Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter is designed to be a broad overview of selected systemic therapies for dermatologic diseases. It provides an historical perspective for each drug, a discussion of its mechanism of action and side effects, and touches briefly on indications and clinical use. This information should serve as a starting point, allowing the reader to readily compare and contrast treatment options. It is not a substitute for the in-depth knowledge and experience necessary to implement these therapies, nor is it a complete library of systemic drugs used in dermatology. A number of systemic medications are reviewed in other chapters ( Table 130.1 ) and will not be discussed here.
| SYSTEMIC MEDICATIONS COVERED IN OTHER CHAPTERS | |
|---|---|
| Antihistamines | Ch. 18 |
| Antimicrobials | Ch. 127 |
| Biologic immunomodulators, including rituximab, secukinumab, TNF-α inhibitors, ustekinumab | Ch. 128 |
| Cytokines, including G-CSF, GM-CSF, interferons | Ch. 128 |
| Glucocorticosteroids | Ch. 125 |
| Interleukin (IL)-1 and IL-1R antagonists, including anakinra, canakinumab, rilonacept | Chs 45 & 128 |
| Intravenous immunoglobulin (IVIg) | Ch. 128 |
| Ivermectin | Ch. 84 |
| JAK inhibitors, e.g. tofacitinib | Ch. 128 |
| Psoralens | Ch. 134 |
| Psychotropic agents, including pimozide, atypical antipsychotic agents | Ch. 7 |
| Retinoids, including acitretin, bexarotene, isotretinoin | Chs 8 , 36 & 126 |
| Spironolactone | Ch. 36 |
Systemic drugs used in dermatology may be subdivided into broad categories, such as immunosuppressive, cytotoxic, and antiproliferative ( Table 130.2 ). Drugs in a single category act in a relatively similar fashion and generally have similar important side effects. For example, immunosuppressive drugs suppress the body's ability to recognize or eliminate infections and neoplastic cells. Patients may be at increased risk for opportunistic infections and selected lymphoproliferative malignancies as well as squamous cell carcinomas. Given this increased risk for infection, patients with either an active infection or one that may reactivate (e.g. tuberculosis, hepatitis B viral infection) should be cautiously given these drugs.
| CATEGORIES OF SYSTEMIC DRUGS USED IN DERMATOLOGY BASED UPON MECHANISM OF ACTION | |||
|---|---|---|---|
| Immunosuppressive/Anti-inflammatory | Cytotoxic | Antiproliferative | Miscellaneous |
|
|
|
|
Most of the drugs discussed herein lack Food and Drug Administration (FDA) approval for dermatologic indications. However, in most instances, their use is based on the mechanism of action of the drug and the presumed pathogenesis of the disease, although in some instances, it is based upon anecdotal observations. When choosing therapy for a patient, certain broad general principles apply. First, the physician should be aware of all common therapeutic modalities available that are likely to yield optimal results. Certain systemic medications will not be utilized frequently enough for a given clinician to become comfortable with their use; therefore, patients requiring more specialized drugs should be referred to colleagues experienced with their use. Patients should be advised of all reasonable therapeutic choices as well as the risk–benefit profile of each modality. Non-compliance is a relative contraindication for all medications discussed in this chapter.
Prior to initiation of several of these medications, based upon potential side effects, the patient should have a complete history and physical examination, with specific emphasis on organ systems that may be affected by the particular drug. Tuberculin skin testing and/or interferon-gamma release assays (IGRAs; e.g. QuantiFERON®-TB Gold test, T-SPOT®.TB) should be performed prior to using some of the immunosuppressive medications, specifically corticosteroids (particularly when a prolonged course is anticipated) and biologic immunomodulators (see Ch. 128 ). Suggested screening for hepatitis B and C viruses is outlined in Table 128.8 . Consultation with appropriate generalists or specialists in selected situations may be required prior to initiating therapy as well as for periodic screening for treatment complications. Table 130.3 outlines suggested monitoring guidelines for systemic medications discussed in this chapter. These tests may need to be performed more frequently in high-risk patients or in patients with abnormal results. Additionally, each outpatient visit should include an appropriate review of systems and physical examination.
