Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Imaging plays a central role in the initial assessment and serial monitoring of chronic pediatric liver disease. While liver biopsy remains the accepted gold standard test for diffuse parenchymal liver disease, a single biopsy specimen only samples 1/50,000 or 0.002% of the liver, and heterogeneity in severity and distribution of disease can result in sampling errors. Liver biopsy is invasive even when done percutaneously, and up to 3% of patients require hospitalization after the procedure. There is also significant variability in interpretation of liver biopsy specimens by pathologists with discordance in up to 33% of cases. For these reasons, noninvasive methods, mainly ultrasound ( e-Fig. 89.1 ) and magnetic resonance imaging (MRI), of assessing response to therapy and disease progression have come to play a prominent role in the management of chronic pediatric liver disease.
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic pediatric liver disease, with some series listing a prevalence of approximately 10%. NAFLD is defined as the presence of microvesicular/macrovesicular fat in more than 5% of the hepatocytes after the exclusion of significant alcohol consumption, drug use, or other recognizable secondary causes that may result in fatty liver. The prevalence of pediatric fatty liver increases with age and is seen in up to 17% of adolescents. NAFLD is usually diagnosed between 10 and 13 years of age. The majority of patients with NAFLD also have insulin resistance or obesity. NAFLD can range in severity from simple steatosis and nonalchoholic steatohepatitis (NASH), through fibrosis and cirrhosis, but it also may be associated with a number of metabolic processes and toxins ( Box 89.1 ). In addition to fat deposition, NASH patients show hepatic cell injury and inflammation. NASH significantly increases the risks of cirrhosis, hepatic failure, and hepatocellular carcinoma (HCC). At the time of diagnosis, 25% to 50% of children will have NASH, while 10% to 25% will have advanced fibrosis.
Obesity
Extreme malnutrition
Total parenteral nutrition
Cystic fibrosis
Familial hyperlipoproteinemia
Glycogen storage disease
Wilson disease
Galactosemia
Reye syndrome
Severe hepatitis
Poorly controlled diabetes mellitus
Chronic tuberculosis
Chronic congestive heart failure
Alcohol
Chemotherapy
Carbon toxins
Phosphorus
Amiodarone
Many pediatric patients with NAFLD are either asymptomatic or present with vague clinical complaints (abdominal pain, fatigue). On physical exam, hepatomegaly and acanthosis nigricans are seen in up to 40% of patients. Elevation of liver enzymes is common in NAFLD patients, particularly alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Some NAFLD patients also come to clinical attention after incidental detection of a fatty liver on abdominal ultrasounds performed for other reasons.
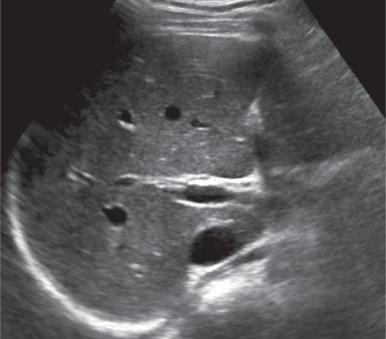
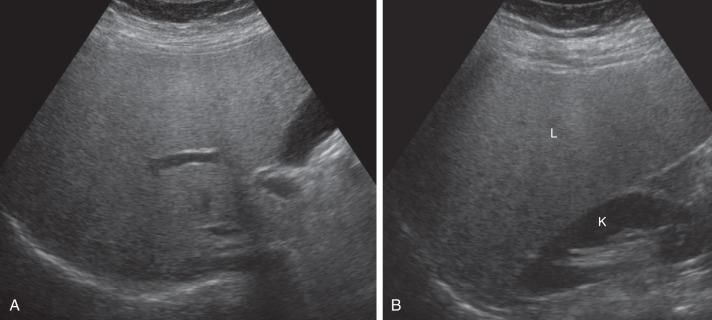
Diagnostic confidence for fatty liver on ultrasound improves with increasing severity of fatty infiltration, with a sensitivity and specificity of over 90% for moderate steatosis. Deposition of fat in hepatocytes causes increased echogenicity of the liver, manifested as difficulty visualizing the portal triads ( Fig. 89.2 ). There is increased attenuation of the sound beam as it propagates through the liver, causing loss of signal in the posterior portions of the liver and inability to visualize the hemidiaphragm. The liver may be substantially more echogenic than the adjacent right kidney (see Fig. 89.2 ). Areas of focal fatty sparing manifest as hypoechoic foci within the fatty liver, without mass effect, which could potentially be misdiagnosed as a mass. Ultrasound elastography is a noninvasive method of assessing liver stiffness, which is a correlate for fibrosis. This method has shown promise for severe fibrosis and advanced cirrhosis but is limited in whole liver assessment due to the small acoustic window used for scanning.
