Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
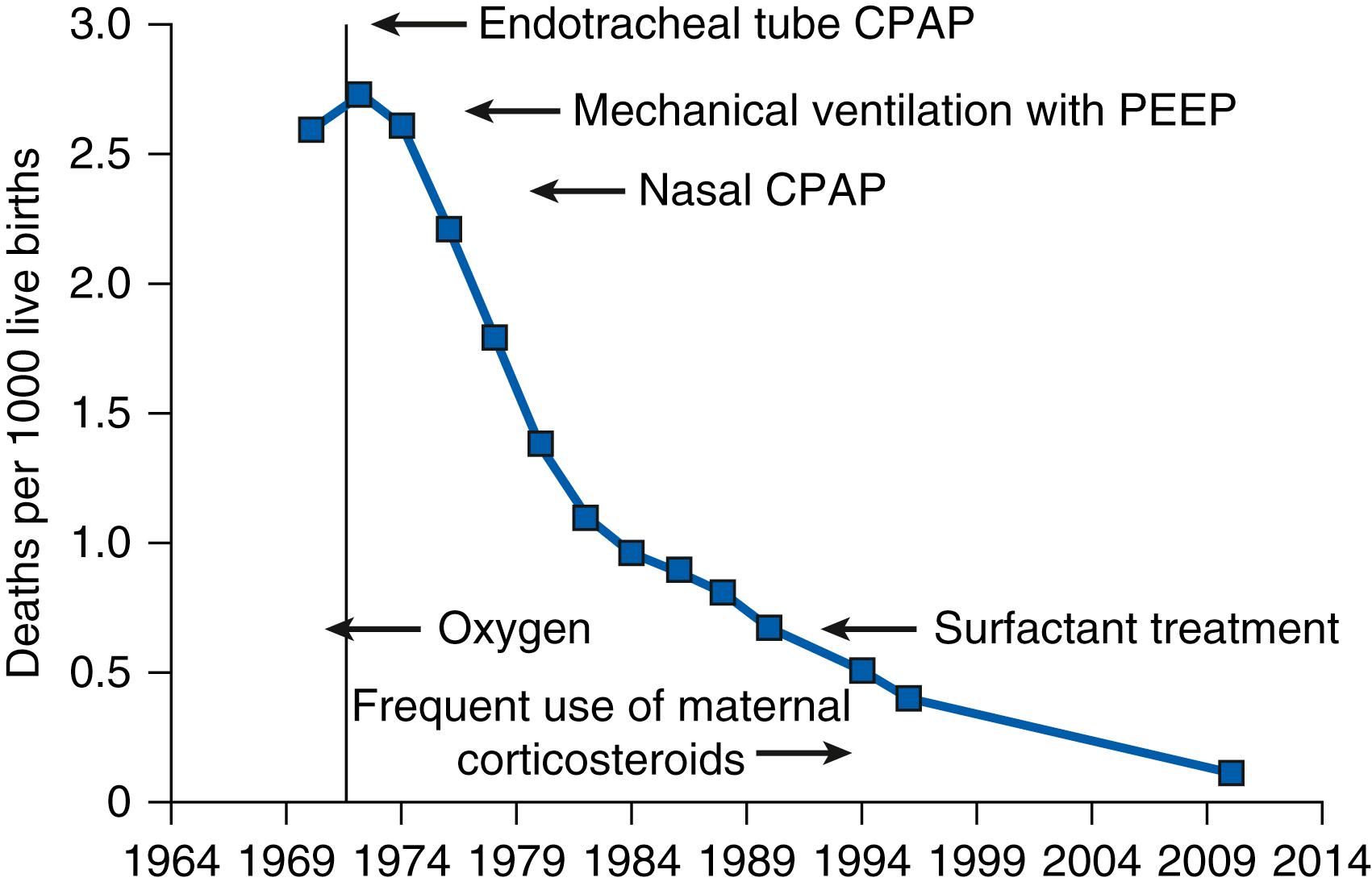
Respiratory distress syndrome (RDS) in preterm infants is the disease most identified with the development of neonatal intensive care. Before the late 1960s, the only therapy for preterm infants who developed progressive respiratory failure shortly after birth was supplemental oxygen; most of these infants died. At autopsy the lungs were atelectatic and had epithelial injury with hyaline membranes, resulting in the name hyaline membrane disease (HMD). Avery and Mead reported in 1959 that saline extracts of lungs of infants with HMD had high minimum surface tensions in contrast to lungs of infants without HMD. The HMD lungs also inflated poorly and collapsed to low volumes at low transpulmonary pressures. Saline lavages of the air spaces recovered small amounts of surfactant lipids that had poor function in vitro . Although mechanical ventilation was used in the 1960s, the mortality rate was high. The first successful therapy was continuous positive airway pressure (CPAP) first described by Gregory and colleagues in 1971. Concurrently, antenatal tests to predict the risk of what is now called RDS were developed using amniotic fluid. Liggins and Howie reported that the risk of having RDS could be decreased with antenatal corticosteroid treatments. These innovations, together with the development of infant ventilators, a better understanding of their use and the physiology of the preterm lung, and improved intensive care for preterm infants (temperature control, nutrition, infection control), resulted in a striking decline in deaths from RDS in the United States from 1970 to 1990 ( Fig. 157.1 ). The care of infants with RDS was simplified and mortality further decreased after 1990 with surfactant therapy (reviewed in Chapter 79 ) and with the widespread use of antenatal corticosteroids (ANS). The strikingly improved outcomes for infants with RDS is the great success story in neonatology. By 2015 and after, an infant in the United States should not die of RDS unless the disease is complicated by severe prematurity or other lung pathologies.
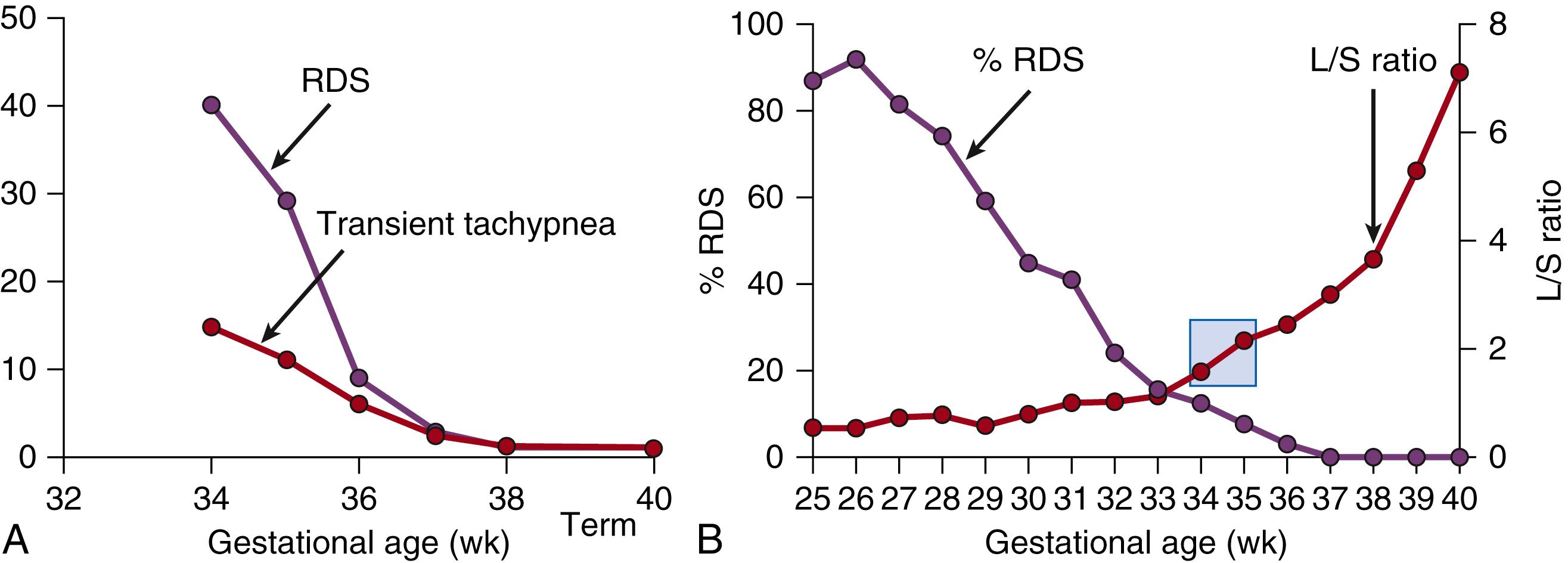
RDS is closely associated with preterm birth, with the incidence increasing as gestational age decreases. The standard diagnosis for RDS requires progressive respiratory failure beginning at or shortly after birth. The respiratory failure is characterized by respiratory distress, clinically identified by tachypnea, grunting, nasal flaring, and chest wall retractions and an increasing oxygen requirement. The chest film shows poor inflation with a uniform hazy and granular appearance on air bronchograms. The odds ratio for RDS at 34 weeks’ gestational age is 40 relative to term infants ( Fig. 157.2A ). The risk is much higher and approaches 100% at gestations below 34 weeks (see Fig. 157.2B ). Furthermore, the risk of RDS is inversely proportional to the lecithin/sphingomyelin ratio. , The human lung is not sufficiently consistently mature to avoid RDS until a gestational age of about 35 weeks, but in clinical practice the incidence of RDS at 35 weeks is only about 20%. This discrepancy is explained by the human lung’s remarkable capacity to induce lung maturation, driven either by abnormalities associated with preterm birth or in response to antenatal corticosteroid treatments.