| MONITORING GUIDELINES FOR SYSTEMIC MEDICATIONS | |||
|---|---|---|---|
| Drug | Initial screening | Follow-up monitoring | Special considerations |
| Antimalarials | Ocular: Slit lamp and fundoscopic examination: assessment of visual acuity and visual field testing Laboratory:
|
|
|
| Retinopathy risk is greatest for those on treatment for at least 5 years (especially with chloroquine) and if maximum daily safe maintenance dose has been exceeded | |||
| Azathioprine |
|
|
|
| Cyclophosphamide |
|
|
|
| Cyclosporine |
|
|
|
| Dapsone |
|
|
|
| Methotrexate |
|
|
|
| Mycophenolate mofetil |
|
|
Discontinue or decrease dose if WBC declines to <3500–4000 cells/mm 3 |
| Thalidomide |
|
|
|
The use of each drug during pregnancy and lactation is reviewed. Wherever possible, this has been referenced with the ninth edition of Drugs in Pregnancy and Lactation by Briggs and colleagues. Additionally, in 2015, under the Pregnancy and Lactation Labeling Rule (PLLR), the FDA abolished the letter rating system for drug safety in pregnant women and during lactation; the letters are to be replaced with narrative-based labeling . Drugs approved prior to 2015 have three years to comply with the new format while drugs approved thereafter must comply from the onset. This text will, when possible, include both when applicable. In general, one should be circumspect in prescribing systemic medications to women of childbearing potential. It is not adequate to merely ask the patient if she is utilizing birth control; the patient must be made acutely aware of the risks associated with medication use during pregnancy and risks of continuing therapy if she becomes pregnant. The prescribing physician also needs to be aware that birth defects have sometimes been ascribed to systemic medications despite lack of strong scientific evidence. Family planning consultation and communication with the patient's obstetrician or primary care physician can prove helpful.
A few of the medications reviewed in this chapter, including mycophenolate mofetil and thalidomide and its derivatives, are subject to FDA-mandated Risk Evaluation and Mitigation Strategies (REMS). In addition to specifically designed medication guides for patients, there are educational programs for health care providers and restrictive computerized programs to ensure compliance and safe usage of the medications, especially as it pertains to pregnancy.
Lastly, several drugs discussed herein have parenteral formulations. Clinicians who administer parenteral medications in the ambulatory setting should be current in Advanced Cardiac Life Support (ACLS) training and keep emergency resuscitation supplies available.
Quinine and its derivatives have been used since the 1600s to treat malaria. Quinine, derived from the bark of the cinchona tree in South America, was first used in dermatology by Payne in 1894 to treat discoid lesions in patients with lupus erythematosus (LE). The most common antimalarials are hydroxychloroquine (Plaquenil®), chloroquine (Aralen®), and quinacrine; the latter, which can lead to yellow skin discoloration, is only available in the US via compounding pharmacies and will not be discussed in detail.
The antimalarials are absorbed extensively into tissues and slowly released, leading to a half-life of 40–50 days. A steady state is achieved slowly, and it may take 3–4 months to see adequate clinical effects. Hydroxychloroquine is catabolized into two metabolites, desethylhydroxychloroquine and desethylchloroquine. Chloroquine is metabolized only into the latter. The initial metabolites then undergo further change into the primary amine form. Overall, 50% of each drug undergoes renal excretion .
The mechanisms of action of antimalarials are complex and incompletely understood. Known endpoints include the following: stabilization of lysosomes within injured cells; inhibition of antigen presentation, cell-mediated immunity, and the synthesis of proinflammatory cytokines; and antithrombotic/antiplatelet effects . The photoprotective effect attributed to the antimalarials may result from their anti-inflammatory properties .
Most conditions will respond to dosages between 200 and 400 mg/day of hydroxychloroquine or 250 mg/day of chloroquine, with a maximum safe chronic dose (from an ocular standpoint) being 5 mg/kg/day and 2.3 mg/kg/day, respectively, utilizing real body weight . After a suitable therapeutic response has been achieved, the response may be maintained with hydroxychloroquine 100–200 mg daily. If available, quinacrine (100 mg/day) can be added to 200 mg twice daily of hydroxychloroquine, to maximize the clinical benefit without increasing the risk of ocular toxicity. Lower doses of chloroquine (125 mg twice weekly) or hydroxychloroquine (100 mg three times weekly) must be used in patients with porphyria cutanea tarda, in order to minimize the risk of a toxic reaction (e.g. hepatotoxicity) in addition to a marked increase in urinary uroporphyrin output and flare of cutaneous disease ( Fig. 130.1 ).
If no response is noted after 3–4 months, the specific antimalarial has failed and should be discontinued; however, a different antimalarial can be tried. In patients with porphyria cutanea tarda, the antimalarial can slowly be increased to daily dosing if necessary and if laboratory monitoring of transaminases allows.
Monitoring guidelines are outlined in Table 130.3 and in the next section.
