Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The pancreas arises early in gestation from the posterior foregut endoderm. A dorsal and a ventral bud first form before migrating and eventually fusing to form a single tissue. Specifically at day 26 of gestation, the foregut evaginates into a condensation of overlying mesenchyme to form the first morphological evidence of the dorsal bud; 6 days later, a ventral bud forms. At 5 to 6 weeks of gestation, the ventral pancreas rotates around the duodenum and fuses with the dorsal pancreas ( Fig. 80.1 ).
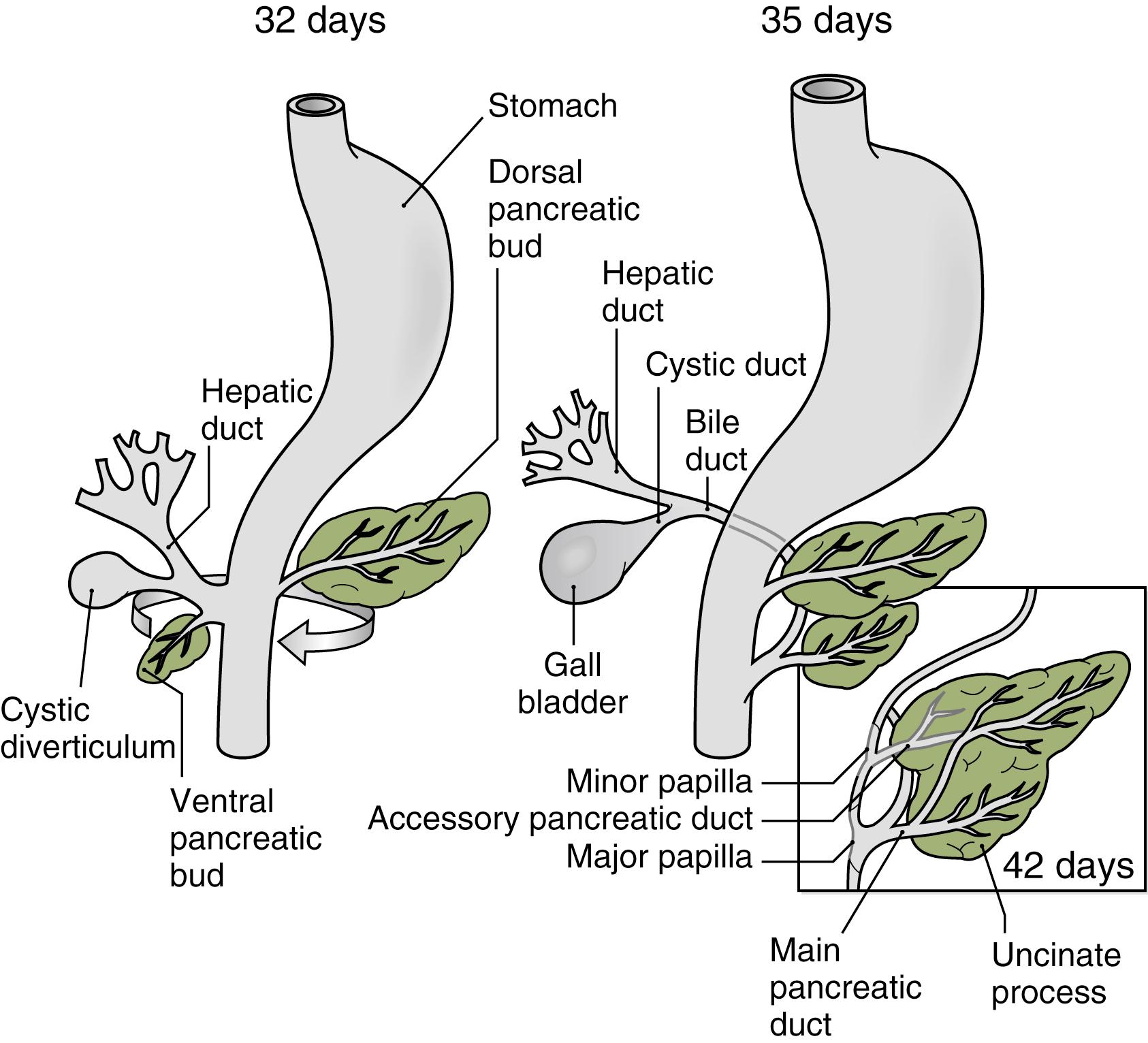
The adult pancreatic ductal system is contributed by each of the two buds. The dorsal bud forms the distal portion of the main pancreatic duct (of Wirsung) and the entire portion of the minor accessory duct (of Santorini) (see Fig. 80.1 ). The ventral bud provides the proximal portion of the main pancreatic duct. Pancreas divisum, discussed further in Chapter 81 , occurs when the two buds and their ductal systems fail to fuse.