New approaches to the diagnosis of RDS are point-of-care ultrasound of the newborn lungs, analysis of gastric aspirates for indications of surfactant, or (in the future) maternal plasma blood to identify mRNA indications of lung maturation. Amniotic fluid can be used to determine mRNA markers of lung maturation.
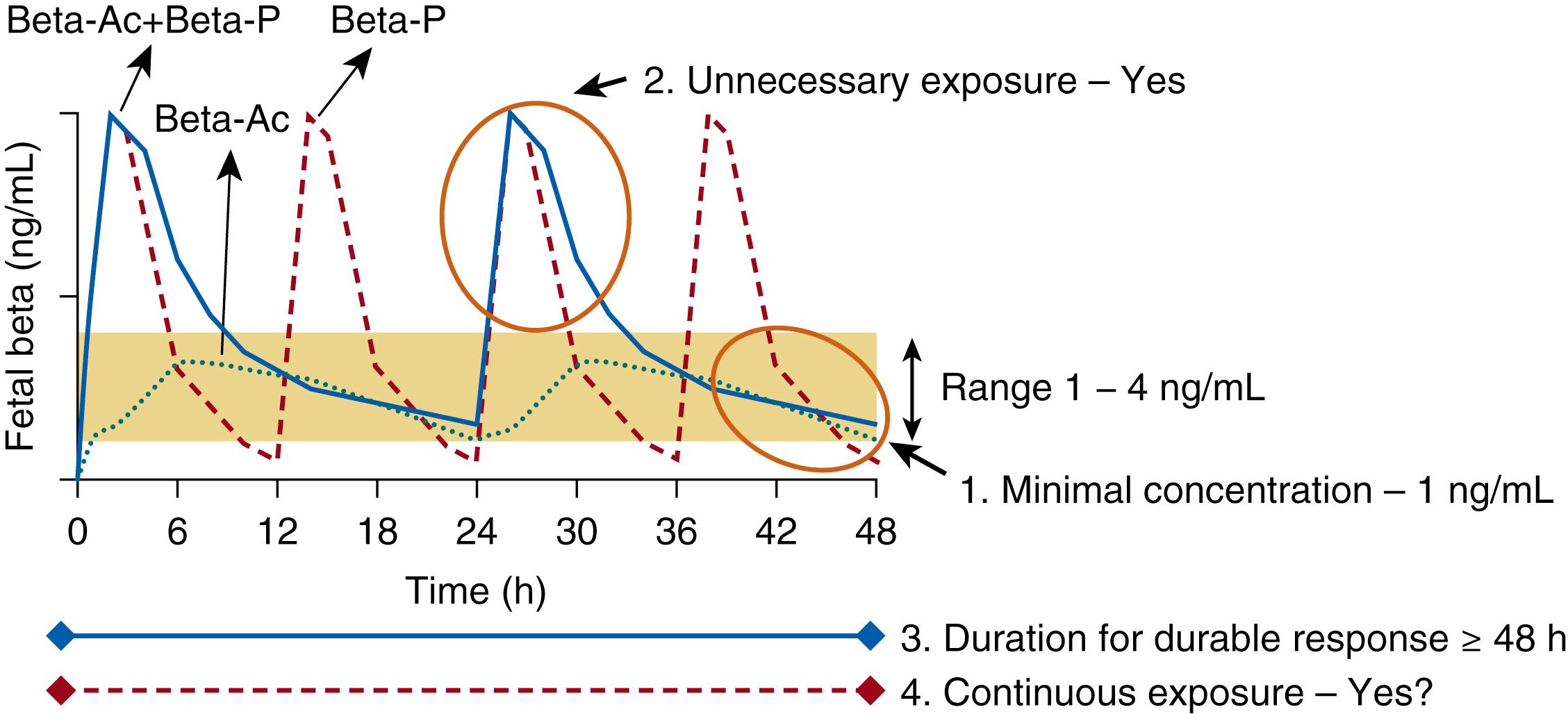
ANS are standard of care at 24 to 37 weeks for women at risk of preterm delivery. However, the standard-of-care treatment is the dosing initially used in 1972 by Liggins and Howie. There has not been an interest in evaluating the treatment, as it is off label and not FDA approved. We have been concerned that the dose is too high and have formally tested lower doses in fetal sheep and rhesus macaque models, together with pharmacokinetic (PK) and pharmacodynamic (PD) studies in reproductive-age, nonpregnant women in India. The concern is to model development of the human fetus for developmental disorders of health and disease (DoHAD)—for very delayed effects on cardiovascular function and metabolic syndrome, as occur in animal models. Fig. 157.3 is a sketch of maternal blood levels for the frequently used treatments. The standard of care in the United States is a 2 to 12 mg dose of a 1:1 mixture of β phosphate + betamethasone acetate (BetaAc). The BetaAc functions as a slow-release reservoir of betamethasone. We tested only the BetaAc form of betamethasone, which is effective for lung maturation in fetal sheep and monkeys, as is the betamethasone phosphate, which yields much higher fetal plasma levels. Therefore, phosphorylated betamethasone or dexamethasone simply exposes the fetus to excess drug. Using infusions of betamethasone phosphate, we determined that fetal exposure of 1 ng/mL was sufficient for lung maturation in sheep or monkeys. A durable response requires greater than 48 hours exposure for lung maturation to have a permanent effect. The exposure needs to be continuous for 48 hours or longer without interruption. , Of interest for low- and middle-income countries (LMIC), oral doses of very inexpensive and stable oral drugs are as effective as intramuscular injections. A concern for the drug used routinely in the United States is that betamethasone is measurable in the maternal blood for greater than 14 days, causing prolonged adrenal suppression and fetal exposures. , To change standard of care, new lower dosing strategies will need to be tested. Our conclusion is that we have used the wrong dose of the wrong drug for 40 years.
This epidemiology and the diagnosis of RDS can be confounded by a number of factors frequently encountered in neonatal practice. Low-birth-weight infants at high risk of RDS often are intubated in the delivery room and treated with surfactant, which prevents a diagnosis of surfactant-deficiency RDS. The management strategy of initiating CPAP therapy in the delivery room to assist newborn transition can mitigate the early respiratory distress and the need for oxygen. , Further, for epidemiologic purposes, the NICHD Neonatal Research Network (NRN) has simplified the clinical diagnosis of RDS. For the interval 1997 to 2002, the NRN diagnosis of RDS required oxygen use for the interval of 6 to 24 hours after birth with some respiratory support to 24 hours and a chest film consistent with RDS. In that era, the incidence of RDS was 63% for infants weighing 500 to 1000 g. In contrast, for the years 2003 to 2007, the diagnosis of RDS was given to 95% of 22 to 28 weeks’ gestational age infants based only on the need for oxygen for more than the first 6 hours of life. In contrast, 69% of 309 patients with birth weights of 0.5 to 1 kg were successfully managed with CPAP and without surfactant in South Africa. The delivery room management of the very preterm infant with CPAP likely can decrease the frequency of the diagnosis of RDS. In contrast, intubation and ventilation in the delivery room are likely to result in a diagnosis of RDS even if the infant has relatively clear lungs and no oxygen requirement. Further, the smallest and most immature infants may need CPAP to maintain functional residual capacity (FRC) and to decrease apnea. These infants may be given a diagnosis of RDS even if surfactant is adequate.