Antimalarials are highly concentrated in the iris and choroid, reaching levels 480 000 times that of plasma . However, irreversible retinopathy rarely occurs when dosages remain within the recommended range and patients are monitored by an ophthalmologist experienced with the ocular effects of antimalarials ( Table 130.4 ). As noted in Table 130.4 , the risk of retinopathy is much less with hydroxychloroquine than with chloroquine.
| RISK FACTORS AND TYPES OF RETINOPATHY ASSOCIATED WITH ANTIMALARIAL THERAPY | ||
|---|---|---|
| Hydroxychloroquine | Chloroquine | |
| Risk factors | ||
| Dosing risk | ||
| Daily dose | >400 mg/day (>5 mg/kg/day, based upon real body weight) | >250 mg/d (>2.3 mg/kg/day based upon real body weight) |
| Total cumulative dose | >1000 g | >460 g |
| Other risk factors | ||
| Ocular |
|
|
| Systemic |
|
|
| Age |
|
|
| Types of retinopathy | ||
Reversible ocular toxicity:
|
|
|
Irreversible ocular toxicity (true retinopathy):
|
|
|
Because of the risk of dose-related ocular toxicity, referral to an ophthalmologist is necessary for a baseline examination. The revised American Academy of Ophthalmology (AAO) guidelines recommend this baseline examination be performed within the first year of antimalarial use. With the exception of higher risk individuals (e.g. the elderly, history of maculopathy) and those patients with symptoms, annual screening then begins after five years of continuous use . This is in distinction to previous guidelines in which screening was performed every 6–12 months. The basis for the revised guidelines is the negligible risk of retinopathy during the first five years of therapy with commonly employed doses (see Table 130.4 ) . Of note, recent data regarding long-term usage suggest a significant potential for retinopathy after 10 years of continuous therapy .
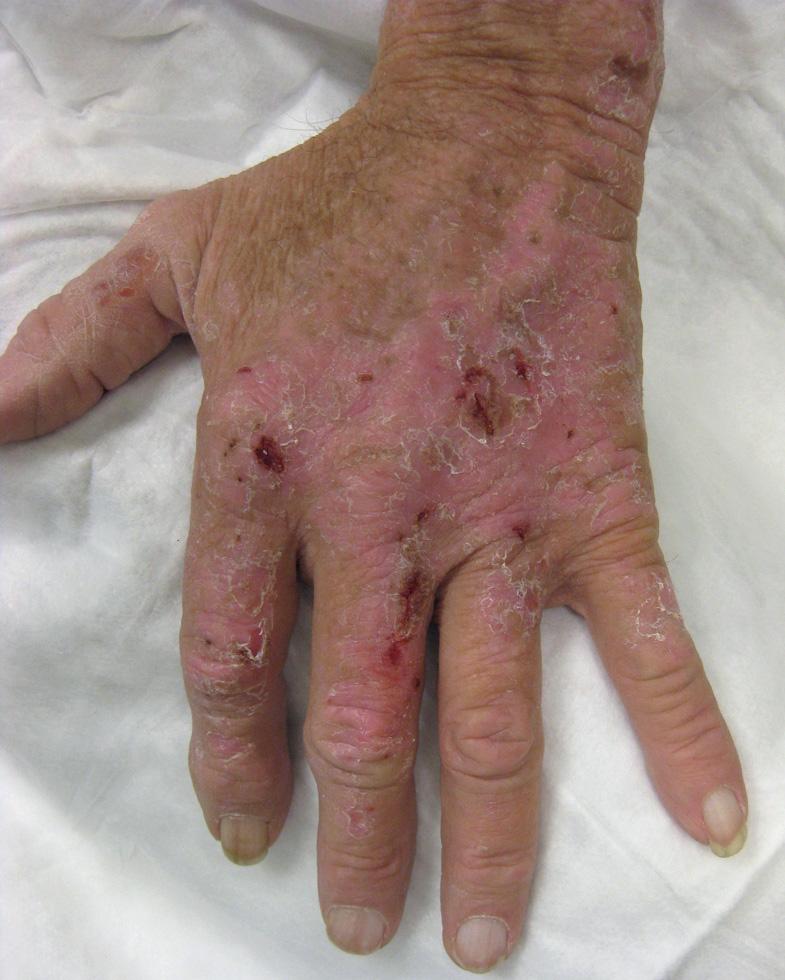
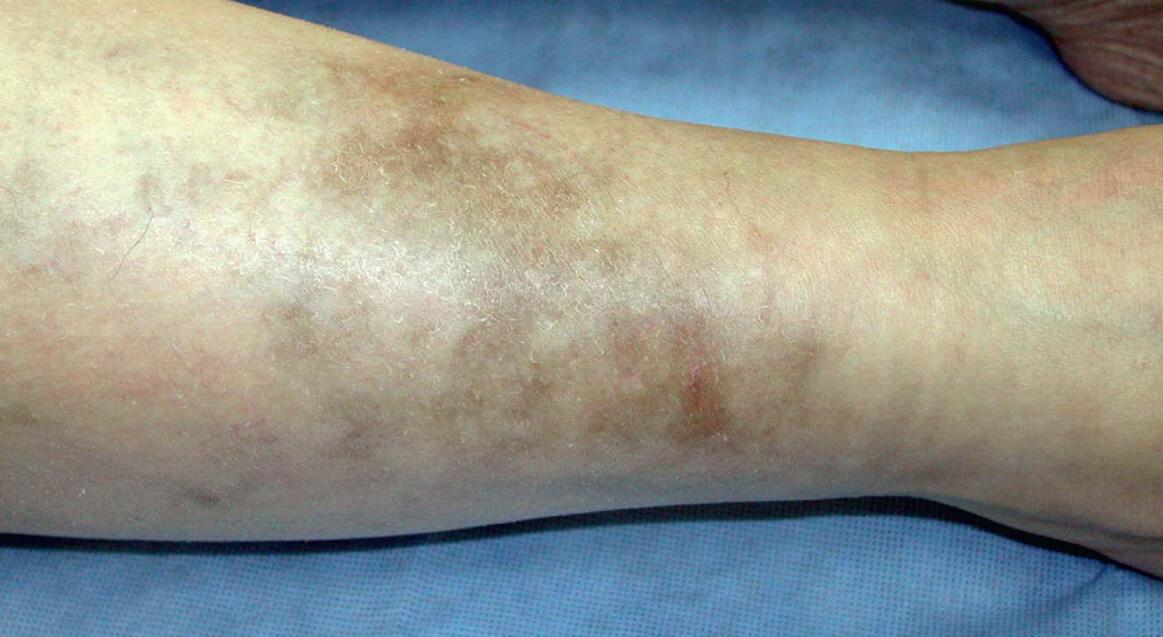
Up to one-third of patients who receive antimalarials for over 4 months will develop a blue–gray to black hyperpigmentation on their shins ( Fig. 130.2 ), face, palate and/or nail beds ( Table 130.5 ). The discoloration fades after cessation of therapy, but may take months to years to resolve completely. Reversible bleaching of the hair roots (achromotrichia) occurs in up to 10% of patients, presumably due to interference with melanosomal function. Another 10–20% may develop an exanthem, ranging from urticaria to lichenoid reactions to exfoliative erythroderma. Of interest, morbilliform and urticarial exanthems have been observed with greater frequency in individuals with dermatomyositis as compared to those with LE.