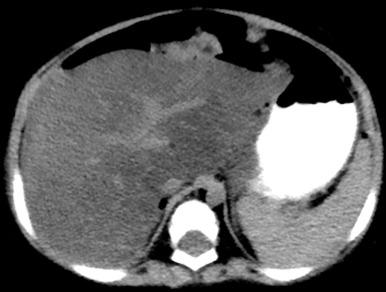
Computed tomography (CT) is not routinely used for the primary diagnosis of fatty liver, but this finding may be seen incidentally. Normally, the liver attenuation is at least the same as the spleen. This relationship is reversed in hepatic steatosis, and the liver is of lower attenuation than the spleen on noncontrast CT ( e-Fig. 89.3 ).
Fat deposition in hepatocytes can readily be detected on conventional MR, with a high degree of sensitivity and specificity. More specifically, the amount of fat in the liver can also be quantified using either MR spectroscopy or chemical shift imaging. MR spectroscopy is the most accurate method of hepatic fat quantification and is very sensitive to small changes in hepatic fat concentration. However, the complexity of analysis and lack of reproducibility are barriers to its widespread clinical adoption.
Chemical shift imaging is the mainstay of hepatic fat quantification on MR because it is quick and easy to perform. Chemical shift imaging takes advantage of the different precession frequencies of hydrogen protons in fat and water. When fat and water are present within the same voxel, there is signal loss on the opposed-phase images. Comparing the signal intensity on the opposed-phase image (short TE image) with the in-phase image (long TE image) allows for calculation of the hepatic fat fraction ( Fig. 89.4 ). Hepatic iron deposition may limit evaluation for steatosis on opposed-phase images, as signal loss also occurs from iron-related susceptibility effects. Chemical shift imaging can help resolve confusing patterns of focal fat deposition or fatty sparing in the liver when present in atypical locations. Focal fat deposition commonly occurs in specific locations, usually around the gallbladder, adjacent to the falciform ligament, and in subcapsular regions. Unlike a mass, vessels course through regions of fatty deposition unperturbed.