Annular pancreas is an anomaly in which the ventral pancreas fails to fully rotate and instead forms a ring of pancreatic tissue that constricts the duodenum. This condition presents in roughly half of patients during the neonatal period with high-grade small bowel obstruction. An alternative hypothesis for annular pancreas formation is that the pancreas abnormally hypertrophies around the duodenum during development. For example, mice lacking the signaling molecule indian hedgehog (Ihh) develop increased ventral pancreatic mass, and just under half form an annulus around the duodenum. The clinical features of annular pancreas are detailed in Chapter 81 .
A remarkable feature of pancreatic differentiation is that all three major parenchymal cell types—including the two exocrine cells, the acinar and duct cells, and the endocrine islet cells—originate from a common progenitor ( Fig. 80.2 ). Development of the pancreas occurs through a stepwise process that is divided into three distinct transitions. These distinct developmental time periods include the primary transition (embryonic day 9 to 12) when pancreatic buds appear. The buds consist of stratified epithelium of a centrally located lumen generated from pancreatic progenitor cells. The secondary transition (embryonic day 12 through 15) is marked by intense proliferation and the branching of the pancreatic buds. Pancreatic cells in this phase of development have become more differentiated as endocrine or exocrine cells and develop distinct transcriptional profiles necessary for their respective cellular phenotype. During this stage, only a small number of cells are thought to remain multipotent. The final stage of pancreatic development starts at embryonic day 15 and continues postnatally. By the third stage of development, cells are thought to be unipotent. Specific defects during these stages of development can occur, and the impact on the pancreas is defined by the stage of pancreatic development in which they occur.
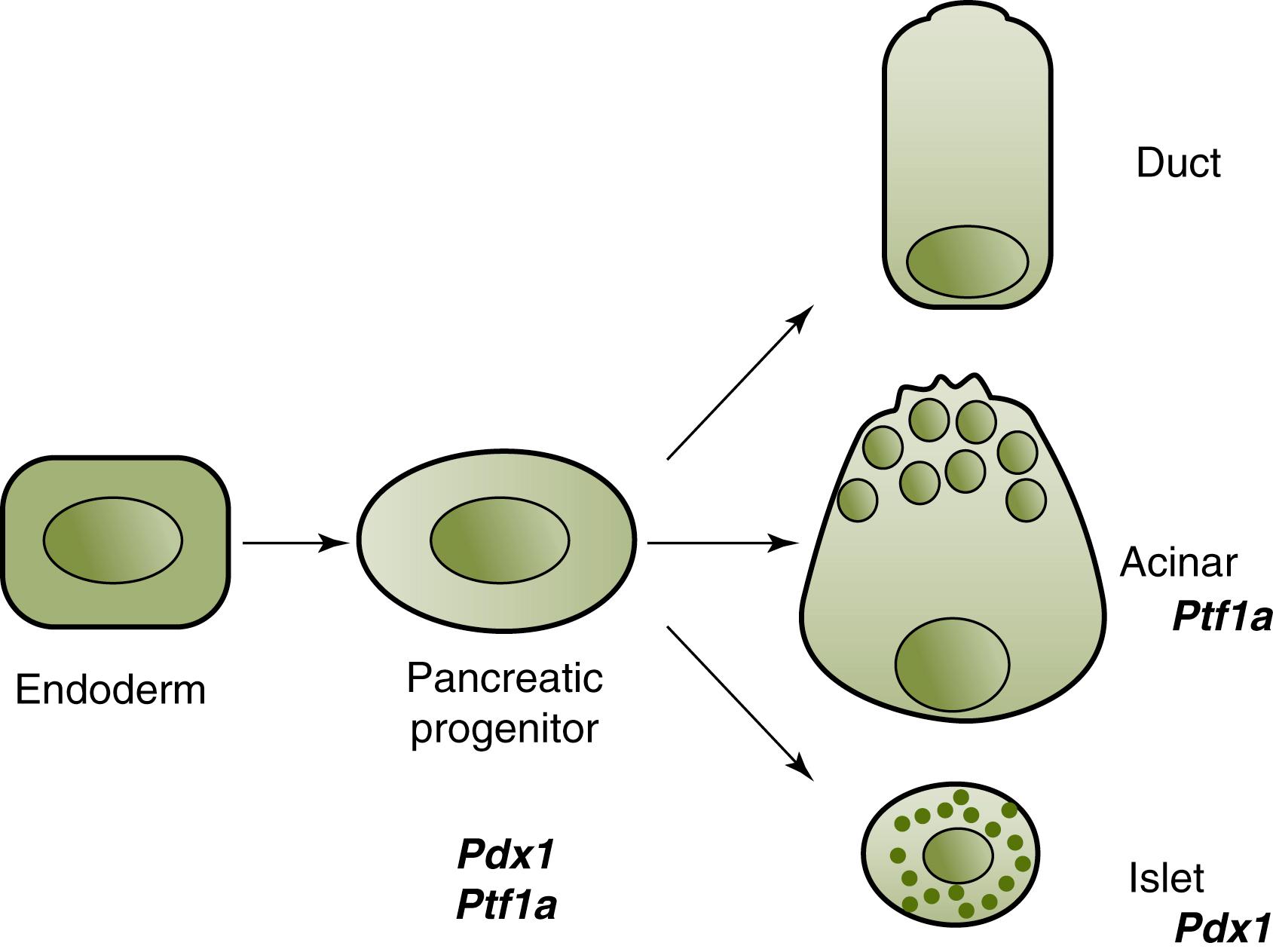