| SIDE EFFECTS OF SYSTEMIC DRUGS USED IN DERMATOLOGY | |||
|---|---|---|---|
| Drug | Common | Uncommon | Rare |
| Antimalarials | Derm : blue–gray to black discoloration; yellowing from quinacrine |
|
|
| Apremilast |
|
Neuro: headaches | Psych: depression and suicidal ideation |
| Azathioprine |
|
|
|
| Cyclophosphamide |
|
|
|
| Cyclosporine |
|
|
|
| Dapsone | Heme : hemolysis, methemoglobinemia | GI : dyspepsia, anorexia |
|
| Hydroxyurea | Heme : anemia, megaloblastic changes |
|
|
| Methotrexate | Heme : leukopenia |
|
|
| Mycophenolate mofetil | GI : diarrhea, cramps, nausea, vomiting |
|
|
| Saturated solution of potassium iodide (SSKI) |
|
|
|
| Tacrolimus |
|
|
|
| Thalidomide |
|
|
|
* Not permanent during short-term treatment if guidelines are followed.
Antimalarials have been reported to worsen psoriasis in some patients, even though in the past they were commonly used to treat psoriatic arthritis. Psoriatic patients traveling to malaria-endemic areas may take these drugs prophylactically.
Laboratory abnormalities do not commonly occur, but it is the practice of the authors to monitor patients as outlined in Table 130.3 . An overdose of antimalarials can be fatal, and although pediatric usage is safe and effective, patients should be warned to keep the drug out of the reach of small children.
The most common dermatologic use for antimalarials is as second-line therapy for cutaneous LE, after topical or intralesional corticosteroids. Antimalarials are especially useful in patients with widespread discoid lesions and in those with the annular or papulosquamous lesions of subacute cutaneous LE (SCLE). Antimalarial use has also been credited with fewer thromboembolic events in patients with systemic LE (SLE) . Additional cutaneous disorders that may respond to antimalarial therapy are listed in Table 130.6 .
| CUTANEOUS DISORDERS THAT CAN BE TREATED WITH SPECIFIC SYSTEMIC DRUGS | ||||||||
|---|---|---|---|---|---|---|---|---|
| Antimalarials | Azathioprine | CTX | Cyclosporine | Dapsone | Methotrexate | MMF | SSKI | Thalidomide |
|
|
|
|
|
|
|
|
|
# Cutaneous drug reactions more common than in other autoimmune connective tissue diseases.
The only true contraindication is hypersensitivity to the drug. Caution should be used in patients with severe blood dyscrasias or hepatic disorders, because bone marrow suppression and hepatitis can occasionally occur. Should ophthalmologic changes of pre-maculopathy develop, an alternative medication should be considered. Ocular changes at this stage are potentially reversible, but could progress if the drug were continued.
