Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Australian ophthalmologist Tom Spring is widely credited as being the first to observe an allergic-like reaction of the upper tarsal conjunctiva, which was later to become known as ‘giant papillary conjunctivitis’. In his 1974 letter to the editor of the Medical Journal of Australia , Spring reported observing the presence of large tarsal papillae, accompanied by discomfort and excessive mucus production in 43% of patients wearing soft lenses.
The term ‘giant papillary conjunctivitis’ was coined by Allansmith et al. to describe papillary changes on the tarsal conjunctiva that were likened to a cobblestone formation ( Fig. 14.1 ). However, this condition can take on a variety of different appearances, depending on the level of severity and whether it relates to soft or rigid lens wear. In its mild form, this condition has been termed ‘lid roughness’ and ‘papillary hypertrophy’. Even in advanced stages, the papillary formations may be extensive but not necessarily ‘giant’. Thus, a more appropriate term that encompasses all of the possible manifestations of the same condition is ‘contact lens–induced papillary conjunctivitis’ (CLPC).
In soft lens wearers, CLPC may take as little as 3 weeks to develop or as long as 4 years to manifest. In rigid lens wearers, CLPC typically appears after about 12 months.
It is difficult to characterise the prevalence of a condition that (a) has a variable time of onset, (b) varies in severity throughout the seasons of the year (because of its allergic nature) and (c) varies over the years as different lens care regimens and lens wear modalities come into and go out of vogue. It is also apparent that the definition of CLPC has changed over time, with the earlier literature (say, pre-1985) focusing on the appearance of very large papillae (consistent with the notion of ‘giant’ papillae) and the subsequent literature paying more attention to CLPC in its more subtle variants.
In his original article, Spring reported a prevalence of CLPC in 43% in hydrogel lens wearers; however, this was during the days when patients were using thermally disinfected hydroxyethyl methacrylate (HEMA) lenses. Before the introduction of disposable contact lenses, the prevalence of overt CLPC in those using hydrogel lenses on a daily-wear basis was reported to be 2% to 18%.
Grant reported a CLPC prevalence of 19% in patients using non-planned replacement extended-wear hydrogel lenses versus 3% with disposable hydrogel extended-wear lenses. However, Poggio and Abelson found no difference in CLPC between patients wearing those lens types (1.9% and 2%, respectively). Other authors reported somewhat higher incidence figures of 6.4%, 6.7%, and 6% to 12% for disposable hydrogel extended-wear lenses.
Reviewing chart records of subjects participating in clinical trials of various silicone hydrogel/care solution combinations, Carnt et al. reported CLPC occurrence at a rate of 0.4 cases per 100 participant-months. There was no difference in this response rate between among brands of silicone hydrogel lenses, although solution type had a small effect.
Radford et al. did not find a significant difference in the frequency of CLPC among users of silicone hydrogel lenses versus hydrogel lenses. However, Maldonado-Codina et al. reported increased grades of papillary conjunctivitis among patients wearing silicone hydrogel lenses of high modulus (Focus Night & Day) versus those of low modulus (Acuvue Advance). Santodomingo-Rubido et al. reported the following number of adverse events in 53 subjects followed up for 18 months: Focus Night & Day daily wear – 2, extended wear – 5; Pure Vision daily wear – 2, extended wear – 4. This represents an overall response rate of CLPC of 16% per year.
Alemany and Redal found a lower incidence of overt CLPC in patients wearing daily-wear rigid lenses compared with conventional daily-wear soft lenses. Grant et al. reported that the incidence of CLPC in patients wearing rigid lenses on an extended-wear basis was 2%, versus 6% to 12% for soft disposable extended wear. Fonn et al. reported the results of a 4-month clinical trial in which 6 (33%) of 18 patients wearing silicone hydrogel lenses on an extended-wear basis developed CLPC; two of these cases were removed from the study because of the severity of their conditions.
Tagliaferri et al. observed that 25% of subjects fitted with lotrafilcon A silicone hydrogel lenses for up to 30 days of continuous wear developed CLPC after 12 months.
Surveys of contact lens wearers presenting to hospital clinics have revealed the following prevalence figures for CLPC: China – 5% ; India – 6% ; Singapore – 16% ; Nepal – 37% ; and United States – 58%.
The normal tarsal conjunctiva can take on a variety of forms, which may be categorised in different ways. Allansmith described three forms of normal tarsal conjunctival appearance: (a) satin or smooth (14%); (b) small, uniformly sized ‘micropapillae’, which are less than 0.3 mm in diameter (85%); and (c) non-uniform papillae (< 1%), where some papillae can be as large as 0.5 mm in diameter.
