Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hypertension implies an increase in either cardiac output or, more typically, in systemic vascular resistance (SVR). Essential hypertension developing in young adults may be initiated by an increase in cardiac output, associated with signs of overactivity of the sympathetic nervous system; the blood pressure (BP) is labile, and the heart rate is increased. Later, the BP increases further because of a rise in SVR, with return to a normal cardiac output. Most patients in clinical practice with sustained hypertension have an elevated SVR. Over time, vascular remodeling and microvascular rarefaction (loss of vessels and capillaries) contribute a structural component.
The abrupt left ventricular systole creates a shock wave that is reflected back from the peripheral resistance vessels and reaches the ascending aorta during early diastole and is visible in tracings of aortic pressure as the dicrotic notch. With aging, there is loss of elasticity of the conduit vessels that transport the pressure wave more rapidly and an increase in the tone of the resistance vessels that provides the reflected shock wave. Eventually, this shock wave in the aorta coincides with the upstroke of the aortic systolic pressure wave, leading to an abrupt increase in the height of the systolic BP. This accounts largely for the frequent finding of isolated, or predominant, systolic hypertension in the elderly. In contrast, systolic hypertension in the young usually reflects an enhanced cardiac contractility and output.
The integration of cardiorenal function is illustrated by the response of a normal person to standing. Upon standing, there is an abrupt fall in venous return and, hence, in cardiac output; this elicits a baroreflex response, as resistance vessels constrict to buffer the immediate fall in BP, and capacitance vessels contract to restore venous return, resulting in only a small drop in the systolic BP with a modest rise in heart rate. During prolonged standing, increased renal sympathetic nerve activity enhances the reabsorption of sodium chloride (NaCl) by the kidney tubules, as well as the release of renin from the juxtaglomerular apparatus. Renin release and the subsequent generation of angiotensin II and aldosterone maintain systemic BP and circulating volume. In contrast, the BP of patients with autonomic insufficiency declines progressively upon standing, sometimes to the point of syncope. Patients with autonomic failure vividly illustrate the crucial importance of a stable BP for efficient function of the brain and kidneys. Therefore, it is no surprise that evolution has provided multiple, coordinated BP-regulatory processes. The understanding of the cause of a sustained change in BP, such as hypertension, requires knowledge of a number of interrelated pathophysiologic processes. The most important and best understood of these are discussed in this chapter.
The kidney has a unique role in BP regulation. Kidney salt and water retention sufficient to increase the extracellular fluid (ECF) volume, blood volume, and mean circulatory filling pressure enhances venous return, cardiac output, and BP. The kidney is so effective in excreting excess fluid during periods of surfeit, or retaining fluid and electrolytes during periods of deficit, that the ECF volume and, specifically, the blood volume normally vary less than 10% with changes in salt intake. Consequently, the role of body fluids in hypertension is subtle. For example, a 10-fold increase in daily NaCl intake in normal subjects increases ECF volume by only about 1 L (about 6%) and normally produces no change, or only a small increase, in BP. Conversely, a diet with no salt content leads to the loss of approximately 1 L of body fluid over 3 to 5 days with only a trivial fall in BP. Different effects can be seen in patients with chronic kidney disease (CKD) whose BP often increases with the level of salt intake. This “salt-sensitive” component to BP increases progressively with the loss of kidney function. Among normotensive subjects, salt-sensitive BP is apparent in about 30% and appears to have a genetic component. Salt sensitivity is almost twice as frequent in patients with hypertension and is particularly common among Black persons, the elderly, and those with CKD. It is generally associated with a lower level of plasma renin activity (PRA).
What underlies salt sensitivity? Normal kidneys are exquisitely sensitive to BP. A rise in mean arterial pressure (MAP) of as little as 1 to 3 mm Hg elicits a subtle increase in kidney NaCl and fluid elimination. This “pressure natriuresis” also works in reverse and conserves NaCl and fluid during decreases in BP. It is rapid, quantitative, and fundamental for normal homeostasis. It is primarily a result of changes in tubular NaCl reabsorption rather than total renal blood flow (RBF) or glomerular filtration rate (GFR). Indeed, kidney autoregulation maintains RBF and GFR remarkably constant during changes in BP. It is the pressure natriuresis mechanism that accurately adjusts salt excretion and body fluids in persons with healthy kidneys across a range of BPs. Two primary mechanisms of pressure natriuresis have been identified.