Timing of the defect during a particular stage of pancreatic development can lead to deficiencies in all (defect seen during the primary transition) or only one type of pancreatic cell (defect seen after the secondary transition). Johanson-Blizzard syndrome is a rare autosomal recessive syndrome that is characterized by dysmorphic nasal alae, ectodermal abnormalities, early growth failure, and exocrine pancreatic deficiency. A set of mutations have been identified and are associated with a defect in the UBR1 gene. This defect has been associated with intrauterine-onset distinctive pancreatitis and leads to the destruction of the exocrine pancreas. Other syndromes associated with early onset pancreatic insufficiency include Shwachman-Diamond syndrome (SDS), campomelic dysplasia (CD), and Pearson marrow syndrome.
Two major types of signals control the delicate morphological changes in the pancreas as well as the cellular growth, expansion, and differentiation of pancreatic progenitors. They include (1) a repertoire of transcription factors and (2) inductive signals from adjacent structures and tissues.
Transcription factors are identified by their DNA-binding domains and they either activate or suppress gene transcription. A summary of a few key factors is provided below.
The transcription factor PDX1, or pancreatic and duodenal homeobox 1, identifies early pancreatic progenitors. , Absence of PDX1 expression results in pancreatic agenesis. ,
During adulthood, PDX1 expression becomes largely confined to the beta cells, where it binds to and activates the insulin promoter. Conditional inactivation of Pdx1 in adult mouse beta cells results in diabetes. In summary, PDX1 is required for proper pancreatic development as well as beta cell function.
Pancreas specific transcription factor 1a (PTF1a), or the alpha subunit of pancreas-specific transcription factor 1, is a basic helix-loop-helix transcription factor that initially contributes to the specification of all three pancreatic precursors ; however, later in development its serves to promote acinar cell maturation. ,
Ptf1a deficiency in mice results in pancreatic agenesis with complete absence of exocrine development, but ectopic endocrine cells can still be found. In a human pedigree, a truncating single nucleotide mutation in PTF1a led to pancreatic and cerebellar agenesis.