An alternative model for classifying the normal tarsal conjunctiva was proposed by Potvin et al., who conducted a computer-assisted morphometric examination of photographic images of the fluorescein-stained tarsal conjunctiva of eight asymptomatic non–lens wearers. The eight subjects were classified into two distinct groups: (a) those displaying ‘small feature’ tarsal plates (with a modal feature area of 25,000 to 35,000 μm 2 and a restricted range of areas); and (b) those displaying ‘large feature’ tarsal plates (with a modal feature area of 50,000 to 70,000 μm 2 and a wide range of areas). The cells generally appear to be pentagonal or hexagonal in shape.
The three-category model of Allansmith is of more immediate relevance to clinicians because it is based on the appearance of the tarsal conjunctiva under low magnification with the use of the slit lamp biomicroscope.
It is important that an assessment is made only of the central region of the tarsal plate, for the following reasons: (a) there is often increased ‘roughness’ of the conjunctiva at the lateral extremities of the everted lid that is unrelated to lens-induced pathology; (b) the process of lid eversion causes the conjunctiva to artificially appear distorted and irregular along the margin of the lid eversion fold (i.e. anatomically superior to the tarsal plate, but paradoxically ‘inferior’ as the everted lid is viewed); and (c) the conjunctiva just inside the lid margin (i.e. anatomically inferior to the tarsal plate) is rarely affected by lens wear.
Allansmith noted that the appearance of CLPC was different in soft lens wearers versus rigid lens wearers. In soft lens wearers, papillae are more numerous and are located more towards the upper tarsal plate (i.e. closer to the fold of the everted lid); and the apex of the papillae take on a rounded flatter form ( Fig. 14.1 ). In rigid lens wearers, papillae take on a crater-like form and are located more towards the lash margin, with few papillae being present on the upper tarsal plate ( Fig. 14.2 ). Skotnitsky et al. suggested that patients wearing silicone hydrogel lenses for extended periods are more predisposed to developing localised CLPC as a result of the stiffer modulus of these lenses interacting with the superior palpebral conjunctiva.
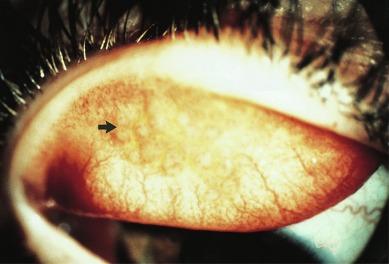
In the early stages of development, the tarsal conjunctiva in patients suffering from CLPC may be indistinguishable from the normal tarsal conjunctiva. An important early distinguishing feature is increased redness of the tarsal conjunctiva ( Fig. 14.3 ). This change can be detected with reference to the lower palpebral conjunctiva, which is usually unaffected and can therefore act as a ‘baseline’ against which any change is measured.
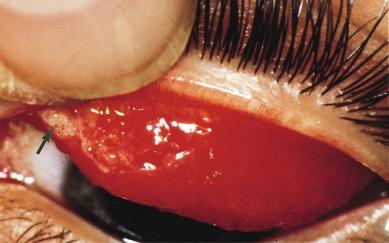
In advanced cases, papillae can exceed 1 mm in diameter and often take on a bright red/orange hue. Evidence of papillae often appears in the form of irregular light reflexes ( Fig. 14.4 ). The hexagonal/pentagonal shape may be lost in favour of a more rounded appearance. The pattern of distribution of papillae may reflect the underlying anatomy of the tarsus; for example, Fig. 14.5 is a non-uniform CLPC that has developed in a soft lens wearer. Three-dimensionally, giant papillae can be said to take on a ‘mushroom’ form, with a flattened or even slightly depressed apex or tip.
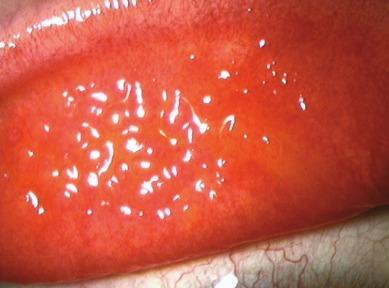
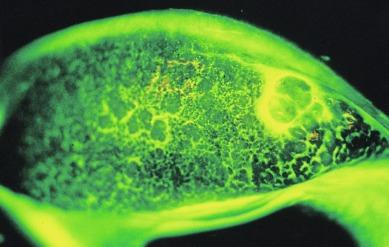
Because the conjunctiva is thickened, oedematous and often hyperaemic, fine vessels which can normally be observed to traverse the conjunctival surface are obscured, although deep vessels remain visible over the tarsal plate. A tuft of convoluted capillary vessels is often observed at the apex of papillae; this vascular tuft will generally stain with fluorescein.