RDS is a diagnosis of exclusion, particularly in the very preterm infant ( Box 157.1 ). Very preterm infants are born because the pregnancy is not normal. A frequent cause of very preterm birth is preeclampsia, which can result in fetal growth restriction and abnormal lung parenchymal and microvascular development in animal models and in infants. The associated respiratory abnormalities may coexist or may mimic RDS. Severe pulmonary hypoplasia is a distinct diagnosis resulting from space-occupying masses in the chest, which inhibit fetal breathing, or a lack of amniotic fluid (Potter syndrome, prolonged rupture of membranes). However, milder variants of pulmonary hypoplasia are probably frequent and difficult to distinguish from RDS. The majority of preterm infants born at less than 30 weeks’ gestational age will have been exposed to histologic chorioamnionitis, and the inflammation in amniotic fluid results in inflammation in the fetal lungs even if frank pneumonia or positive cultures are not identified. Tracheal aspirates of these infants contain inflammatory cells and increased levels of cytokines. The organisms are often low-grade pathogens such as Ureaplasma that do not grow using standard microbiologic techniques (see Chapter 79 ).
Fetal growth restriction/preeclampsia
Chorioamnionitis/fetal lung inflammation
Neonatal pneumonia
Pulmonary hypoplasia
Transient tachypnea of the newborn
Proinflammatory mediators such as lipopolysaccharide, interleukin-1, or live organisms can inhibit alveolar development and cause microvascular injury in the preterm fetal lung. Similarly, ANS inhibit saccular and alveolar development in multiple animal models. In fetal sheep some inflammatory stimuli and ANS increase surfactant and thus mature the fetal lungs. However, the adverse effects of inflammation and interference with lung structural development may contribute to the variable presentations and progression of RDS. Some infants will have frank pneumonia at birth from pathogens such as Group B streptococcus and Escherichia coli with clinical presentations that mimic severe RDS.
The incidence of the diagnosis of transient tachypnea of the newborn increases as gestational age decreases (see Fig. 157.2A ). Transient tachypnea is respiratory distress resulting from delayed clearance of fetal lung fluid from the airways and lung parenchyma. This abnormality is diagnosed primarily in moderately preterm infants or term infants delivered by cesarean section before labor. However, the sodium transporters that help keep the air space free of excess fluid following delivery are developmentally regulated, and low function in the preterm lung likely contributes to RDS. Gastric aspirates from infants with a diagnosis of transient tachypnea also have decreased lamellar body counts and surfactant function. Therefore, RDS likely includes the pathophysiology of delayed fluid clearance and may be indistinguishable from severe transient tachypnea.
RDS is in part a diagnosis of exclusion because the clinician relies only on clinical and radiologic findings. Any cause of respiratory compromise will result in tachypnea, retractions, and flaring in the term and preterm infant. The hazy lungs by radiologic assessment can reflect surfactant deficiency, pneumonia, hypoplasia, or excess fluid, or simply the lungs imaged on expiration in the early hours of life. Although not widely used, a more specific diagnosis can result from analyses of gastric fluid aspirated soon after birth by counting lamellar bodies or measuring the stability of bubbles. , For infants thought to have RDS, perhaps the best diagnostic test is the clinical response to surfactant treatment characterized primarily by an acute increase in oxygenation. Infants can have RDS and other lung abnormalities at the same time. For example, fetuses exposed to chorioamnionitis and funisitis—an indicator of a fetal inflammatory response—will have decreased clinical response to surfactant treatment.
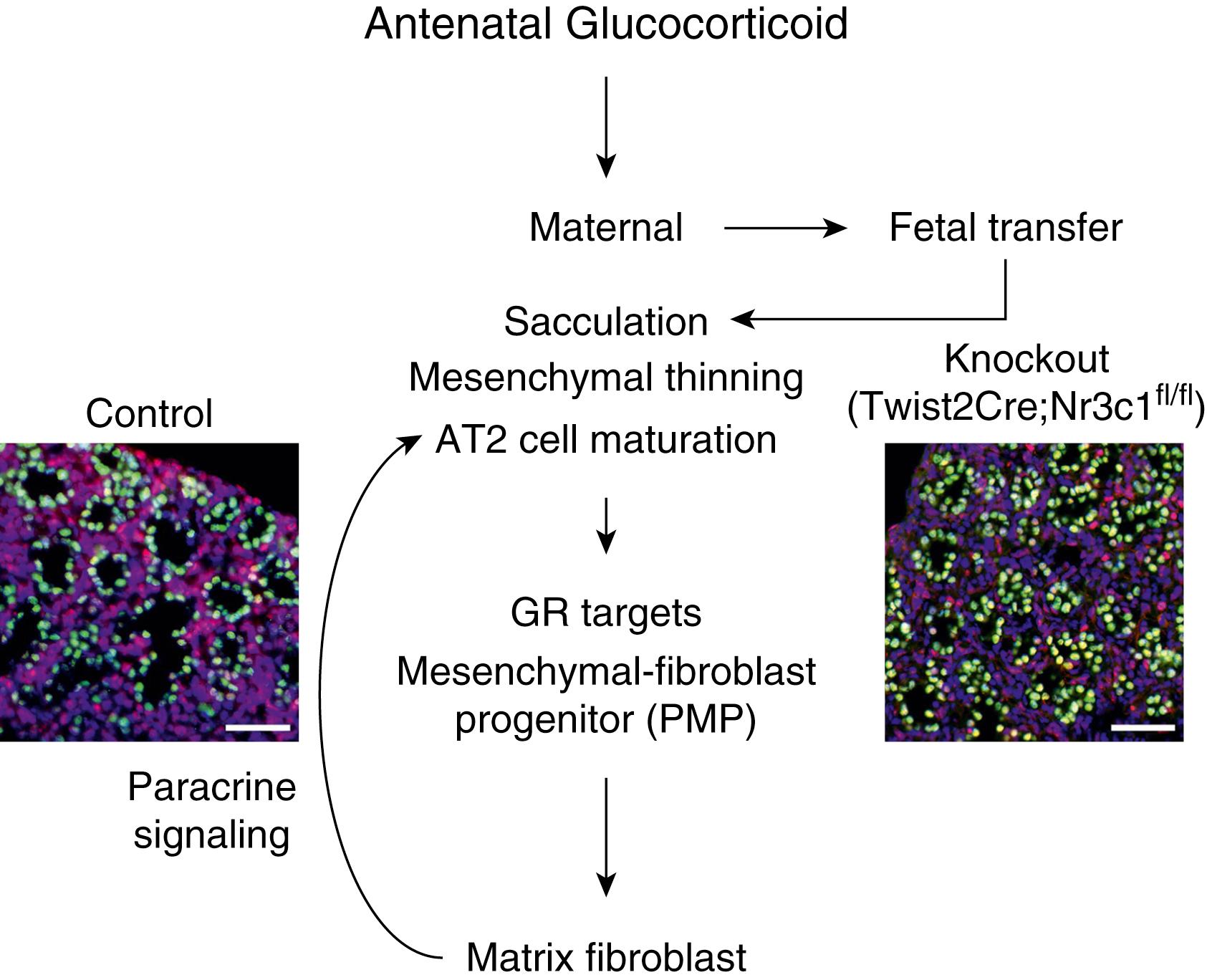
Great progress has been made in elucidating the molecular mechanisms mediating the effects of antenatal glucocorticoids on lung maturation. Transgenic manipulation of gene expression in mouse models has been used to identify cell-specific pathways activated by glucocorticoid signals, which activate fetal lung fibroblasts, in a paracrine fashion, to induce perinatal lung maturation. Likewise, cellular and molecular insights into the pathogenesis of congenital lung malformations and large airway developmental abnormalities are enabled by recent technical advances in cell and molecular biology. Relevant to the effects of ANS are recent data demonstrating that glucocorticoids signal differentiation through a newly identified pulmonary mesenchymal progenitor cell (PMP), which differentiates into matrix fibroblasts by modulation of VEGF, JAK-STAT, and WNT pathways to signal, in a paracrine fashion, to SOX9 + -alveolar epithelial progenitor cells to enhance maturation of alveolar type 2 (AT2) cells and alveolar type 1 (AT1) cells in the fetal lung ( Fig. 157.4 ). This sequence of paracrine signaling differs from previous concepts that considered AT2 epithelial cells as the primary target of ANS to increase surfactant synthesis—an effect on surfactant pool sizes that requires several days. The initial increases in lung gas volume caused by ANS result primarily from the direct effects of glucocorticoids on lung mesenchymal cells.