Nuclear receptor subfamily 5, group A, member 2 (NR5A2) is an orphan nuclear receptor induced by Ptf1a . Nr5a2 has been shown to be important for pancreatic progenitor expansion early in development but is also restricted to the epithelial tips during the secondary transition where it is required for acinar cell specification and terminal differentiation. Deletion of Nr5a2 in experimental animals leads to agenesis of the exocrine pancreas, but endocrine function remains. Nr5a2 directly influences expression of acinar zymogens and gene products critical for acinar secretion.
The muscle, intestine and stomach expression 1 (MIST1) is a basic helix-loop-helix transcription factor whose expression, in the postnatal state, is restricted to acinar cells. MIST1 is thought to function in the maintenance of acinar cell polarization. Ablation of Mist1 still allows the development of acinar cells, but they are more sensitive to injury and more prone to neoplastic transformation.
The SRY-box 9 (SOX9) is a high mobility group protein. This transcription factor is essential for the secondary transition in pancreatic development. Lineage tracing experiments demonstrate that Sox9 is expressed in pancreatic progenitor cells , ; however, Sox9 expression in the mature pancreas is limited to the duct epithelium. SOX9 haploinsufficiency in humans is associated with CD, a skeletal malformation syndrome that is associated with the heterozygous loss of function mutation in SOX9. Individuals with this syndrome have reduced islet cell mass and cystic ducts, indicating a role for duct cell maintenance by SOX9. , In experimental animals, knockout of pancreas-specific Sox9 results in pancreatic hypoplasia.
Additional transcription factors involved in endocrine specification include MAFA, ISL1, BRN-4, NEUROD, NKX6.1, NKX2.2, PAX4, PAX6, and ARX.
In addition to the critical role of transcription factors expressed from within the developing pancreas, fluctuating levels of signals from adjacent tissues and structures also control pancreatic development. Early on, pancreas specification from endoderm requires suppression of WNT and fibroblast growth factor 4 (FGF4) signaling from adjacent mesoderm. , Additionally, mesodermal retinoic acid signals refine the anterior-posterior position in which the pancreas develops.
The notochord, which early in development is in proximity to the dorsal pancreatic bud, permits bud specification by suppressing the antipancreatic factor sonic hedgehog (Shh). , By contrast, ventral bud development requires withdrawal of FGF signaling from nearby cardiac mesenchyme.
At a later period, endothelial tissue, such as dorsal aorta and the vitelline veins, influence pancreatic development. , For example, the dorsal aorta could induce Pdx1 and insulin expression in isolated endoderm. In addition to points of endodermal-endothelial contact, sphingosine-1-phosphate secreted by endothelial cells in the circulation promotes dorsal pancreatic budding.
Growth factors secreted by pancreatic mesenchymal cells are also important to the development of the endodermal primordium. The presence of mesenchyme shifts the growth state of pancreatic progenitors from differentiation to proliferation. Notably, the mesenchymal factor FGF-10 promotes proliferation. Fgf-10 -deficient mice exhibit severe growth retardation of the pancreas. Conversely, overexpression of Fgf-10 controlled by the Pdx1 promoter results in sustained proliferation rather than differentiation. , Fgf-10 signaling causes activation of the Notch pathway. Mice deficient in various Notch signaling components show accelerated differentiation and less proliferation, resulting in pancreas hypoplasia. Overexpression of Notch1 in pancreatic epithelium prevents endocrine and exocrine differentiation. The Notch target Hes1 prevents p57-mediated cell-cycle exit and is required for differentiation during the early stages of pancreas formation. The importance of ensuring pancreatic progenitor proliferation is underscored by the finding that the final size of the pancreas is determined by the original number of progenitor cells present at an early embryonic stage. Stromal components also influence the pancreas during development. For example, neural crest cells that migrate into the pancreas influence the number of beta cells. Finally, it is also likely that, conversely, the pancreas contributes instructive signals to its adjacent tissues; this postulate, however, has not been well characterized.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here