Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The natural history of aortic dissections associated with aortic rupture or end-organ malperfusion invites a careful study of the structural properties of the false and true lumens (why the false lumen ruptures) and of the mechanisms of branch artery obstruction (how the dissection causes malperfusion). These same structural properties of the dissected aortic wall also have significant implications for effective open and endovascular treatment of aortic dissections, which are discussed in another chapter.
The pathognomonic feature of aortic dissection is hemorrhage within the aortic wall, typically near the junction between the inner two thirds and outer one third of the media. This creates a new vascular space within the aortic media called the false lumen. In approximately 95% of patients, the hemorrhage is associated with an entry tear extending through the intima to the plane of hemorrhage within the media. The false lumen might enlarge by propagating circumferentially and longitudinally. If the false lumen tears back into the original (true) lumen or tears into the origins of branch arteries that it encounters during propagation, it can begin to conduct blood flow like the true lumen. If it does not establish significant flow, it can thrombose.
The first type of dissection is the double-barrel aorta, in which both the true and false lumens are patent. Clinicians usually use the phrase aortic dissection to refer to this classic type of dissection. Typically, the entry tear is between the aortic root and the proximal third of the descending aorta and lies on a background of a featureless intima. Despite the catastrophic and often bizarre clinical presentation, fulminating clinical course, and obvious gross pathologic findings in most cases of aortic dissection, the initiating event is most often unknown.
The two hallmarks of dissection, the propagating wedge of intramural hemorrhage and the intimal tear, have each been invoked as the primary culprit. Yet the first minutes of an aortic dissection remain purely speculative: If the intimal flap tears first, what disrupted it? If the vasa vasora undergo disruption with hemorrhage into the media first, why did they rupture? Much has been learned about aortic wall biology and the pathoanatomy and natural history of dissection, and considerable progress has been made in the endovascular treatment of dissections, without answering the etiologic questions. A better understanding of the initating events at the onset of an aortic dissection might suggest a specifically targeted preventive therapy.
In the second type of dissection, the false lumen is thrombosed, a condition clinically referred to as intramural hemorrhage or intramural hematoma (IMH). Although IMH is classically described as aortic dissection without an entry tear, in practice most cases are classified based on cross-sectional imaging, whose specificity for revealing existing tears is unknown. In these cases the etiology is thought to be hemorrhage from the ruptured vasa vasorum.
In a model of aortic dissection with no blood flow and equal pressures in the true and false lumens, the true lumen immediately collapses and the false lumen immediately becomes ectatic and larger, resulting in an overall increase in aortic cross section. In this hydrostatic model of dissection, immediate false lumen expansion depends on the percentage of circumference of the wall involved by the dissection and absolute blood pressure. Immediate true lumen collapse depends on the percentage of circumference of the wall involved by the dissection, but it is comparatively insensitive to absolute blood pressure. Both false lumen expansion and true lumen collapse also depend on the depth of the dissection within the media because this determines the amount of elastin distributed between the outer wall of the false lumen and the dissection flap, although this feature has not been assessed in a hydrostatic model. In a way that is incompletely understood at present, the size and location of entry and reentry tears and outflow into peripheral vessels also contribute to true lumen collapse.
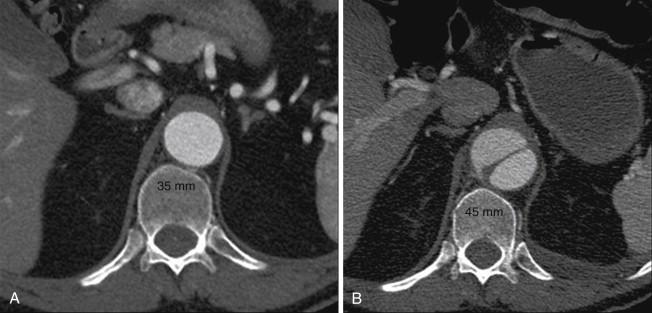
Anecdotal review of computed tomography (CT) scans in patients who fortuitously had a study at the time of acute dissection as well as one shortly before the dissection show that the dissected aorta can acutely grow to a diameter 25% greater than its baseline ( Figure 1 ). The anatomy of the two lumens explains this phenomenon. The normal aorta expands as a result of blood pressure until wall tension generated by elastic recoil of the mural elastin and collagen balances blood pressure. In aortic dissection, the dissection flap typically contains the intima and two thirds of the media, and the outer wall of the false lumen contains the remaining one third of the media and the adventitia. Being thinner and less elastic than the outer wall of the undissected aorta, the outer wall of the false lumen must expand to a larger diameter to generate, at a given blood pressure, the same wall tension. The dissection flap, which lies between isobaric lumens, has been released from transmural pressure and therefore undergoes radial elastic collapse. Thus false lumen dilation and true lumen collapse are to be expected, given the structural characteristics of the aortic wall.
The availability of stent grafts invites us to consider the reversal of this process: What would happen if a covered stent graft were deployed across the communication between the lumens, covering the tear and sequestering the false lumen? In this case, transmural pressure would be restored to the dissection flap and partially removed from the outer wall of the false lumen. The dissection flap would expand, the outer wall of the false lumen would contract, and, assuming the false lumen could vent its contents, the false lumen would disappear. Current endograft trials will validate or nullify this hypothesis.
Immediately following a double-barrel aortic dissection, the inner surface of the false lumen wall, which is lined by bare media, is highly thrombogenic. Additional factors favoring early thrombus formation are the areas of stasis in the false lumen, where the flap meets the outer wall of the aorta and in the blind-sock extremes of a dissection, as well as areas in the true lumen distal to an obstructing flap.
In the chronic phase of a double-barrel aortic dissection, the patient is left with a false lumen of which the outer wall is histologically indistinguishable from an aneurysm, except where the pathognomonic junction of flap with outer wall is present in the section. Like any other aneurysm with greatly attenuated wall components, the false lumen is prone to wall degeneration, gradual increase in size, and rupture.
Ischemic complications ( Box 1 ) can be divided into obstructions directly caused by the dissection flap and those not directly resulting from it. Obstructions directly caused by the flap are divided into a static or fixed obstruction resulting from the flap entering and narrowing the origin of a vessel; a dynamic obstruction, in which the flap prolapses across the vessel like a washcloth across a bathtub drain; and a mixed static and dynamic obstruction ( Figure 2 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here