Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A 61-year-old woman with no remarkable past medical history developed, over a few months, weakness of her legs with difficulty getting in and out of chairs and climbing stairs. She also experienced significant fatigue. This persisted for 6 months, and then, she developed dysarthria, mild dysphagia, blurred vision, and mild proximal upper extremity weakness. She also endured dry eyes and dry mouth for nearly 1 year.
Examination was remarkable for normal cranial nerves. On motor exam, she had moderate weakness of neck flexion and hip flexion. Reflexes were trace and symmetric at the biceps, triceps, brachioradialis, patella, and Achilles bilaterally, and became easier to elicit after 10 seconds of maximal contraction of the muscle. Gait exam was normal.
Repetitive nerve stimulation testing of the right median and ulnar nerves demonstrated low-amplitude compound muscle action potential amplitudes, which was facilitated following 10 seconds of isometric exercise. Acetylcholine receptor and muscle specific tyrosine kinase antibodies were negative. Voltage-gated calcium channel antibodies were found to be elevated. Computed tomography (CT) of the chest was unremarkable. 18 F-fluorodeoxyglucose positron-emission tomography (FDG-PET) was normal.
The patient is now 3 years out from her symptom onset and has not developed any malignancy. She has been receiving 3,4-diaminopyridine and has improved dramatically. Her examination is now normal.
Lambert-Eaton myasthenic syndrome (LEMS) is the most common presynaptic neuromuscular transmission disorder in adults with an annual incidence of approximately 0.5 × 10 −6 . It has a 10 times lower annual incidence rate than myasthenia gravis, the most common postsynaptic neuromuscular transmission disorder. Roughly 50% of LEMS patients will have an underlying malignancy, which is usually small cell lung cancer (SCLC). Those without malignancy are presumed to have a sporadic autoimmune etiology. Both paraneoplastic and nonparaneoplastic forms are associated with the P/Q type voltage-gated calcium channel (VGCC) antibodies. Other antibodies to presynaptic proteins that have been associated with LEMS include ERC1, synaptotagmin I, laminin β2, and presynaptic muscarinic acetylcholine receptor. SOX-1 antibodies have been identified in the majority of paraneoplastic LEMS patients, but are not associated with nonparaneoplastic LEMS. The age of disease onset varies between the paraneoplastic and nonparaneoplastic LEMS. In the paraneoplastic form, the average age of onset is 60 years. There is a bimodal peak of incidence for the nonparaneoplastic form with one peak at approximately 35 years and a second peak around 60 years. LEMS often precedes tumor detection in the paraneoplastic form. The immune response producing LEMS begins early in the tumor evolution.
The neuromuscular junction is composed of the presynaptic nerve terminal, junctional cleft, and the postsynaptic muscle endplate, which houses the acetylcholine receptors ( Fig. 69.1 ). When an action potential propagates down the motor axon to the motor nerve terminus, depolarization opens the VGCC, resulting in an influx of calcium into the presynaptic membrane. The VGCC are heteromeric multisubunit complexes that are composed of Ca 2+ selective pore-forming α1 subunit, a largely extracellular α2δ subunit, an intracellular β subunit, and sometimes a γ subunit. The autoantibodies target multiple VGCC subunits including α and β subunits. Sometimes the α2δ subunit is also targeted but not in isolation. The intracellular calcium binds to calmodulin and activates calcium-dependent signaling pathways, which mobilizes acetylcholine vesicles from “active zones” into the synaptic cleft. The acetylcholine binds to the acetylcholine receptors, which causes sodium and potassium movement across the endplate membrane. When the endplate potential depolarizes and reaches the threshold, a muscle fiber action potential is initiated causing muscle contraction. In LEMS, antibodies block the P/Q subtype of VGCC preventing calcium influx and less acetylcholine vesicles are released from motor and autonomic cholinergic nerve terminals. There is a lower probability of reaching the endplate depolarization threshold and generating a muscle fiber action potential.
