( Fig. 3.4 ). It is suggested that the resulting separation of smooth muscle cell hemidesmosomes causes inefficient smooth muscle contraction and increased venous distensibility. However, some varicose veins are capable of constricting in response to an infusion of dihydroergotamine. This venoconstriction is even more pronounced than that occurring in normal veins. The reason for this paradoxical effect is unknown, but a varicose vein appears to be a dysplastic vein characterized by malformations. Whether this is the result of continual high venous pressure or whether it is the primary etiologic event in the development of valvular incompetence is also unknown.
Elastin and collagen are known to play an important role in maintaining structural integrity of blood vessel walls. Normally when the wall is stretched, elastin generates a shortening force that opposes the traction exerted by the side branches and perivascular connective tissue and the lengthening force caused by pressure in the lumen. Type I collagen is believed to confer tensile strength to the vessel wall, whereas type III collagen may be involved in extensibility . In dilated and morphologically normal segments of varicose veins type I collagen is present in a greater amount than type III. Furthermore, varicose veins contain more type I and type III collagen than do normal veins. It has been found that the elastin content is significantly reduced in dilated segments of varicose veins when compared with both normal veins and normal segments of varicose veins. Microscopically, the ratio of collagen to elastin appears to be significantly increased in the dilated segments of varicose veins. These findings tend to emphasize the important role of elastin in providing a retractile force that opposes development of dilation and tortuosity of the vein wall.
Although collagen accumulation is thought to separate smooth muscle cells within the varicose vein wall, the collagen content of varicose veins is less than that found in normal veins. The bulk of the varicose vein wall is made up of mucopolysaccharides and other ground substances. Varicose veins contain 67% more hexosamine (which comprises about 0.3% of normal vein dry material) than is found in normal veins.
Dysplasticity of the varicose vein wall may explain why varicose veins have an even greater susceptibility to pressure-induced distension than do nonvaricose veins. This anatomical–pathophysiologic correlation has been demonstrated by pharmacologic studies that show reduced maximal contraction of varicose veins compared with control veins. They have also been investigated with in vitro techniques measuring distensibility as a function of infused volumes of saline. However, some investigations have failed to discover a significant difference in the degree of intimal fibrosis between varicose and nonvaricose veins. Therefore fibrosis of the vein wall alone is not totally responsible for the development of varicose veins.
In studying smooth muscle reactivity, the three main vasoconstrictor agents—norepinephrine, angiotensin II, and endothelin-1—were compared. In diseased vein segments, a significant reduction in response to angiotensin II and norepinephrine was seen. It was also noted that there was a reduction in response to endothelin-1. The reduction in angiotensin II affinity appeared at an early stage of varicose disease and supports the hypothesis that such an abnormality within the venous wall could play a role in the pathogenesis of primary varicose veins.
A decrease in tocopherol concentration has been noted in varicose veins. A significant correlation also seems to exist between the inhibition of vessel wall tissue lipoperoxidation and their tocopherol concentration independent of serum concentrations. This may be the result of the protective effect of blocking peroxidation of membrane-associated fatty acids by tocopherol and other antioxidants to prevent vein wall damage. It is clear that the dysplasticity of varicose veins correlates with the changes in their pharmacodynamics and histochemistry. Varicose veins have a demonstrated loss of contractility.
Varicose veins are often complicated by local inflammation and thrombosis. This may be the result of venous hypertension and an inherent histochemical abnormality in the varicose vein/endothelial wall. The formation of arachidonic acid-derived prostanoids was investigated in segments of varicose and nonvaricose veins. Venous production of prostacyclin was decreased, whereas that of thromboxane A 2 and prostaglandin E 2 was increased in the varicose vein segments, regardless of whether they were macroscopically affected or unaffected. It is unknown whether this change in the cyclooxygenase pathway in the varicose vein wall is the cause or effect of its dysplasticity. In addition, histochemical examination discloses a marked increase in the activity of lysosomal enzymes, acid phosphatase, β-glucuronidase and anaerobic isoenzymes (lactodehydrogenase) in primary varicose veins. These enzyme patterns suggest a decline in energy metabolism and an increase in cellular damage in the varicose veins. It has also been found that varicose veins accumulate and metabolize norepinephrine less efficiently than normal veins.