First, a rise in kidney perfusion pressure increases blood flow selectively through the medulla since medullary blood flow is not as tightly autoregulated as cortical blood flow. These increases in pressure and flow enhance kidney interstitial hydrostatic pressure throughout the kidney, which is an encapsulated organ. This rise in interstitial pressure reduces proximal tubule reabsorption, increases salt excretion, and impairs fluid return to the bloodstream. Second, the degree of stretch of the afferent arteriole regulates the secretion of renin into the bloodstream and, hence, the generation of angiotensin II. Thus, an increase in BP that is transmitted to this site reduces renin secretion. Angiotensin II coordinates the body’s salt and fluid retention mechanisms by stimulating thirst and enhancing NaCl and fluid reabsorption in the proximal and distal nephron segments. By stimulating secretion of aldosterone and arginine vasopressin, and inhibiting atrial natriuretic peptide (ANP), angiotensin II further enhances the reabsorption in the distal tubules and collecting ducts. Thus, during normal homeostasis, an increase in BP is matched by a decrease in PRA that reduces sodium reabsorption. It follows that a normal or elevated value for PRA in hypertension is effectively “inappropriate” for the level of BP, and is thereby contributing to the maintenance of hypertension.
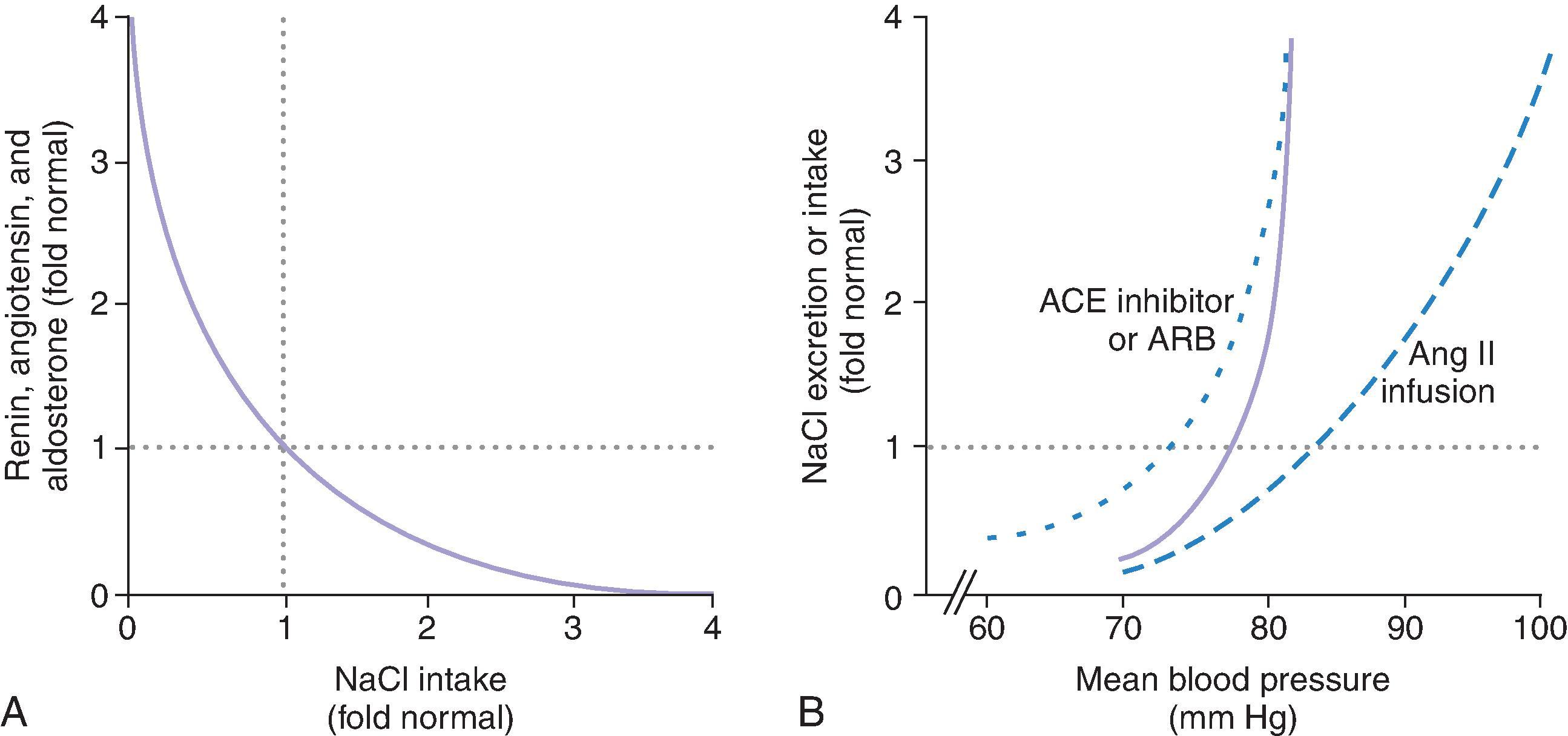
The relationships among long-term changes in salt intake, the renin-angiotensin-aldosterone system (RAAS), and BP are shown in Fig. 63.1 . Healthy people regulate the RAAS closely with changes in salt intake. An increase in salt intake brings about only a modest rise in MAP, because the RAAS is suppressed and the highly effective pressure natriuresis mechanism rapidly increases NaCl and fluid elimination in the urine sufficiently to restore a near-normal blood volume and BP. Expressed quantitatively in Fig. 63.1 , the slope of the long-term increase in NaCl excretion with BP is normally almost vertical. One factor contributing to the steepness of this slope, or the gain of the pressure natriuresis relationship, is the reciprocal changes in the RAAS with BP that dictate appropriate alterations in salt handling by the kidney. Therefore, when the RAAS is artificially fixed, the slope of the pressure natriuresis relationship flattens, resulting in salt sensitivity, displacement of the set point, and a change in ambient BP. For example, an infusion of angiotensin II into a normal subject raises the BP. Because angiotensin II is being infused, the kidney cannot suppress angiotensin II levels appropriately by reducing renin secretion. Therefore, one component of the pressure natriuresis mechanism is prevented and the BP elevation is sustained without an effective kidney compensation. In contrast, normal individuals treated with an angiotensin-converting enzyme (ACE) inhibitor to block angiotensin II generation or an angiotensin receptor blocker (ARB) to block AT 1 receptors have a fall in BP. Again, the kidney cannot stimulate an appropriate effect of angiotensin II and aldosterone that would be required to retain sufficient NaCl and fluid to buffer the fall in BP. Therefore, when the RAAS is fixed, the BP changes as a function of salt intake and becomes highly “salt sensitive” (see Fig. 63.1 ). These studies demonstrate the unique role of the RAAS in long-term BP regulation and its importance in isolating BP from NaCl intake.