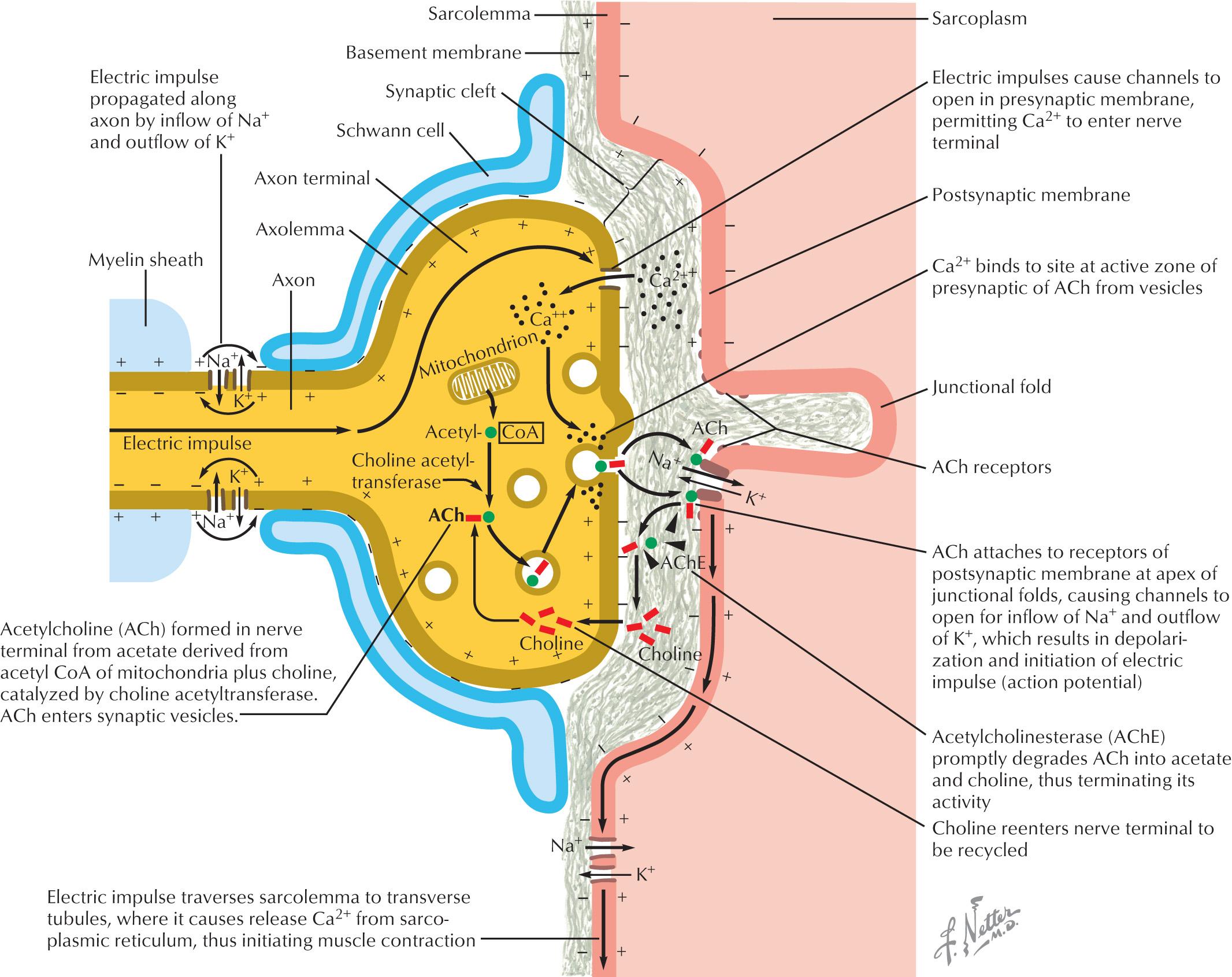
VGCCs are expressed on SCLC and other cancer cells in addition to the presynaptic motor nerve terminal membrane. These channels present in SCLC tumor cells have antigenic potential and stimulate autoantibody production. In nonparaneoplastic LEMS patients, nearly 65% will have the HLA-B8-DR3 haplotype. Ten to 15% of patients with LEMS do not have detectable P/Q-type VGCC antibodies. These seronegative patients have clinical and electrophysiologic features that are indistinguishable from seropositive patients.