The lung undergoes dramatic changes in cell number, differentiation, and structure in late gestation, a process that continues after birth. The lung of the term infant contains approximately 30% of the 500 million alveoli that are present in the adult lung ( Fig. 157.5 ). At the margin of viability at 24 weeks’ gestation, the lung has just matured beyond the canalicular stage to the early saccular stage of development. The saccular lung has undifferentiated distal air saccules with a poorly developed capillary microvasculature. The potential surface area for gas exchange of the developing human lung does not increase much before 30 weeks’ gestation. Septation of the distal saccules to form alveoli does not begin until after 34 weeks. The rate of septation to form alveoli is high until term. After birth, the rate of alveolar septation decreases through early infancy, but alveolar development and remodeling probably continue at a slow rate throughout life. Precocious maturation that commonly occurs in preterm infants with maternal glucocorticoid treatment or after exposure to chorioamnionitis accelerates mesenchymal involution and increases surfactant synthesis, but may disrupt alveolar septation, a process with potential long-term consequences on lung growth and structure. ,
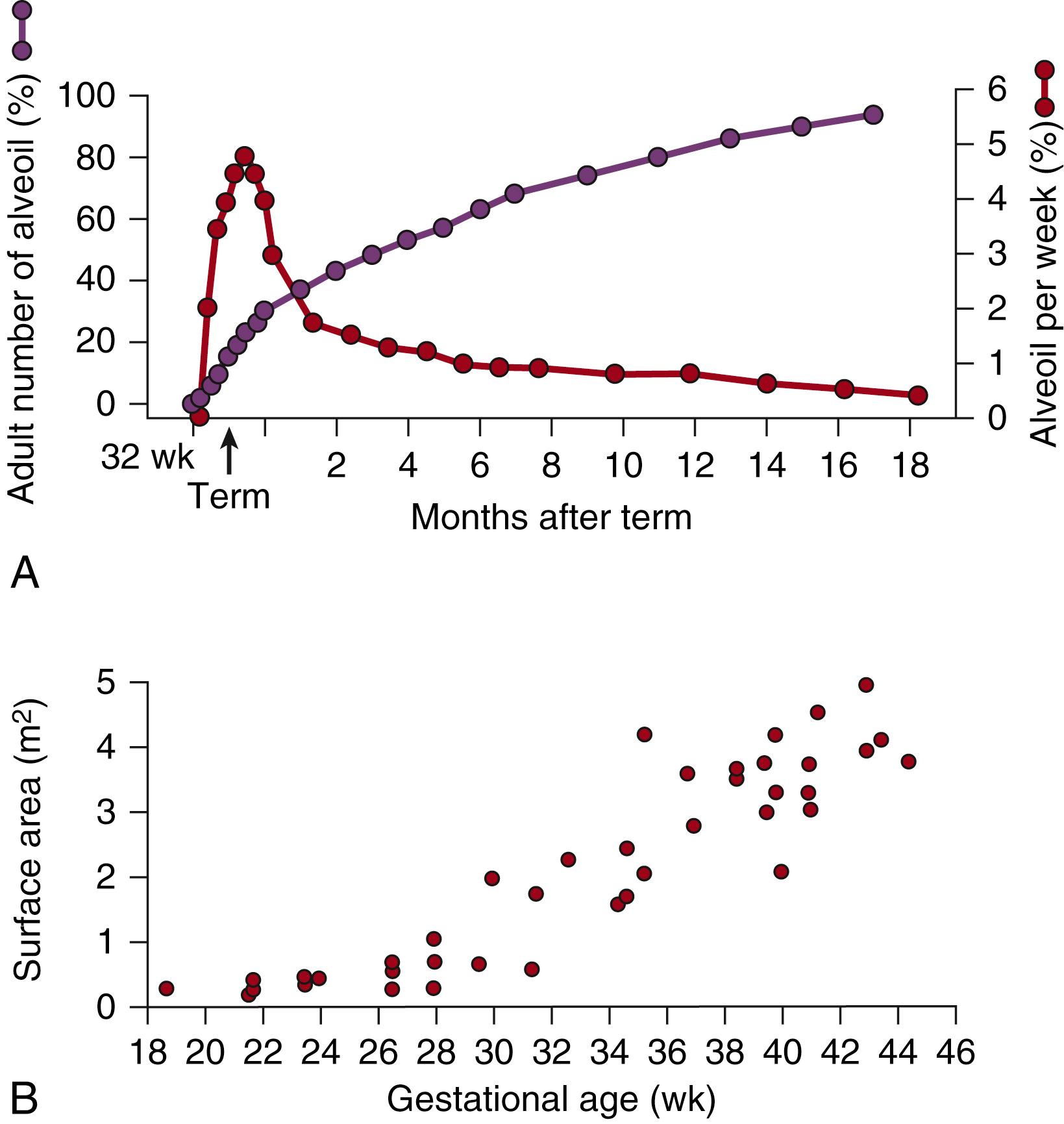
A major factor determining how the preterm lung responds to treatment and injury is the structural maturation of the lung at delivery. The preterm lung with RDS has lung gas pressure-volume characteristics quite distinct from the term or adult lung. As illustrated in Fig. 157.6 , all lung volume variables are less for the preterm infant with RDS than in the adult lung. FRC and total lung capacity (TLC) are lower. Pressures needed to achieve lung opening and TLC are higher for the premature lung. Pressure-volume characteristics resulting from the immature air space structure explain why the preterm lung is more easily injured by mechanical ventilation.
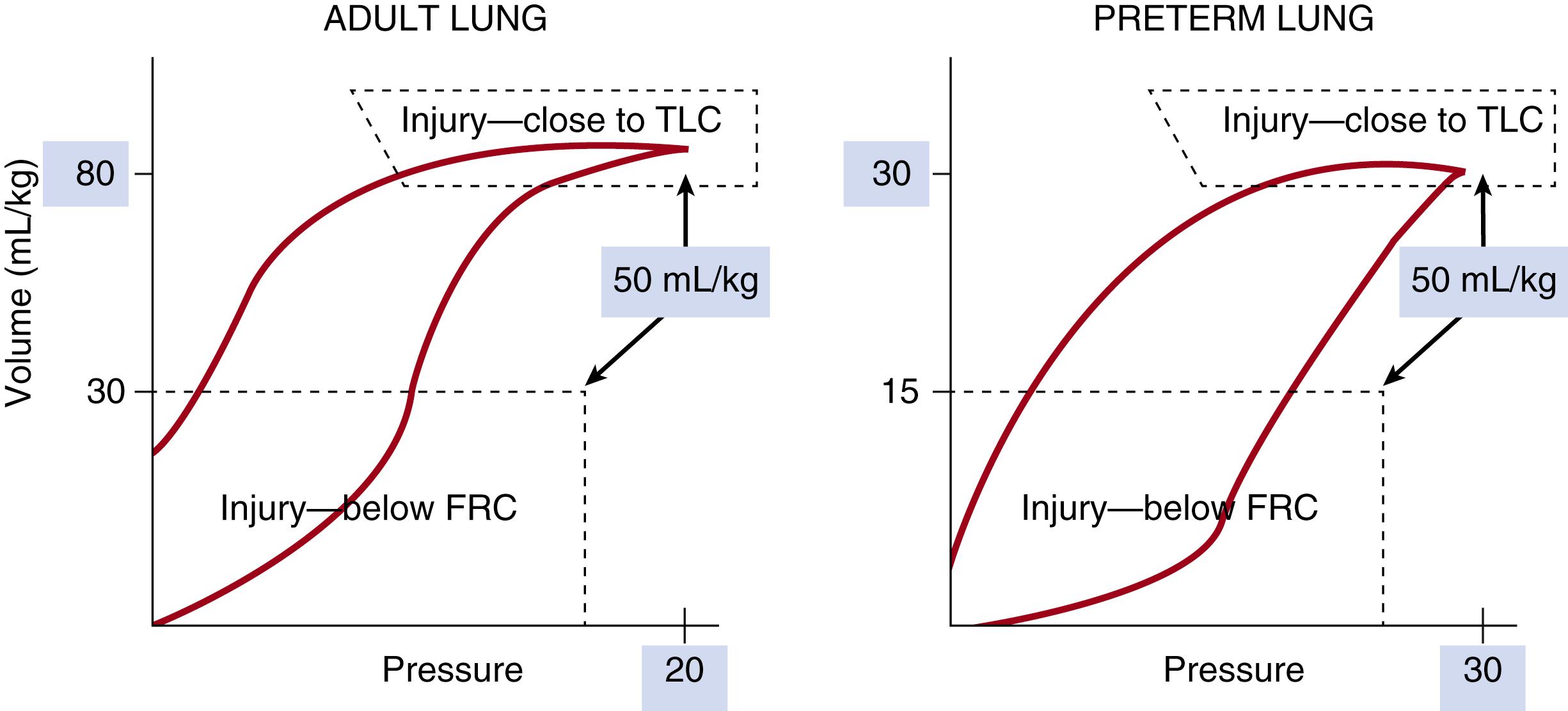
The lungs of infants who have died from RDS are atelectatic with evidence of alveolar and interstitial edema, hyaline membranes, hemorrhage in distal saccules, and distorted small airways. Hyaline membranes are coagula of cell debris, surfactant, and serum proteins caused by epithelial injury, and result in large amounts of soluble and insoluble proteins in the air spaces. The earliest anatomic lesion identified in the lungs of infants who died of RDS shortly after birth is epithelial disruption in the small airways. In preterm surfactant-deficient rabbits, bronchiolar epithelial injury develops within minutes of delivery and ventilation. This injury can be prevented with surfactant treatment ( Fig. 157.7 ). The epithelial damage occurs because the small airways of the immature lung are compliant and distort during ventilation. Lung fluid clearance is not complete, and small airways are fluid-filled at end-expiration. Ventilation requires high peak pressures in the noncompliant lung to achieve relatively normal tidal volumes and carbon dioxide removal.