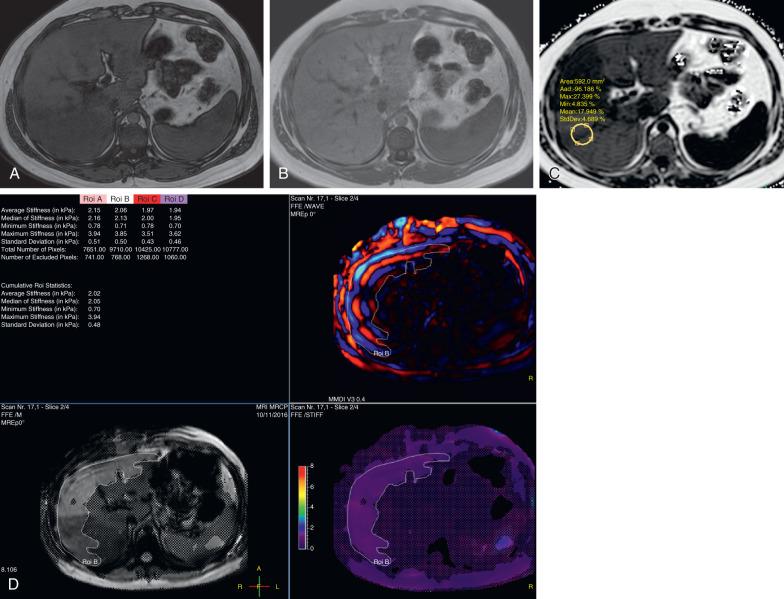
MR elastography (MRE) measures hepatic parenchymal stiffness using special hardware that generates shear waves and measures their propagation through the liver. The hardware used in pediatric MRE is the same type used in adults, except the driver is set to lower power/amplitude settings. An active driver outside the scanner transmits vibrations at 60 Hz to a passive driver on the patient's right upper quadrant. Four magnitude and four phase images are obtained at four different levels in the liver, and these images are then used to generate qualitative and quantitative stiffness maps using specific postprocessing software. For MRE, liver stiffness values greater than 2.74 kiloPascal (kPa) are suggestive of steatohepatitis and greater than 2.93 kPa is diagnostic for liver fibrosis. Compared with ultrasound elastography, MRE has been shown to be more accurate, samples a larger portion of the liver, has greater reliability, and is less limited by ascites or obesity.
Lifestyle modification aimed at weight loss is the first-line therapy for NAFLD, and even mild reduction in weight can improve histologic findings of steatosis and steatohepatitis. Drugs being investigated include antioxidants such as vitamin E, metformin, and thiazolidinediones. The need for accurate and reliable noninvasive monitoring of NAFLD is imperative given the recent discovery that fibrosis and early cirrhosis may be reversible in some patients.
Hepatic iron overload is the result of abnormal intracellular accumulation of iron within the hepatocytes. Normally, excess iron is stored bound to intracellular ferritin. In cases of increased and sustained iron overload, the capacity of ferritin to collect and store iron is surpassed, resulting in deposition of unbound iron primarily in the liver, pancreas, spleen, and bone marrow. Unbound iron may result in cellular injury by reacting with hydrogen and lipids to produce free radicals. In the liver, this can lead to fibrosis and cirrhosis.
There are two main patterns of iron deposition: parenchymal and reticuloendothelial. Parenchymal iron deposition occurs in hereditary hemochromatosis (HH), an autosomal recessive disorder with a gene locus (HFE) on the short arm of chromosome 6. There is increased gastrointestinal absorption of iron with eventual pathologic deposition in the liver, pancreas, heart, pituitary, synovium, and other parenchymal organs. Abnormal parenchymal iron deposition results in oxidative injury and organ dysfunction over time, with patients developing liver fibrosis/cirrhosis, cardiomyopathy, and endocrinopathies including diabetes mellitus. Iron deposition in the pancreas is specific for HH, whereas splenic and bone marrow involvement is usually seen with transfusional/reticuloendothelial iron deposition. A neonatal form of primary hemochromatosis exists in which newborns present with fulminant hepatic failure, along with pancreatic and cardiac involvement.
Reticuloendothelial iron deposition occurs mainly after multiple blood transfusions. This form of iron deposition is also referred to as secondary hemochromatosis, and it is more common than the hereditary form. Iron in transfused red blood cells is taken up by reticuloendothelial cells in the liver, spleen, and bone marrow after phagocytosis. Unlike HH, organs other than the liver, spleen, and bone marrow are affected relatively late in the disease. The pancreas is usually spared in patients with reticuloendothelial iron deposition.