Some recent findings add complexity to these simple relationships. Renin is also generated within the connecting tubule and collecting ducts. This kidney renin may contribute to the very high level of angiotensin within the kidney that does not share the same relationship with dietary salt. Animal models of diabetes mellitus and chronic kidney disease (CKD) demonstrate an increase in local angiotensin generation and action in the kidneys that may contribute to the beneficial effects of ACE inhibitor and ARB therapy, despite often low circulating renin levels. Other studies have shown that prorenin, although not itself active, becomes activated after binding to a renin receptor in the tissues, notably the kidneys. This is important because conventional RAAS antagonists may not block these actions of prorenin.
Four compelling lines of evidence implicate the kidney and RAAS in long-term BP regulation. First, kidney transplant studies in rats showed that a normotensive animal that received a kidney from a hypertensive animal becomes hypertensive, and vice versa. Similarly, human kidney transplant recipients frequently become hypertensive if they receive a kidney from a hypertensive donor. Apparently, the kidney in hypertension is programmed to retain salt and water inappropriately for a normal level of BP, thereby resetting the pressure natriuresis to a higher level of BP and dictating the appearance of hypertension in the recipient, even if the neurohumoral environment is that of normotension. Nevertheless, recent studies in gene-deleted or transgenic mice subjected to kidney transplantation concluded that the increase in BP during prolonged infusion of angiotensin II was mediated by the combined effects of angiotensin on angiotensin receptors within the kidney and the systemic circulation, most likely involving the kidney afferent arterioles and the brain. A second observation was that the BP was normally reduced 5% to 20% by an ACE inhibitor, an ARB, an aldosterone receptor antagonist, or a renin inhibitor. The fall in BP was greatest in those with elevated PRA values, and it was enhanced by dietary salt restriction or concurrent use of diuretic drugs that stimulate the RAAS (see Fig. 63.1 ). Third, almost 90% of patients approaching end-stage kidney disease (ESKD) whose kidneys have lost most of their capacity to accurately adjust salt excretion have hypertension. Fourth, the major monogenetic causes of human hypertension involve genes that activate RAAS signaling (such as glucocorticoid-remediable hypertension) or kidney sodium transport (such as Liddle syndrome).