Differences in expression and microscopic localization of matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinases between normal and varicose veins may explain the variability of disease between vein segments. MMP-2 has been found to cause relaxation of contracted vein segments, which could lead to progressive venous dilation, varicose vein formation and chronic venous insufficiency. Whether the abnormal level and/or action of MMP is the contributing factor, or whether protracted increases in venous pressure lead to an increase in MMP expression is unknown. Therefore both anatomical and biochemical abnormalities in the varicose vein wall contribute to its increased distensibility ( Box 3.1 ).
Heredity
Race
Gender
Posture
Weight
Height
Ligamentous laxity (hernia, flat feet)
Occupation
Hormones
Estrogen
Progesterone
Pregnancy
Primary valvular incompetence
Decreased number of valves
Aging
Alcohol and cigarette use
Incompetent perforating veins
Arteriovenous communication
Vein wall weakness
Vein wall metabolic dysfunction
Secondary valvular incompetence
Phlebitis
Deep vein thrombosis
Matrix metalloproteinase activation
Inflammatory mediators
lncRNA and downstream effectors
Genetic factors
There is conflicting evidence regarding the levels of MMP-2 in varicose veins. Increased MMP-2 and MMP-9 has demonstrated the ability to reduce contractility of the vein segment and has been proposed as a mechanism for chronic venous disease. Conversely, decreased MMP-2 levels were found in varicose vein tissue in other studies that could result in extracellular matrix accumulation, hypertrophy, and atrophy in varicose veins. Increased levels of tissue inhibitors of metalloproteinase (TIMP-1) were also evident with decreased MMP-2 levels.
The timing of MMP expression patterns are another important factor in venous disease. Alsaigh et al used microzymographic techniques in vivo to determine that a short rise in blood pressure in combination with a reduction of the fluid sheer stress resulted in early activation of MMP-1, MMP-8 and MMP-9. TIMP-1 and TIMP-2 were also found to be elevated suggesting interplay with MMPs. The damage caused by the early expression of MMPs could be the initiating factor in the inflammatory reaction resulting in venous disease. MMP may also cleave the membrane receptor vascular endothelial growth factor-2 (VEGFR-2) leaning to additional endothelial viability. The shifting equilibrium of MMPs and TIMPs in varicose vein tissues may affect the ability of proteolytic enzymes to degrade components of the extracellular matrix resulting in restructuring of the vein wall.
Inflammatory mediators are another important component in the pathogenesis of varicosities. Transforming growth factor (TGF)-β 1 is a cytokine involved with vascular remodeling that inhibits expression of MMP-1 and collagenase synthesis and increases TIMP expression. Increased levels of TGF-β 1 and isoform nitric oxide synthetase (iNOS) have been shown to be elevated in the tortuous segments of varicosities when compared with nontortuous segments.
Leukocytes use the cellular adhesion molecule L-selectin to bind to the endothelial wall and express integrins to facilitate tissue extravasation. Saharay et al demonstrated increased extravasation and degranulation of leukocytes in patients with venous hypertension. Conversely Junger et al demonstrated decreased expression of L-selectin in lymphocytes in patients with chronic venous disease. The difference in expression of L-selectin could be the result of differences in the disease process of venous hypertension compared with chronic venous disease.
There are a multitude of additional factors that can contribute to the inflammatory process involved with varicose veins. CD4+ T-lymphocytes were found to be significantly elevated in varicosities compared with overall-measured levels of CD4+ cells indicating a possible role in the pathogenesis of chronic venous disease. Additionally, mast cells influence a variety of factors in the inflammatory cascade. Mast cell chymase is an activator for MMP-1 and MMP-3 that can stimulate the release of TGF-β. Mast cells also secrete tryptase that can degrade Type IV collagen, elastin, proteoglycans, and fibronectin resulting in chronic venous disease. Lastly, endothelium exposed to increased pressure results in increased synthesis of proinflammatory cell adhesion molecules such as VCAM, ICAM, and ELAM resulting in the inflammatory state seen in chronic venous insufficiency.