Chloroquine is thought to be safe for treatment and prophylaxis of malaria during pregnancy; however, there have been anecdotal reports of an increase in birth defects in pregnant women being treated for SLE. Hydroxychloroquine is thought to be safer during pregnancy.
Although excreted into breast milk, standard doses of either drug are not harmful to breastfed infants and are approved by the American Academy of Pediatrics for use during lactation .
Cimetidine may increase circulating levels of antimalarials, and antimalarials may increase digoxin levels. Kaolin and magnesium trisilicate, over-the-counter gastrointestinal drugs, decrease absorption of antimalarials. The most significant potential interaction is the additive risk of retinal toxicity when chloroquine and hydroxychloroquine are used concomitantly. Combined therapy consisting of chloroquine or hydroxychloroquine plus quinacrine is acceptable.
In patients with LE, cigarette smoking has been associated with decreased efficacy of antimalarials. Whether this represents a “drug–drug” interaction or decreased compliance (as a manifestation of high-risk behavior) is unknown.
A novel, small-molecule inhibitor of phosphodiesterase 4, apremilast (Otezla®) works intracellularly to reduce the production of proinflammatory mediators and increase those that are anti-inflammatory (see below). When administered orally, apremilast is 70–75% bioavailable, with peak plasma concentrations observed at ~2.5 hours. Nearly 70% of the drug is bound to plasma proteins, and it is metabolized by cytochrome P450 (CYP) enzymes, predominately CYP3A4 (see Ch. 131 ); this is followed by glucuronidation and non-CYP-mediated hydrolysis. Apremilast has a terminal elimination half-life of 6–9 hours and is excreted in the urine and feces.
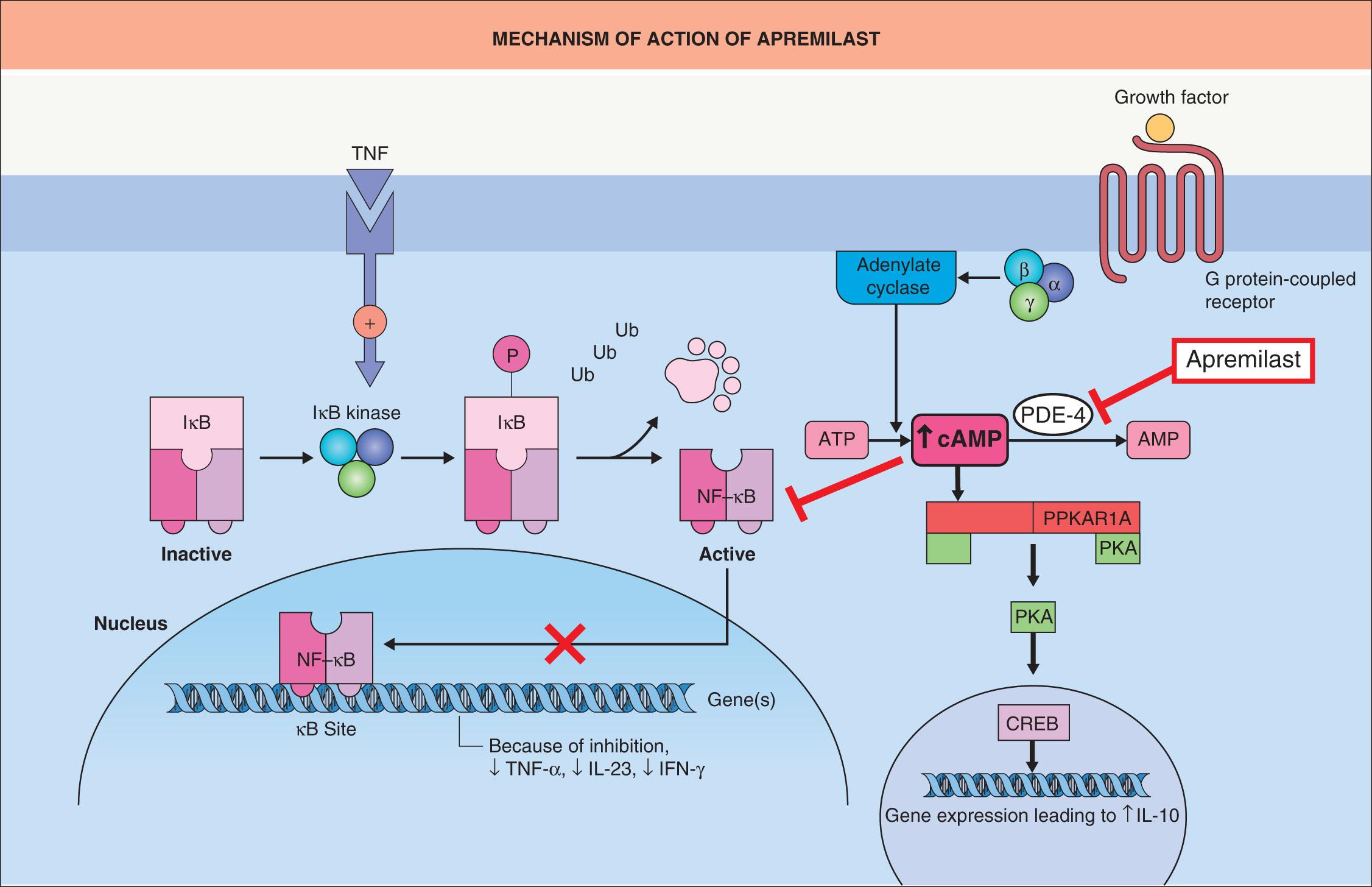
Apremilast inhibits phosphodiesterase 4, which is an intracellular enzyme that degrades cAMP and represents the predominant phosphodiesterase in keratinocytes, dendritic cells, monocytes, and neutrophils. Increasing intracellular cAMP levels activates protein kinase A, leading to enhanced expression of several transcription factors including c AMP- r esponse e lement b inding protein (CREB), while inhibiting others such as nuclear factor kappa B (NF-κB). By inhibiting phosphodiesterase 4 and increasing intracellular cAMP levels, apremilast has multiple downstream effects: it decreases the production of inflammatory mediators such as TNF-α, IFN-γ, and interleukins (IL)-2, -12, and -23; it increases the production of anti-inflammatory mediators including IL-10; and it inhibits natural killer responses ( Fig. 130.3 ) .