Recent studies have suggested that kidney also plays a role in the pathogenesis of hypertension through activation of ENaC in the collecting ducts by proteases including plasmins. Plasmin is a serine protease converted in the kidney from its precursor plasminogen. An ENaC activated by plasmin engages increased kidney sodium and fluid retention and a rise in BP.
An increase in cardiac output necessarily increases peripheral blood flow. However, each organ has intrinsic mechanisms that adapt its blood flow to its metabolic needs. Therefore, over time, this local tissue mechanism translates an increase in cardiac output into an increase in SVR. The outcome is that organ blood flow is maintained, but hypertension becomes sustained. This total-body autoregulation is demonstrated in human subjects who are given salt-retaining mineralocorticosteroid hormones. An initial rise in cardiac output is translated in most individuals into sustained hypertension from an elevated SVR over 5 to 15 days.
Hypertension causes not only remodeling in the distributing and resistance vessels and the heart, but also fibrotic and sclerotic changes in the glomeruli and interstitium of the kidney. Hypertrophy of resistance vessels limits the ratio of lumen to wall and dictates a fixed component to SVR. This is evidenced by a higher SVR in hypertensive versus normotensive individuals during maximal vasodilatation. Moreover, thickened and hypertrophied resistance vessels have greater reductions in vessel diameter during vasoconstrictor stimulation. This is apparent as an increase in vascular reactivity to pressor agents. Remodeling of resistance arterioles diminishes their response to changes in perfusion pressure. This manifests as a blunted myogenic response contributing to incomplete autoregulation of RBF, thereby adding a component of barotrauma to hypertensive kidney damage. Sclerotic and fibrotic changes in the glomeruli and kidney interstitium, combined with hypertrophy of the afferent arterioles, limit the sensing of BP in the juxtaglomerular apparatus and kidney parenchyma. This blunts renin release and pressure natriuresis, thereby contributing to salt sensitivity and sustained hypertension. Rats receiving intermittent weak electrical stimulation of the hypothalamus initially had an abrupt increase in BP followed by a sudden fall after the cessation of the stimulus. However, eventually the baseline BP increased in parallel with the appearance of hypertrophy of the resistance vessels, suggesting that vascular remodeling can sustain hypertension. These structural components may explain why it often takes weeks to achieve maximal antihypertensive action from a drug, a reduction in salt intake, or correction of a renal artery stenosis or hyperaldosteronism. Vascular and left ventricular hypertrophy is largely, but usually not completely, reversible during treatment of hypertension, whereas fibrotic and sclerotic changes are not.
A rise in BP diminishes the baroreflex, thereby reducing the tone of the sympathetic nervous system and increasing the tone of the parasympathetic nervous system. Paradoxically, human hypertension is often associated with an increase in heart rate, maintained or increased plasma catecholamine levels, and an increase in directly measured sympathetic nerve discharge despite the stimulus to the baroreceptors. What is the cause of this inappropriate activation of the sympathetic nervous system in hypertension? Studies in animals show that the baroreflex “resets” to the ambient level of BP after 2 to 5 days. Thereafter, the baroreflex no longer continues to “fight” the elevated BP but defends it at the new higher level. Much of this adaptation occurs within the baroreceptors themselves. With aging and atherosclerosis, the walls of the carotid sinus and other baroreflex-sensing sites become less distensible. Therefore, the BP is less effective in stretching the afferent nerve endings, and the sensitivity of the baroreflex is diminished. This may contribute to the enhanced sympathetic nerve activity and increased plasma catecholamines that are characteristic of elderly hypertensive subjects. Additionally, animal models have identified central mechanisms that alter the gain of the baroreflex process, and, therefore, the sympathetic tone, in hypertension. The importance of central mechanisms in human hypertension is apparent from the effectiveness of drugs, such as clonidine, that act within the brain to decrease the sympathetic tone. The kidneys themselves contain barosensitive and chemosensitive nerves that can regulate the sympathetic nervous system. In one study, hemodialysis patients experienced an increased sympathetic nervous system discharge and increased BP that were not apparent after bilateral nephrectomy. This suggests that the renal nerves were maintaining enhanced sympathetic tone. Based on this pathophysiology, radiofrequency ablation of the renal nerves has successfully improved BP control in some, but not all, studies of patients with drug-resistant hypertension, further illustrating the importance of the renal nerves in setting the long-term level of BP in human subjects.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here