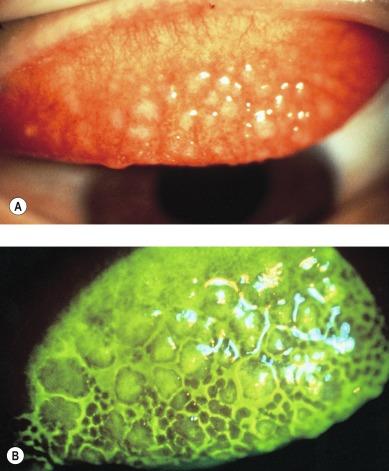
Other signs that can be observed in severe manifestations of CLPC include conjunctival oedema and excessive mucus ( Fig. 14.3 ), which usually forms into strands that lie in the valleys between papillae. Excess mucus will also accumulate at the inner and outer canthi at night and can sometimes be observed as clumps or strands floating across the cornea or on the lens surface ( Fig. 14.6 ). Prolonged oedema may result in a mild ptosis, which is often asymmetrical.
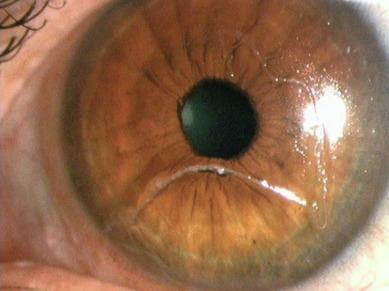
Giant papillae can display infiltrates, and if the condition persists for some time, the conjunctival surface at the apex of the papillae can become scarred and appear cream/white in colour ( Fig. 14.7 ). The cornea may be compromised and display superficial punctate staining and infiltrates superiorly. Injection of the superior limbus may also be apparent.
There is general concordance between the severity of signs and symptoms. In the early stages of CLPC, patients may complain of discomfort towards the end of the wearing period and slight itching. Patients may report an increase in mucus production upon awakening. Intermittent blurring is sometimes noted; this results from mucus being periodically smeared across the lens surface. A slight but non-variable vision loss is attributable to more tenacious lens deposits, such as protein, which is of aetiological significance in this condition (see later).
In more severe cases, the itching and discomfort can become so marked that the patient is forced to remove the lens. Excessive lens movement and decentration can result from a combination of (a) the large papillae creating greater contact and friction with the coated lens surface, and (b) excess mucus acting as an ‘adhesive’ between the tarsal conjunctival and lens surfaces.
The signs and symptoms of CLPC, as they manifest with different grades of severity, are summarised in Table 14.1 .
| Grade | Signs | Symptoms |
|---|---|---|
| 0 |
|
|
| 1 |
|
|
| 2 |
|
|
| 3 |
|
|
| 4 |
|
|
It has been possible to thoroughly document pathological changes that take place in patients with CLPC because it is a relatively simple matter to biopsy tissue from the tarsal conjunctiva. However, contact lens–induced corneal pathology is often poorly understood because researchers rarely biopsy tissue specimens from the living human cornea.
The conjunctiva becomes thicker in CLPC (0.2 mm in CLPC vs. 0.05 mm in normals). Greiner et al. observed dramatic ultrastructural changes in the conjunctival surface in patients with CLPC. Conjunctival surface area is increased by two-fold and epithelial cells are enlarged and distorted, becoming elongated in shape. The microvilli are reduced in number and become distorted, forming aggregated tufts on the surface of papillae. Normally present crypts of Henle are not observed. The number of mucus-secreting non-goblet cells is increased. More dark cells are observed at the apices of the papillae.
CLPC is associated with a dramatic redistribution of inflammatory cells between the epithelium and the stroma of the conjunctiva. Mast cells, eosinophils and basophils are found in the epithelium (they do not normally reside there), and there is an increase in the number of neutrophils and lymphocytes in the epithelium. Eosinophils and basophils are found in the stroma, with an increase in the number of mast cells, plasma cells and neutrophils.
Zhong at al. have demonstrated that membranous epithelial cells (M cells) play a key role in the pathogenesis of CLPC for the binding and translocation of antigen and pathogen.
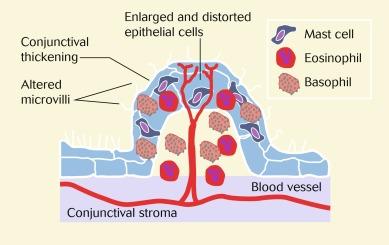
Fig. 14.8 is a schematic diagram of tissue and cellular pathological changes that characterise a papilla.