Changes in RNA expression of a gene can be determined by both genetic and environmental mechanisms. Gene expression can be modified by signaling from other genes and also by regulatory elements on the same gene. MicroRNAs, long noncoding RNAs (lncRNAs), and epigenetics are additional complications with gene modification. lncRNAs are nonprotein coding transcripts longer than 200 nucleotides that target different aspects of RNA transcription. lncRNAs can target various components of the transcription reaction, transcriptional repressors or activators, and the DNA duplex to sharply control gene expression. Silencing of the lncRNA-GAS5 (growth arrest specific transcript 5) promoted cell proliferation, migration and cell cycle of human saphenous vein smooth muscle cells. lncRNA-GAS5 directly binds to a calcium dependent RNA-binding protein, Annexin A2. There is evidence that Annexin A2 reduces human saphenous vein smooth muscle cell proliferation. Therefore a low expression of lncRNA-GAS5 may enable human saphenous vein smooth muscle cell proliferation and migration via Annexin A2 as a pathogenesis of varicosities.
Hypoxia has also been considered as a trigger for varicose veins. Oxygenation of the outer two thirds of the saphenous vein is provided by the vasa vasorum and the inner one third by the luminal blood. Acute hypoxia is a trigger for dilation of the vasa vasorum leading to increased blood flow. Hypoxia-activated leucocytes and endothelium have been shown to release mediators regulating vein wall remodeling similar to those seen in varicosities. In vivo studies using venous endothelial cell culture have demonstrated that hypoxia upregulated angiogenesis through the release of proangiogenic factors such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF).
Venous endothelial cells also respond to hypoxia by upregulating hypoxia-inducible factors (HIFs). HIFs are nuclear transcription factors involved with regulating genes involving oxygen homeostasis. Genes regulated by HIFs have been shown to increase the expression of proinflammatory mediators in hypoxic conditions such as VEGF, endothelial isoform of nitric oxide synthase (eNOS), prostaglandin I2 and cyclooxygenase-2.
Hypoxia-inducible factor-1α (HIF-1α) and metallothionein (MT) also have a higher expression in varicocele and varicose veins compared with normal veins. MT is a metal-binding protein that shields against cell apoptosis under hypoxic stress. This function of MT may lead to decreased vascular cell apoptosis and contribute to the thick dilated walls of varicose veins and varicoceles. The evidence for hypoxia as a causative factor in varicosities remains inconclusive because of the poor design of published in vivo studies and heterogenicity. However, similar oxygen content was found in the blood of varicose and nonvaricose veins (see Fig. 3.1 ).
Approximately 75% of the body's total blood volume is contained within the peripheral venous system. The quantity of blood within the legs is a function of body position. When erect, 300 to 800 mL of extracellular and vascular fluids (the quantity varies according to the experimental method and the size of the subject measured) collects in the legs. This includes a 15% increase of blood volume. Thus the venous system, especially in the legs, is an important component of the cardiovascular system's circulatory reservoir. However, the arterial system plays an equally important role in cardiovascular adaptation to postural changes by virtue of changes in arterial resistance. In fact, studies have demonstrated that reflex changes in venous tone are not essential for this fluid shift.
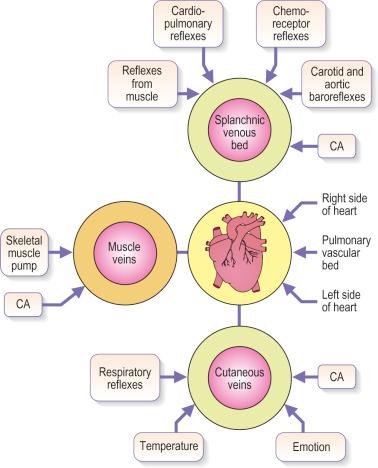
Venous blood pressure is determined by several factors. Among these are pressure generated by the heart, energy lost in the peripheral resistance of arterioles, hydrostatic gravitational forces, blood volume, anatomical composition of the venous wall, efficiency of one-way valves, vein wall distensibility (determined by hormonal, systemic alcohol and other factors), and contraction of venous smooth muscle as influenced by ambient temperature and sympathetic and parasympathetic nerve tone ( Fig. 3.5 ).
Although arterial pressure is one factor in the development of venous pressure, arterial hypertension is a factor associated with the development of varicose veins in some epidemiologic studies, but not others. Curiously, atherosclerotic disease has been linked epidemiologically to varicose veins, although it might be two common conditions occurring concurrently. It is postulated that this coincidence may be related to an atherogenic risk profile, caused primarily by coexistent inactivity, obesity and hypertension. At rest, in the erect position, pressure in the saphenous vein is determined primarily by the height of the column of blood from the right atrium to the site of measurement (90 to 120 mmHg at the ankle) ( Fig. 3.6 ). Contraction of calf muscles generates pressures of between 200 and 300 mm Hg.