The recommended dosage for psoriatic arthritis and psoriasis is 30 mg twice daily. In order to reduce gastrointestinal symptoms, an upward titration of the dose by 10 mg/day is recommended, starting with an initial dose of 10 mg/day. Apremilast is available in 10, 20 and 30 mg tablets. For individuals with severe renal impairment, the recommended maximum daily dose is 30 mg; there is no dosing adjustment for hepatic impairment.
The most common side effects are gastrointestinal, especially nausea and diarrhea, and they are most evident during the first 15 days of administration and gradually resolve over several weeks. Headache and nasopharyngitis are additional potential side effects. Because depression, including suicidal ideation, can be observed in up to 1% of patients, patients with a history of depression should be monitored closely. Loss of ~5–10% of body weight occurs in ~10% of patients. No laboratory monitoring is recommended, but serial measurements of weight can be performed.
Apremilast is FDA-approved for adult patients with active psoriatic arthritis and moderate to severe plaque psoriasis. The ESTEEM trial reported that a significantly greater proportion of patients receiving apremilast (30 mg BID) achieved Psoriasis Area and Severity Index (PASI)-75 (33.1%) and PASI-50 (58.7%) at week 16 compared with placebo (5.3% and 17.0%); quality of life measures and nail and scalp psoriasis were similarly improved . It is currently being investigated for other inflammatory skin diseases including discoid LE, lichen planus, granulomatous dermatoses, and atopic dermatitis.
Apremilast is contraindicated in patients with known hypersensitivity to the drug or its components. Dosing should be adjusted for renal failure (see above). Relative contraindications include a history of depression or suicidal ideation.
Apremilast was formerly pregnancy category C. However, it has not been evaluated in well-controlled studies involving pregnant women and should only be used during pregnancy if the potential benefit justifies the potential risk. It is not known whether apremilast or its metabolites are present in human milk.
Coadministration of apremilast with potent CYP450 inducers including rifampin, phenobarbital, carbamazepine, and phenytoin may significantly diminish apremilast levels and should be avoided (see Ch. 131 ).
Azathioprine was developed in 1959 from its parent drug 6-mercaptopurine (6-MP). After its anti-inflammatory and immunosuppressive effects were noted, dermatologists began to utilize azathioprine for the treatment of inflammatory diseases. With its moderately potent immunosuppressive and anti-inflammatory effects, azathioprine has a reasonable risk–benefit profile. However, it should be reserved for serious, life-threatening or recalcitrant dermatoses after other therapies have failed.