Men with HH become symptomatic at least a decade earlier than women (as a result of the protective effect of menstruation) after total body accumulation of approximately 20 g of iron. The most common presenting symptoms are weakness, lethargy, and elevated liver function tests, occurring in 75% of patients. Patients with transfusional iron overload become symptomatic much earlier than in HH because each blood transfusion provides approximately 200 mg of iron, and there is no mechanism for iron excretion in the body other than through sweat, shedding of skin cells, and menstruation (an iron loss rate of approximately 1 mg per day for men and 2 mg per day for premenopausal women). Elevated serum ferritin levels are seen in patients with iron overload, but this finding may also be seen with any inflammatory process, as ferritin is an acute phase reactant. Transferrin saturation levels can also be performed, and levels greater than 60% are over 90% accurate for identifying HH. These tests are less helpful for transfusional iron overload. Iron overload occurs in virtually all patients requiring life-long blood transfusions, necessitating careful monitoring and treatment of parenchymal iron deposition to limit toxicity and avoid complications.
Ultrasound is not helpful for the detection of iron deposition but may show findings of cirrhosis, portal venous hypertension, or HCC late in the disease. Noncontrast CT may show increased attenuation of the liver (>72 HU), but this is a late finding with a sensitivity of approximately 60% and may be seen with other conditions such as amiodarone therapy or glycogen storage disease (GSD).
MR is the imaging modality of choice for confirming the diagnosis, determining severity, and monitoring therapy in persons with hemochromatosis. Excess iron deposition causes a proportional decrease in signal intensity on T1- and T2-weighted sequences and is most pronounced on gradient echo sequences. Skeletal muscle is relatively unaffected by iron deposition and serves as a good internal reference to compare the signal intensity of affected abdominal organs and bone marrow, which will be hypointense to skeletal muscle. On in-phase and out-of-phase imaging, decreased signal intensity is seen in the affected organs on the in-phase sequence (an opposite finding from steatosis). The key findings in HH are low signal intensity within the liver and pancreas on T2-weighted images ( Fig. 89.5 ). Patients with reticuloendothelial iron deposition demonstrate low signal in the liver, spleen, and bone marrow ( Fig. 89.6 ) with sparing of the pancreas.
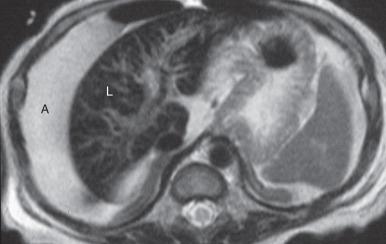
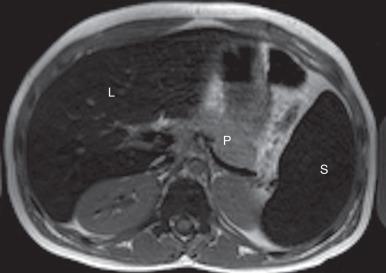
There are two main methods of MRI liver iron quantification: signal intensity ratio and relaxometry. Signal intensity ratio is widely used because it is accurate and easy to perform. This method takes advantage of the effect of iron on adjacent proton spins; iron causes rapid dephasing of spins/loss of coherence with resultant loss of signal on long TE images (T2- or T2*-weighted). This effect is more pronounced on gradient echo images due to the lack of a 180-degree refocusing pulse. Signal intensity ratio is obtained by comparing the signal intensity of the liver with skeletal muscle on gradient echo imaging at varying TE and flip angles, and the values obtained are used to quantify iron deposition. An online calculator is available. The main limitation of this technique is that high levels of iron cannot be measured and are reported as greater than 350 umol/g.
Relaxometry estimates iron content by measuring the loss of transverse magnetization caused by iron susceptibility over increasing values of TE. Signal loss occurs in the liver with increasing TE values, and the rate of signal decay is measured as a constant (T2 or T2* depending on whether spin echo or gradient echo techniques are used). Reciprocal values of the signal decay constant, R2 or R2*, have also been used as they provide direct correlation with liver iron content (LIC). In general, R2* values are more sensitive for low levels of iron and are faster to acquire (performed with GRE techniques) but are inaccurate at higher iron levels, while R2 values are less susceptible to artifact or hardware/scanner differences but take longer to perform. R2 and R2* methods of estimating LIC are statistically equivalent in children when LIC is less than 20 mg/g.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here