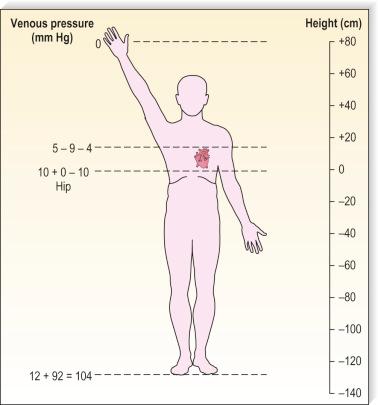
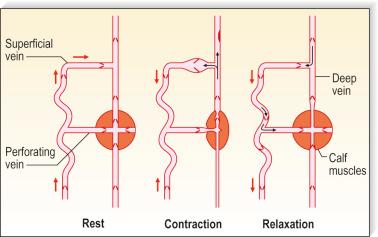
Pressure generated deep within the fascia, outside of muscles, is between 100 and 150 mmHg ; however, with muscular activity, pressure in the normal saphenous vein at the level of the malleoli falls 45 to 68 mmHg below the resting level. It is reduced from 80 to 40 mmHg in the posterior tibial vein. Because of the one-way valves, blood flow is directed from the superficial venous system to the deep venous system through perforating vessels (see Fig. 1.4 ). This has been demonstrated visually by serial phlebography of the normal lower leg. The venous blood then flows towards the heart.
The venous pump in the foot is an important portion of the muscle pump of the lower leg. Weight bearing is usually necessary to propel blood up the leg. Bidirectional ultrasound velocity detector tracings of venous blood flow through the popliteal vein have demonstrated the importance of dorsiflexion of the foot when there is no weight bearing. Therefore full flexion of the foot is important after sclerotherapy to maximize the efficacy of the lower extremity muscle pump.
Respiration produces alterations in intra-abdominal venous pressure. This ‘abdominal venous pump’ contributes to the flow of blood even when an individual is erect. Inspiration produces a rise in venous pressure in the external iliac vein, common iliac vein and inferior vena cava when measured in both the horizontal and erect positions (6.3 mmHg and 8.7 mmHg, respectively).
In the supine position, blood flows evenly along all superficial and deep vessels towards the heart. It is propelled by the relatively small vis-à-tergo (force from behind) from the capillaries, and the respiration-induced aspiration of blood into the abdominal and thoracic veins. In contrast to deep veins, superficial veins have smooth muscle in their walls. This allows contraction of these vessels in response to cold and to drugs such as dihydroergotamine, and allows dilation in response to topical and systemic alcohol, estrogen and light physical trauma. Part of the pathophysiology of varicose veins may be a diminished response of such smooth muscle contraction, as previously described.
Regardless of its cause, chronic venous hypertension in the lower extremities causes an increase in venous diameter. This may lead to valvular insufficiency, which usually causes a reversal of blood flow from the deep veins into the superficial veins through incompetent perforating veins. This ‘private circulation’ may account for as much as 20% to 25% of the total femoral flow involved in a circular retrograde flow ( Fig. 3.7 ).
It has been found that prevalence of reflux in vein segments is correlated with signs of venous insufficiency, but in the general population approximately 12% of limbs with no disease have reflux as detected by duplex ultrasound.
Venous insufficiency has been correlated with standing occupations. A study that compared symptom-free vascular surgeons with normal individuals of nonmedical vocations showed that the superficial system was by far the most common site of venous incompetence in both groups. Vascular surgeons (standing occupation) showed a greater incidence of reflux than the controls. This was true even in subgroups in which reflux was seen in the superficial veins in addition to those with reflux in the deep veins and perforating veins. In patients with symptoms of venous insufficiency, reflux in the great saphenous vein (GSV) territory is found in 85% of limbs, but only 68% of limbs show true saphenofemoral junction (SFJ) incompetence. Reflux is found in 20% of such patients in the small saphenous vein (SSV) territory, and strictly nonsaphenous origin of varicosities is found in 6%. One study demonstrated that 93% of the 10% of patients with nonsaphenous reflux in the study group were women with a mean of 3.2 pregnancies. This implied an association with female sex, hormones and/or number of pregnancies. Studies of causation of reflux focus on venous valves and vein walls. On one hand, an antiproteolytic milieu may favor the deposition of collagen and allow varicosities to develop ; on the other hand, activation of monocytes and conversion to macrophages may cause weakening or destruction of valve segments.