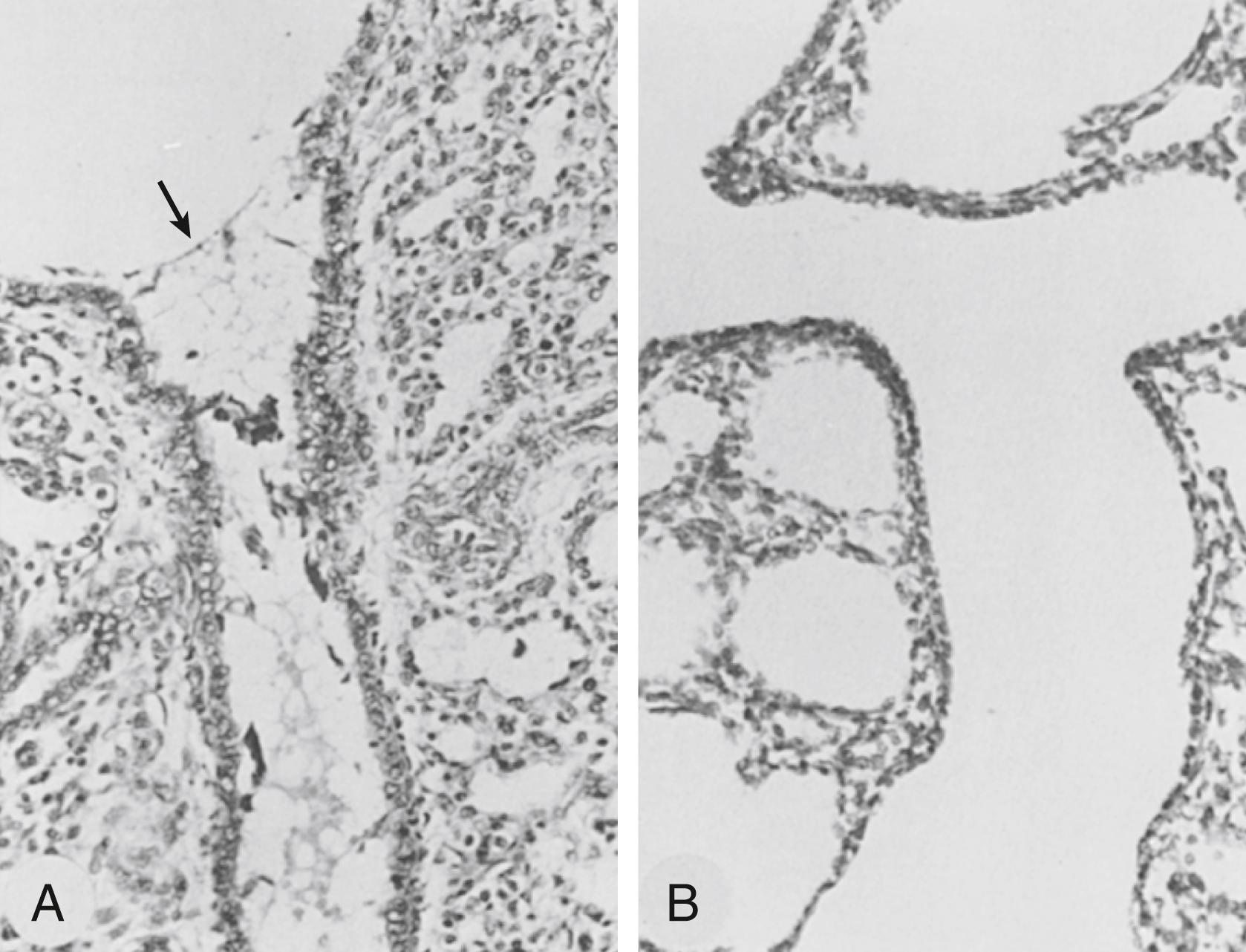
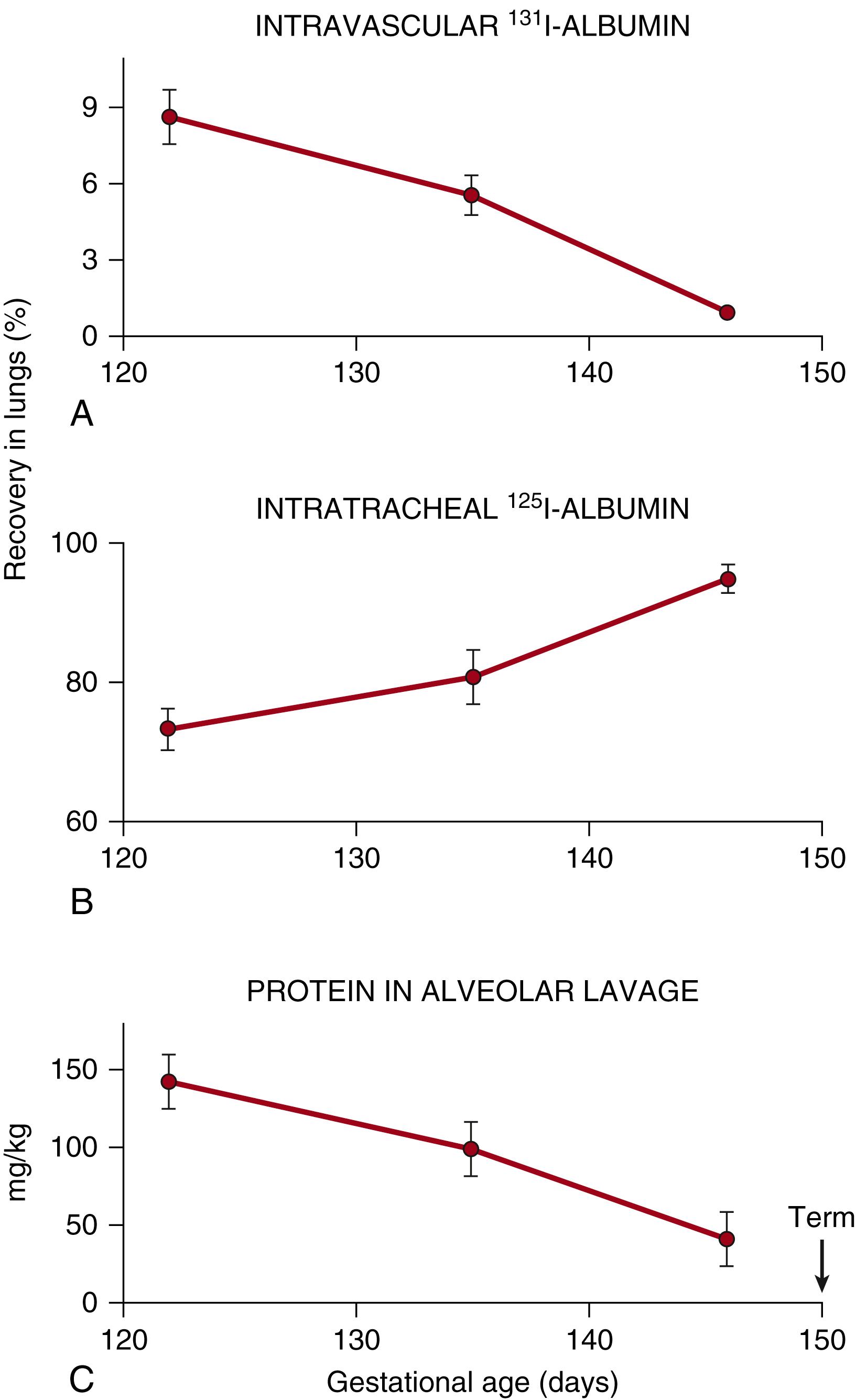
The preterm lung has both endothelial and epithelial abnormalities that cause proteinaceous pulmonary edema after delivery and ventilation. In preterm lambs ventilated for the first 3 hours of life, 11.5% per hour of the labeled albumin mixed with the fetal lung fluid at birth left the lung, and 1.7% per hour of the labeled albumin given intravascularly at birth was recovered from the air spaces, demonstrating the bidirectional movement of albumin across an injured alveolar epithelium. The protein in the alveolar washes increased more than fourfold, indicating that the net protein movement was from the intravascular compartment to the air spaces. The rate of protein accumulation in the air spaces was striking in preterm ventilated rabbits. Approximately 2% to 3% of the intravascularly injected and labeled albumin was recovered from the air spaces within 20 minutes of birth. The leakage was not homogeneous. Leakage involved an increasing number of saccules with time and appeared to occur directly into the alveoli rather than at the bronchiolar level.