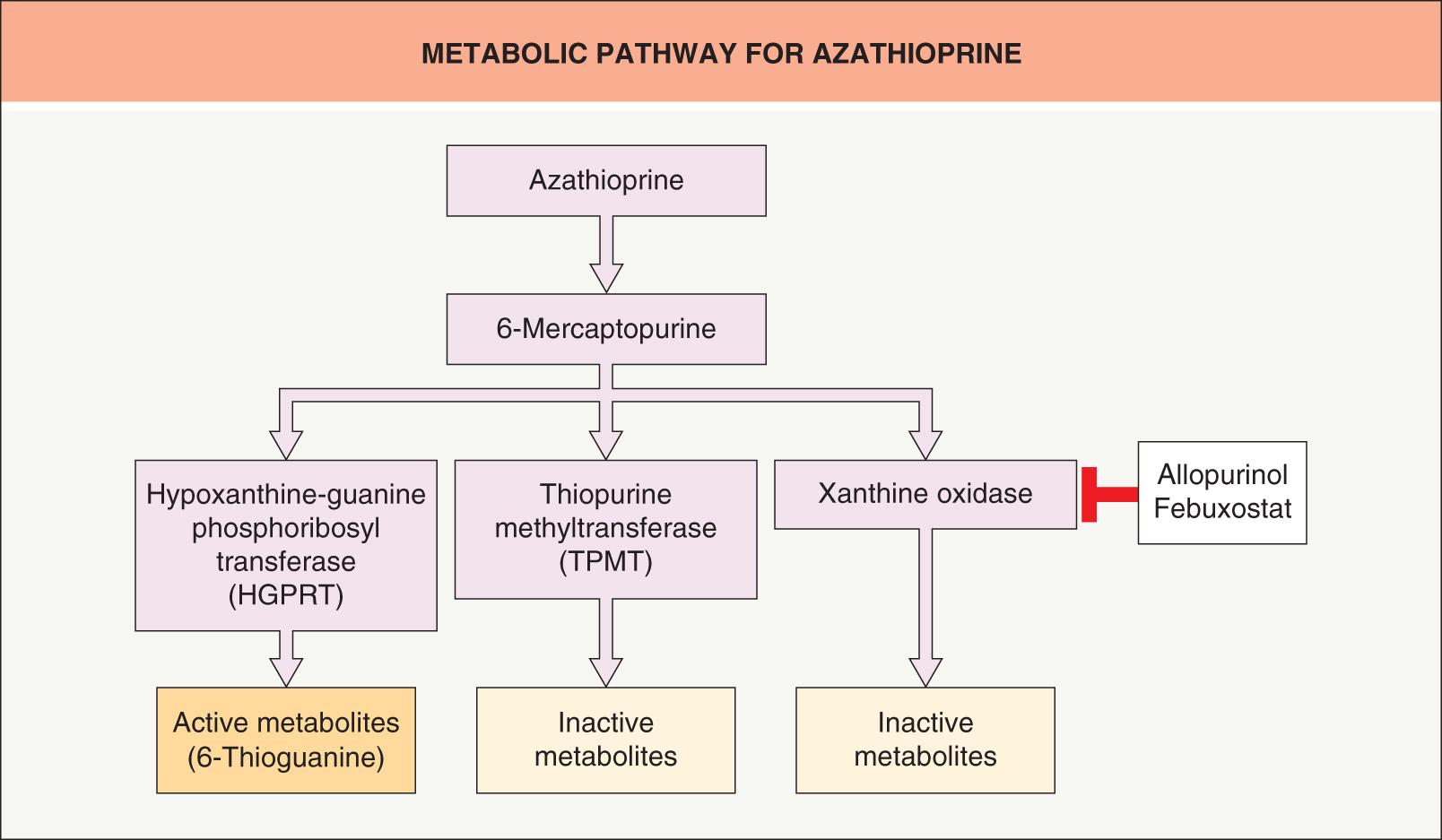
Azathioprine (Imuran®, Azasan®, Azamun™) has an 88% bioavailability. Immediately after absorption, it is converted to 6-MP and subsequently processed through three different, competing pathways ( Fig. 130.4 ). When 6-MP is catabolized via two of these metabolic pathways, by either xanthine oxidase or thiopurine methyltransferase (TPMT), inactive metabolites result. Active metabolites, such as the purine analogue thioguanine monophosphate and others, are produced from the third and only anabolic pathway via hypoxanthine-guanine phosphoribosyl transferase (HGPRT) . Should either the xanthine oxidase or TPMT catabolic pathway be blocked, more 6-MP will be shunted through the anabolic HGPRT pathway, leading to more active metabolites; excessive immunosuppression and pancytopenia may result .