A number of factors have been suggested as playing a role in the aetiology of CLPC, and it is unlikely that any one causative factor can account for all cases. These factors are summarised in the following.
Papillary conjunctivitis of an apparently identical form to that induced by contact lenses has been observed in patients who do not wear contact lenses but whose tarsal conjunctivae have been exposed to various types of mechanical trauma, such as:
plastic ocular prostheses ;
extruded scleral buckle ;
excessive cyanoacrylate glue used to close a perforated cornea ;
protruding nylon sutures ;
rigid contact lens imbedded in upper fornix ;
elevated corneal deposits ; and
epithelialised corneal foreign body.
In many of these cases, the papillary conjunctivitis resolved and patient symptoms were alleviated upon removal of the trauma. The reports of Dunn et al. and Greiner are particularly noteworthy because, unlike the other cases which involve trauma induced by man-made materials, the papillary conjunctivitis was induced by trauma from inert epithelial irregularities.
Trauma is known to cause mast cell degranulation, so the presence of large numbers of degranulated mast cells in the conjunctival epithelium and stroma of patients with CLPC is consistent with trauma being a factor of aetiological significance in this condition. The conjunctivae of patients with CLPC have significantly higher levels of neutrophil chemotactic factor – a substance which is generally released in traumatised tissue. The decay-accelerating factor (DAF), a membrane-associated complement regulatory protein which inhibits the central C3 amplification of the cascade, is present on both the ocular surface and in tears. Szczotka et al. reported that DAF concentrations were significantly reduced in patients with CLPC compared with normal non–contact lens wearing controls. This reduction may be associated with enhanced complement activation contributing to the pathogeneses of CLPC.
This anaphylactic reaction is mediated by immunoglobulin type E (IgE) antibodies which proliferate when the conjunctiva is exposed to certain antigens. The IgE antibodies set off a chain reaction leading to mast cell degranulation and the release of inflammatory mediators and other substances that can affect tissue damage and repair. Patients with CLPC exhibit large numbers of degranulated mast cells in the conjunctival epithelium and elevated levels of IgE in tears.
Protein deposition on the lens has been implicated as the antigenic stimulant to IgE production. More specifically, deposits that form on the anterior lens surface are likely to be more significant in that this surface lies in direct apposition with the tarsal conjunctiva. In support of this lens deposition theory, Ballow et al. demonstrated that when contact lenses from patients suffering from CLPC were placed in the eyes of monkeys, a frank papillary conjunctivitis ensued, with elevated IgE levels. These changes did not occur when new lenses or lenses from patients without CLPC were placed in the eyes of monkeys.
A critical issue in formulating strategies to treat or prevent CLPC is to determine the specific causative antigens. Protein deposition on the lens surface is the most popular candidate; however, protein on lenses of patients with CLPC is indistinguishable from protein on lenses of patients without CLPC. The antigenic stimulus could also be one of a number of other potential lens contaminants, such as lipids, calcium, mucus and albumin. Microorganisms such as bacteria (and bacterial endotoxins) may also trigger CLPC.
The type of plastic used to fabricate the contact lens could theoretically have an antigenic role; however, this is difficult to prove. The success or otherwise of various polymers in alleviating or preventing CLPC probably relates more to the propensity of different materials to become deposited and/or the frequency of lens replacement, rather than any real differences in their intrinsic antigenic potency.
Early-generation preservatives, such as thimerosal and benzalkonium chloride, are known to have a causative role in the development of CLPC. Certainly, treatment is more likely to succeed if lens care systems are free of such preservatives.
In their initial writings, Allansmith et al. likened CLPC to vernal conjunctivitis in view of the similar inflammatory cell profiles of the two conditions; this view still holds today. The unusual presence of large numbers of basophils led Allansmith et al. to suggest that these diseases were of the cutaneous basophilic type. This is a classic skin reaction which has a delayed time course and is mediated by sensitised T lymphocytes and antibodies. In support of this proposed aetiology, Hann et al. induced a CLPC-type reaction in guinea pigs after injection of various antigens into the tarsal plate.
Despite the evidence cited earlier, the proportion of basophils to the total pool of inflammatory cells in CLPC is significantly less than that observed in a typical cutaneous basophilic hypersensitivity reaction. In view of this, Begley suggested that CLPC may better reflect the classic tuberculin type of delayed hypersensitivity reaction in which variable numbers of basophils can be present.
The antigens discussed in the previous section on the immediate hypersensitivity reaction are likely to be the same as those that mediate the delayed hypersensitivity reaction.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here