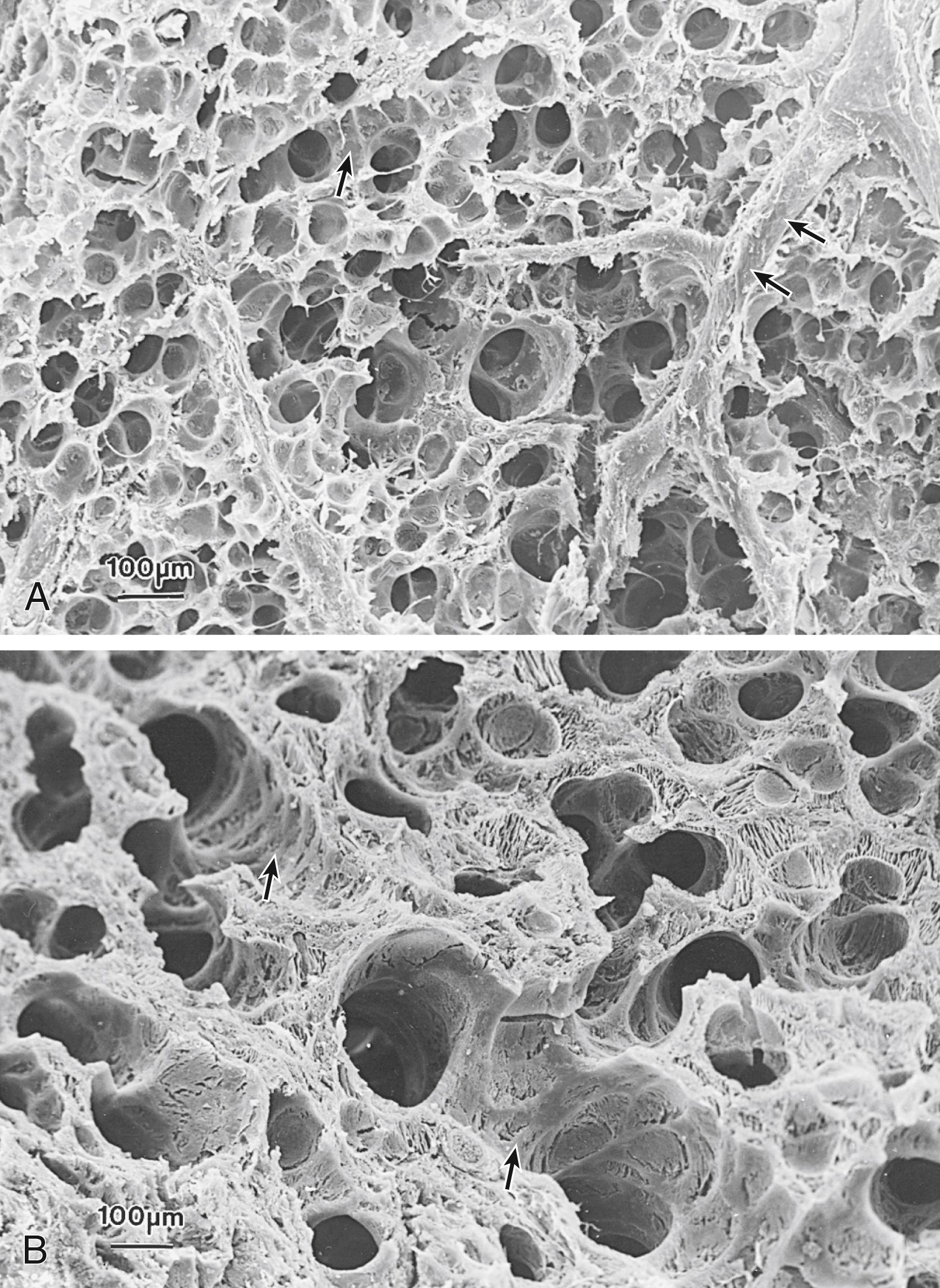
Lung gas volumes of infants with RDS can be low because the lung has not yet developed sufficiently to hold much gas, or because distal air spaces are uninflated (see Fig. 157.6 ). Another cause of the volume loss in the lungs of infants with RDS is alveolar edema. After 3 hours of ventilation, the TLC was 48 mL/kg in preterm monkeys without RDS and 19 mL/kg for monkeys with RDS. The flash-frozen lungs, evaluated by light and scanning electron microscopy, of animals with RDS had overexpanded distal airways and underexpanded and fluid-filled alveolar spaces ( Fig. 157.8 ). By scanning electron microscopy, the alveoli of animals with RDS were filled with proteinaceous liquid, and the interstitium was swollen by edema fluid ( Fig. 157.9 ). The lungs of monkeys with RDS exhibit alveolar and interstitial edema because of the slow clearance of fetal lung fluid after birth and the entrance of proteinaceous edema into the air spaces.
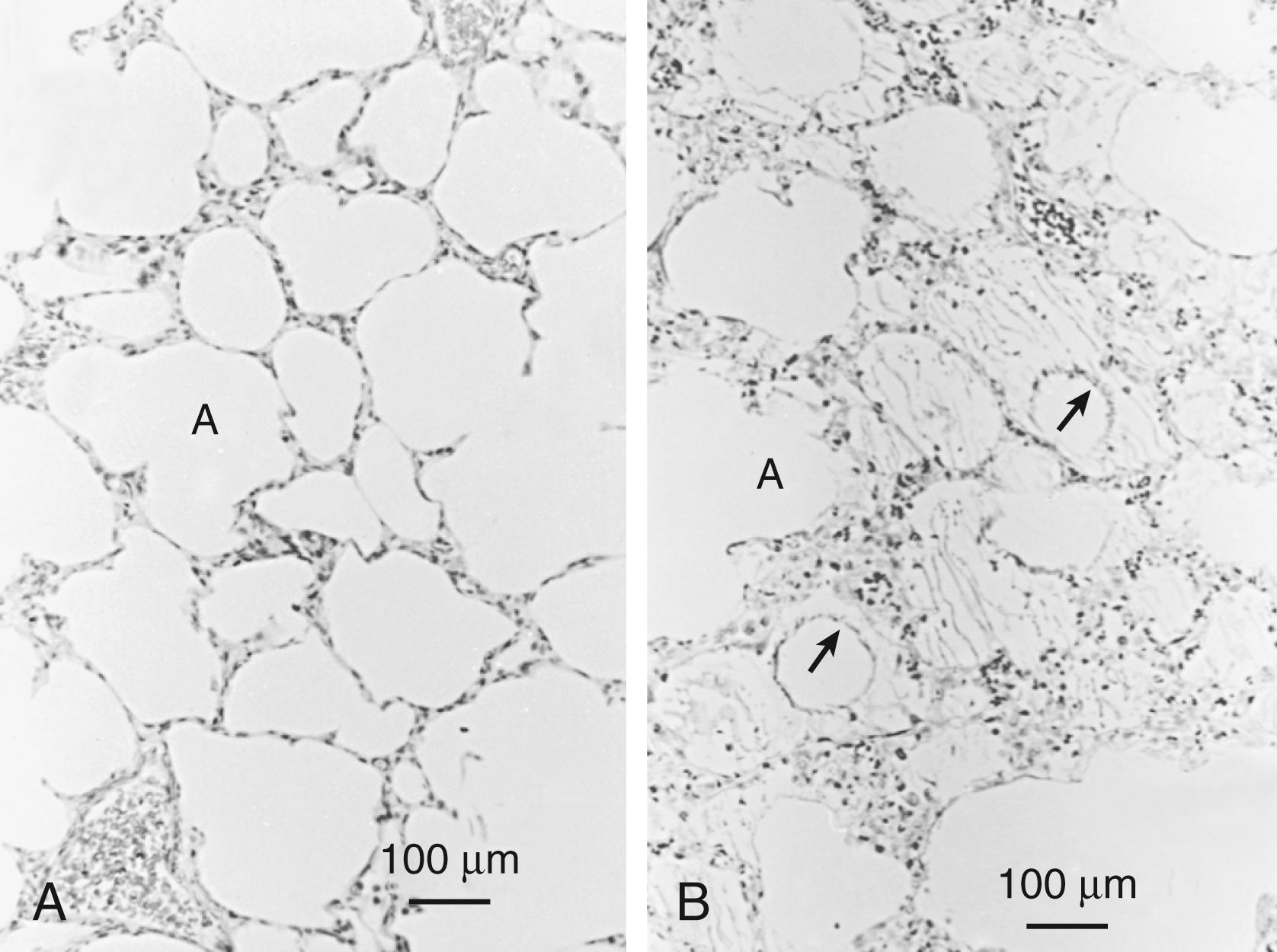
Five clinical variables are important determinants of the amount of edema formation: gestational age, tidal volume, positive end-expiratory pressure (PEEP), antenatal corticosteroid treatment, and surfactant treatment. In preterm animal models, protein leaks from the vascular space to the alveolar space and from the alveolar space to the vascular space increase as gestation decreases ( Fig. 157.10 ). The pressure needed to achieve an adequate tidal volume and gas exchange decreases as gestational age increases. Therefore, a possible explanation of the increased leak is the barotrauma caused