The TPMT pathway is interesting because the enzyme can have variable activity based upon genetic polymorphisms. Three distinct phenotypes exist, and ethnicity may factor into the frequency of certain alleles. Encoded at the 6p22.3 locus, the TPMT activity trait is inherited in an autosomal co-dominant manner . Overall, 89% of Caucasians are homozygous for the high-activity allele and have relatively elevated levels, 11% are heterozygotes and have moderate activity, and 1/300 is homozygous for one of the seven low-activity alleles and has low TPMT activity. Red blood cell (RBC) TPMT activity mirrors systemic activity, and a test for RBC TPMT activity has been developed. Although TPMT activity may vary somewhat between different laboratories and within different batches of the same test kit, knowledge of baseline TPMT activity is clinically useful in the majority of patients who will receive azathioprine. However, vigilance in monitoring for pancytopenia will also identify those with low TMPT activity who will require dose reduction. Likewise, patients with high TMPT activity may need higher doses.
Although decreased xanthine oxidase activity is rarely due to genetic polymorphisms, this enzyme is inhibited by the medications allopurinol and febuxostat (see Fig. 130.4 ). Azathioprine dosage should be decreased by 75% in patients receiving allopurinol or febuxostat.
6–Thioguanine, the active metabolite of azathioprine, is a purine analogue similar in structure to both adenine and guanine. Instead of an amino or hydroxyl group, it contains a thiol moiety. Incorporation of 6-thioguanine into DNA and RNA inhibits purine metabolism and cell division. 6–Thioguanine has other activities which are not well understood, such as suppression of T-cell function and B-cell antibody production . It also decreases the number of Langerhans cells in the skin and inhibits their ability to present antigens .
Available in 25, 50, 75 and 100 mg tablets, empiric dosing is generally started at 50 mg/day and increased to a maximum of 2.5 mg/kg/day according to clinical efficacy and careful monitoring. Maximum doses based on baseline TPMT determination are outlined in Table 130.7 . In either case, renal insufficiency dictates dose reduction (see Table 130.3 ). Baseline evaluation should include a complete medication history because of adverse effects when azathioprine is used concomitantly with allopurinol, febuxostat, captopril or warfarin (see below). Monitoring guidelines are outlined in Table 130.3 .
| MAXIMUM DOSE OF AZATHIOPRINE AS DETERMINED BY BASELINE TPMT ACTIVITY | |
|---|---|
| TPMT level | Maximum dose of AZA |
| <5 U | AZA contraindicated |
| 5–13.7 U | Up to 0.5 mg/kg/day |
| 13.7–19 U | Up to 1.5 mg/kg/day |
| >19 U | Up to 2.5 mg/kg/day |
Major side effects are related to the immunosuppressive effects of azathioprine (see Table 130.5 ). Pancytopenia occurs rarely, particularly when doses are based on TPMT activity. Patients may be at increased risk for malignancies, especially lymphoproliferative disorders and squamous cell carcinomas of the skin and female genitourinary tract, and should be monitored accordingly. Factors influencing a patient's malignancy risk include degree and duration of immunosuppression, skin phototype, and existing comorbidities.
Azathioprine may rarely cause a life-threatening hypersensitivity reaction. It most commonly develops during the first month of therapy and when there is concurrent use of either cyclosporine or methotrexate. The cutaneous eruption is typically morbilliform with areas of confluence. Other components of the syndrome include fever, respiratory and gastrointestinal distress, hepatotoxicity, and possible cardiovascular collapse. This hypersensitivity reaction represents an absolute contraindication as re-exposure may lead to cardiovascular collapse.
Although azathioprine has FDA approval for non-dermatologic uses only, dermatologists have been using this drug for decades to treat severe dermatologic conditions, most often as a corticosteroid-sparing agent in the treatment of immunobullous diseases and various subtypes of cutaneous vasculitis (see Table 130.6 ) . It is inexpensive and has moderate immunosuppressive and anti-inflammatory effects. Clinical benefit of the drug may not be apparent until it has been administered for at least 4 to 6 weeks.
Absolute contraindications to azathioprine therapy include history of a hypersensitivity reaction, as re-challenge may prove fatal. Active serious infection and pregnancy are relative contraindications. Concomitant use of allopurinol or febuxostat requires dose reduction of azathioprine (see above) or selection of an alternative medication.
Azathioprine was formerly pregnancy category D and is associated with preterm delivery, low-birth-weight infants, sporadic anomalies, and hematologic toxicities. Even though it is considered relatively safe for use in transplant patients who become pregnant, for dermatologic purposes it should not be prescribed during pregnancy. There are limited data regarding risk to breastfed infants, but based upon several small series, its use is probably allowable during breastfeeding .
Inhibition of xanthine oxidase by allopurinol or febuxostat (see Fig. 130.4 ) increases the risk of pancytopenia in azathioprine-treated patients. Captopril may increase the risk of leukopenia. Azathioprine can decrease the effectiveness of warfarin and pancuronium, necessitating larger doses of these drugs. Since azathioprine may decrease the effectiveness of intrauterine contraceptive devices, alternative birth control methods should be employed.
The dosage, uses and side effects are outlined in Table 130.8 .
| BLEOMYCIN |
|
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here