Direction of venous flow in varicose veins has been examined by McPheeters and Rice using fluoroscopy. Reversal of flow caused by incompetent perforator valves is beneficial during sclerotherapy. When a superficial varicosity is injected its venous flow is forced distally to the smaller branching veins where it is arrested (see Chapter 8 ). Thromboembolic disease is thereby prevented.
Superficial veins respond to increased pressure by dilating. Valvular incompetence occurs and varicosities appear. In addition, in muscular contraction, high compartmental pressure, which normally occurs within the calf muscle pump, is transmitted directly to the superficial veins and subcutaneous tissues drained by perforating veins. When this occurs, venous pressure in the cuticular venules may reach 100 mmHg in the erect position. This causes venular dilation over a broad area and may cause capillary dilation, increased permeability and a subsequent increase in the subcutaneous capillary bed through angiogenesis. This is expressed clinically as telangiectasia (venous blemishes). Histologically, cutaneous and subcutaneous hemosiderin deposition may also occur. In time this causes cutaneous pigmentation (see Chapter 2 ). However, some patients with chronic venous insufficiency are able to increase their venous blood flow through exercise. It is postulated that various factors (e.g., sympathetic tone, temperature, tissue metabolites) compensate venous hypertension to normalize cuticular blood flow. This finding demonstrates the complexity of the superficial venous system.
A special situation develops in the area of the medial malleolus. In this area, perforating veins are not surrounded by deep or superficial fascia. Therefore any increase in deep venous pressure is transmitted directly through perforating veins to superficial connecting veins. This causes high cutaneous venous pressures and a transudation of extracellular fluid. This, in turn, leads to perivascular fibrin deposition, which has been blamed for decreased oxygenation of cutaneous and supporting tissues; this was thought to contribute to cutaneous ulceration (see Chapter 2 ). This theory has largely been discredited; the ability of any fibrin screen to prevent oxygen diffusion has never been proven.
The effect of temperature variations on the venous system is well studied. The cutaneous vasculature is intimately involved in thermoregulation. An increase in body core temperature results in cutaneous vasodilation. This does not occur as a result of relaxation of venous smooth muscle but is the result of the reduction in the vasoconstrictor impulses to the vein wall. Such vasodilation also occurs in varicose veins. Strain gauge venous occlusion plethysmography has shown an increase in venous distensibility associated with temperature elevation. Similarly, alcohol ingestion may influence the development of varicose veins. Alcohol intake, in the same manner as increased environmental temperature, causes cutaneous vasodilation. In an examination of 136 men with primary varicose veins greater than 4 mm in diameter, it was found that a significantly increased incidence of varicose veins occurred among men who consumed 4 oz (around 120 mL) of alcohol a day. In another examination of 4903 men and women with varicose veins, alcohol was found to increase the risk of varicose veins in women, and smoking increased this risk in both genders. Further experimental evaluations of these associations are warranted.
In summary, pathologic development of varicose veins can be divided into four broad categories, which may overlap and contribute to each other: increased deep venous pressure, primary valvular incompetence, secondary valvular incompetence and hereditary factors (such as vein wall weakness). All of these categories coexist and are influenced by temperature, alcohol, hormonal and other vasodilatory stimuli ( Box 3.2 ).
Pelvic obstruction (indirect)
Intra-abdominal pressure secondary to Valsalva, leg crossing, constrictive clothing, squatting
Obesity
Saphenofemoral incompetence
Venous obstruction
Perforator valvular incompetence
Venous obstruction
Venous obstruction (thrombosis)
Destruction of venous valves (thrombophlebitis)
Congenital absence of venous valves
Decreased number of venous valves
Vein wall weakness
Deep venous obstruction
Increased venous distensibility
Hormonally induced through pregnancy, systemic estrogens, and progesterones (concentration- and ratio-dependent)
Hereditary Factors
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here