by the ventilatory requirements needed to support the more immature lung. In the mature lung, pulmonary edema occurs after lung overdistension using high tidal volumes (volutrauma). High pressures or volumes are not required to injure the immature surfactant-deficient lung because nonuniform inflation causes focal overdistension. Without surfactant treatment, pulmonary edema occurs with relatively low tidal volume ventilation. The end-expiratory volume of the preterm lung also is an important injury variable. Ventilation of preterm lambs with the same tidal volume results in different amounts of protein recovery from the airways, depending on the PEEP used to support end-expiratory lung volume. Antenatal treatment with ANS also can greatly decrease injury and edema. ,
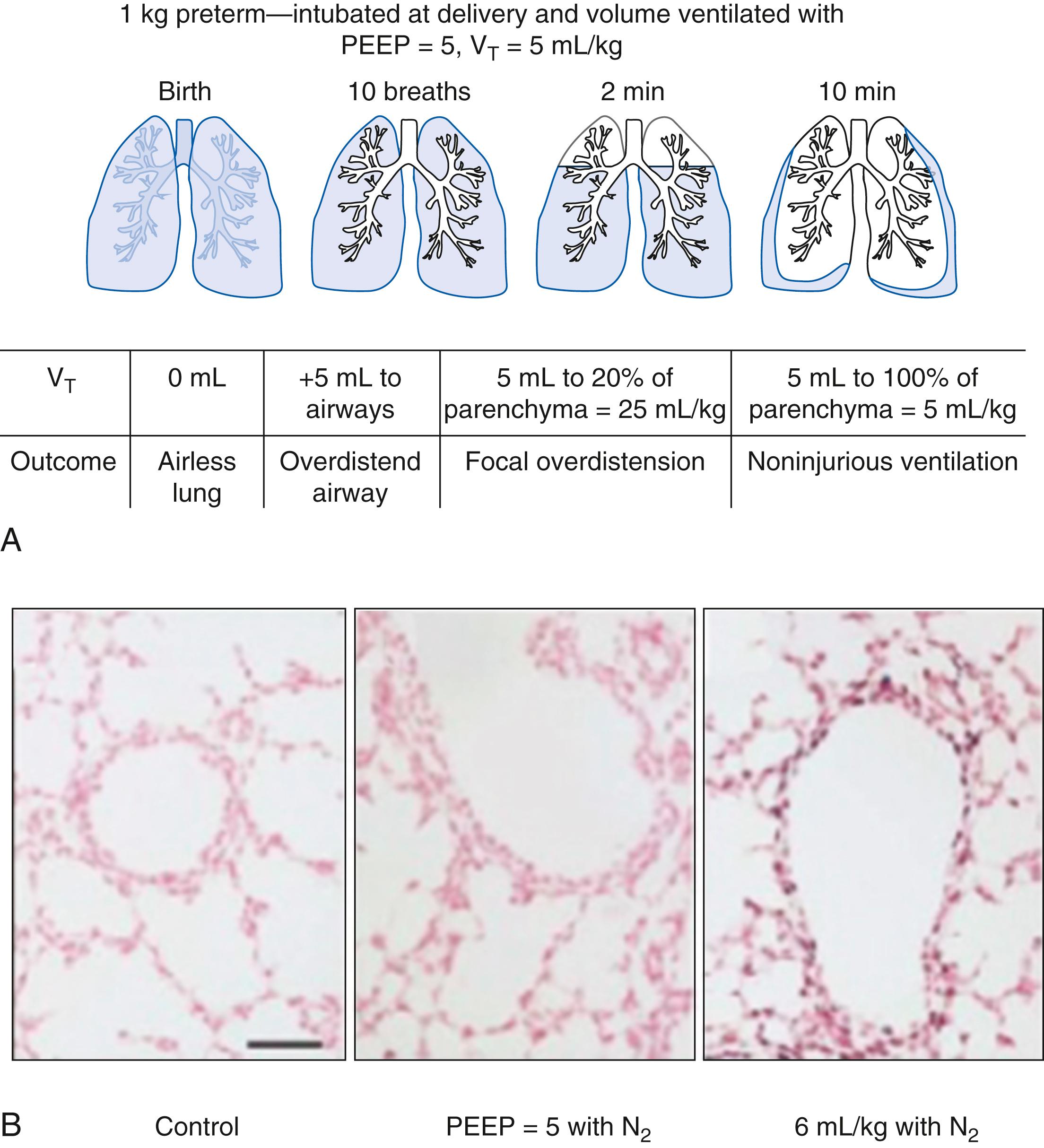
These observations describe outcomes of preterm animals ventilated from birth for several hours. The preterm lung may be most easily injured with the initiation of ventilation at birth because the air spaces are fluid-filled and the clinician wants to establish gas exchange quickly. Tidal volumes increased to 15 mL/kg severely injure the airways of preterm lambs, and subsequent ventilation amplifies and propagates that injury to the lung parenchyma ( Fig. 157.11 ). , The injury can be decreased with lower tidal volumes, the use of PEEP to maintain FRC, and surfactant treatment. These experiments in animal models provide the context for understanding why the gentle recruitment of tidal volume in the delivery room when supported by CPAP can decrease the number of infants diagnosed with RDS.
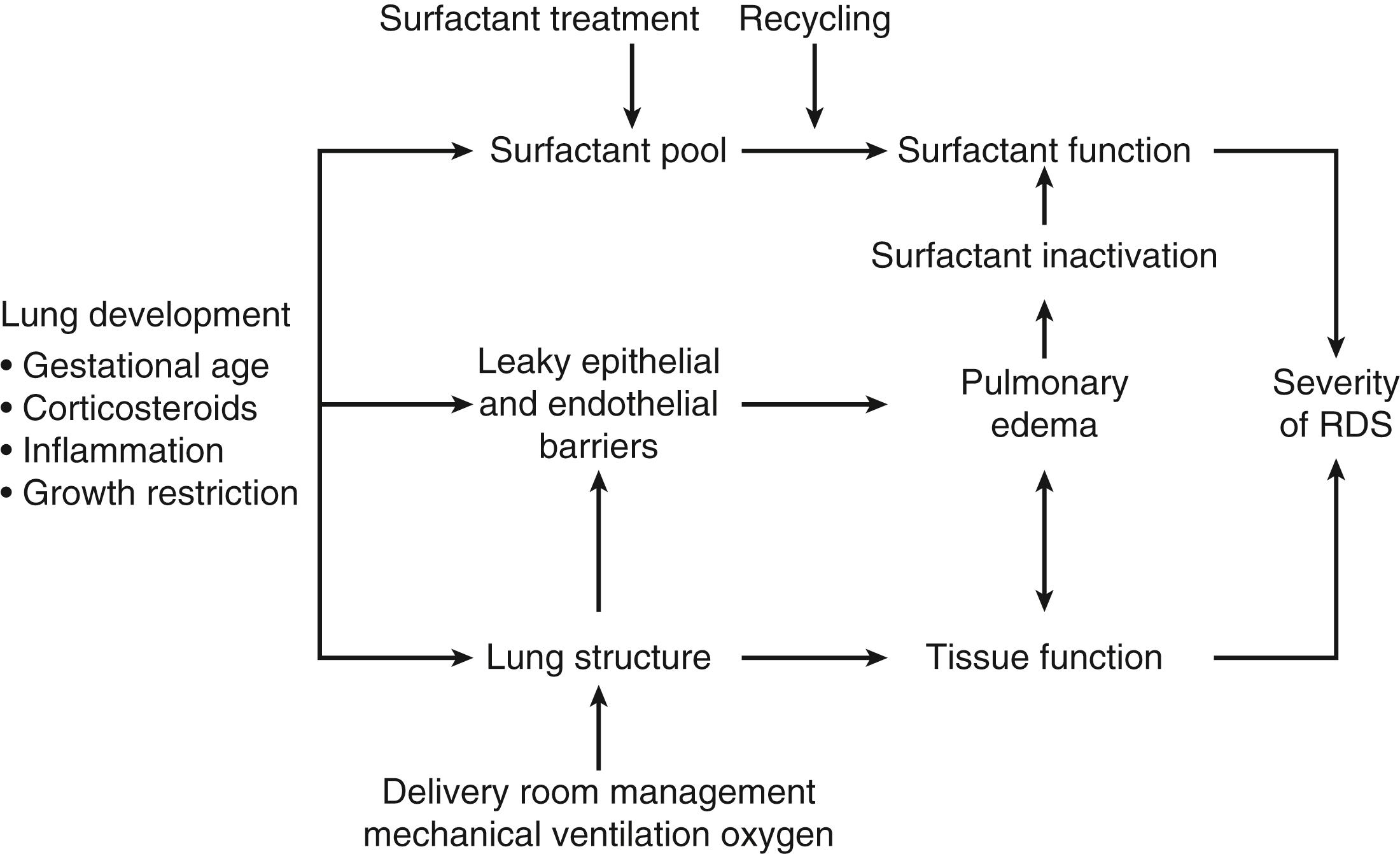
An integrated perspective on the major variables that determine the pathophysiology of RDS is shown in Fig. 157.12 . Lung development as modified by gestational age and exposures such as ANS, inflammation, and growth restriction determine the lung structure and the amount of surfactant that the preterm infant has at birth. The preterm lung structure can be injured to alter tissue function, primarily by epithelial disruption causing pulmonary edema. An inadequate surfactant pool can be corrected with surfactant treatment. The integration of these variables primarily determines the severity of RDS.
Surfactant metabolism in the adult lung is the basis for the discussion of surfactant metabolism in the preterm lung with RDS ( Fig. 157.13 ). Extensive descriptions of the surfactant lipids and proteins, the biophysics of surfactant function, and regulation of synthesis and secretion are found in Chapter 73, Chapter 74, Chapter 75, Chapter 76, Chapter 77, Chapter 78, Chapter 79, Chapter 80 . Surfactant lipids are synthesized from glucose and lipid precursors by type II cells in the alveoli. The lipids are processed via the Golgi to multivesicular bodies that aggregate the lipids with the surfactant protein SP-B and SP-C. The surfactant is stored in membrane-enclosed lipid and protein structures called lamellar bodies in the type II cells. Lamellar bodies are secreted by exocytosis either constitutively or when type II cells are stimulated by secretagogues such as β-agonists and purines or by mechanical stretch. The other SPs, SP-A and SP-D, are primarily secreted by type II cells independently of the lamellar bodies.
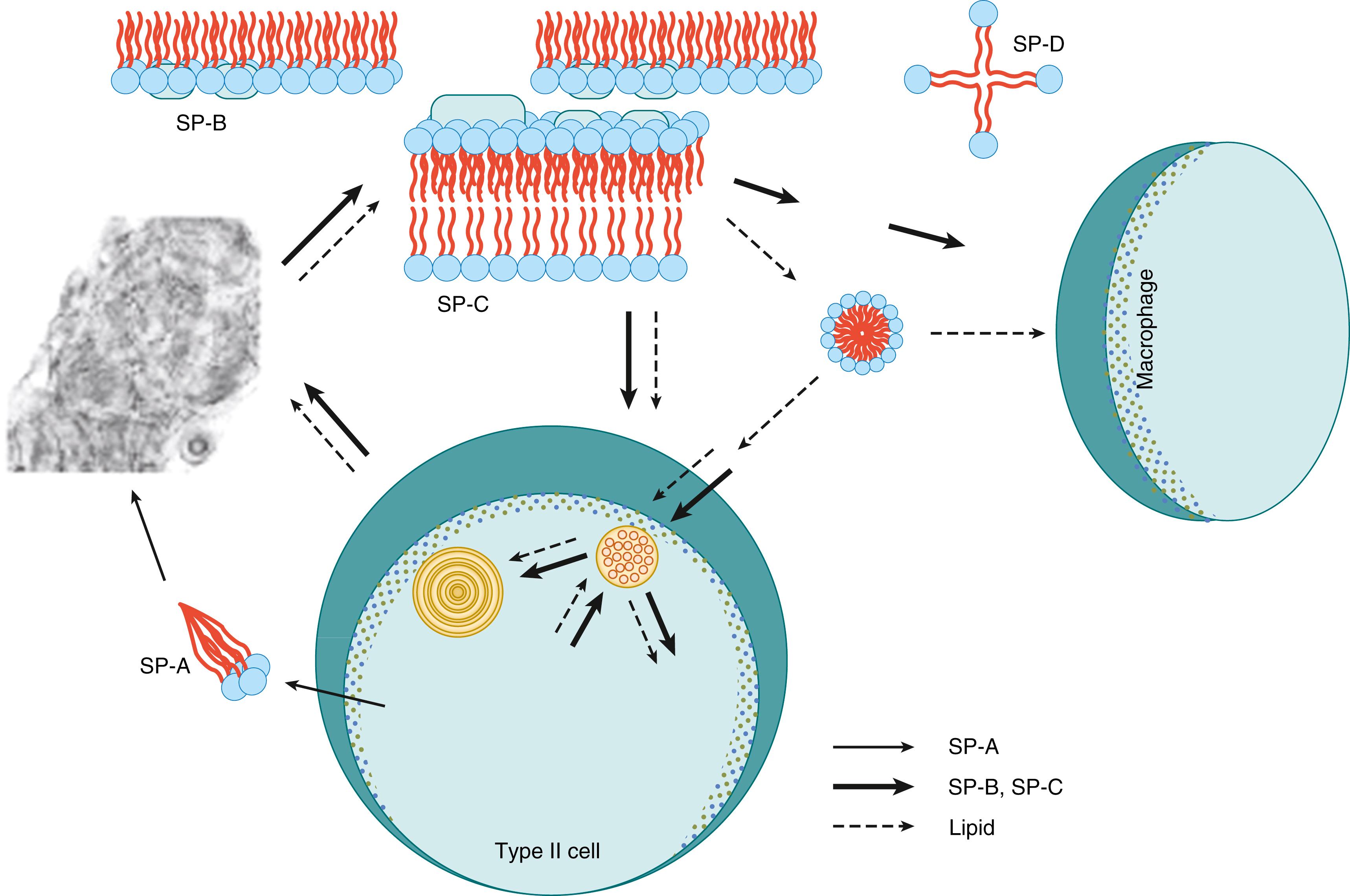
Surfactant has an extracellular life cycle once secreted to the thin fluid hypophase lining the alveoli and small airways of the healthy lung. The SP-A associates in the hypophase with the lipids plus SP-B and SP-C to form tubular myelin and other loose lipid arrays that are macroaggregated lipoprotein forms. These large lipoprotein aggregates are the forms of surfactant that adsorb to the air-water interface. The surfactant film is multilayered and continuously replenished from surfactant in the hypophase. Lipids are cleared from the air spaces as small liposomal vesicles that contain the phospholipids but essentially no SPs. The SPs are cleared separately by macrophages and type II cells. Approximately half of the surfactant components are catabolized by alveolar macrophages, and the other half are catabolized by type II cells in the adult mouse lung. However, the type II cells also take up phospholipids, SP-B, SP-C, and SP-A and recycle them via multivesicular bodies back to lamellar bodies for resecretion. At steady state, the alveolar surfactant pool is very metabolically active and is replaced every 5 hours. The airway clearance rates and efficiencies of recycling for SP-A, -B, and -C are similar to the lipids.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here