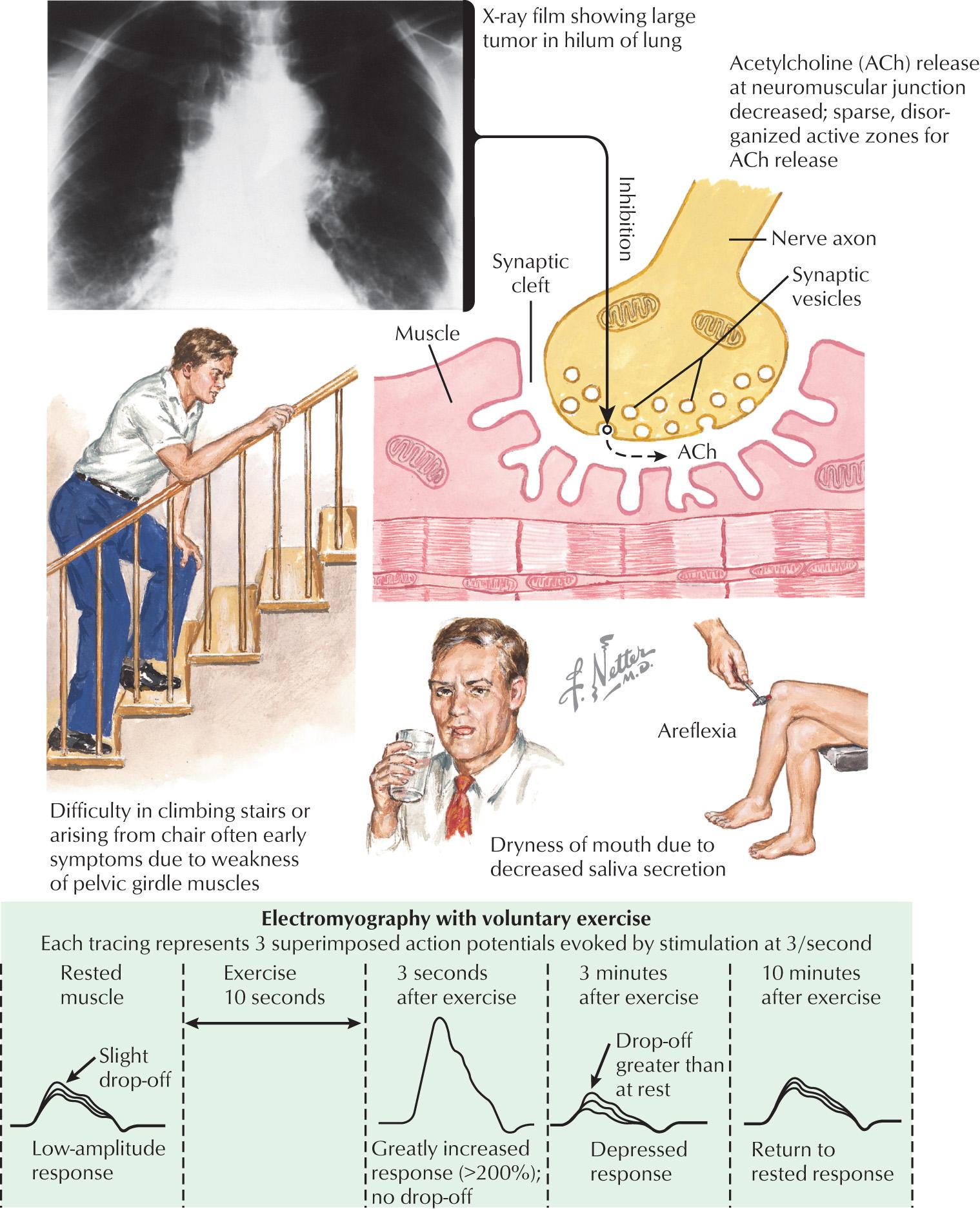
LEMS has a similar presentation regardless of the presence of an underlying malignancy ( Fig. 69.2 ). The clinical triad in LEMS is proximal muscle weakness, autonomic dysfunction, and areflexia. The most common presenting symptom is leg weakness followed by generalized weakness, muscle pain or stiffness, and dry mouth. Rarely, patients present with arm weakness, diplopia, or dysarthria. Some patients have no detectable weakness on exam on manual motor testing. The physician must rely on history of impaired function or evidence of difficulty rising from a chair or climbing stairs. Patients often have severe fatigue. The majority of patients with LEMS will develop autonomic dysfunction including dry mouth, blurred vision, hypohidrosis, constipation, and orthostatic hypotension. Dry mouth is the most common autonomic symptom. Deep tendon reflexes are classically diminished. In nearly half of patients, diminished reflexes will facilitate and become more pronounced following brief exercise. Respiratory symptoms are unusual but may occur later in the disease course. Interestingly, some patients with LEMS will complain of vague sensory symptoms such as numbness and tingling.
The primary diagnosis considered in the differential for LEMS is myasthenia gravis. In contrast to LEMS patients who present with leg weakness, most myasthenic patients will first develop ocular symptoms, including diplopia and ptosis. In LEMS, the weakness typically spreads rostrally, whereas weakness in myasthenia spreads caudally. The reduction in reflexes and autonomic dysfunction are also distinctive features of LEMS. Because LEMS typically presents with proximal weakness, this may simulate a myopathy. Myopathic patients would not typically have areflexia or autonomic dysregulation. LEMS may mimic chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) given the weakness and areflexia. The absence of sensory involvement and presence of autonomic dysfunction differentiates LEMS from CIDP.
Serologic testing in LEMS includes a creatine kinase and a paraneoplastic panel, which includes P/Q-type VGCC antibodies. Although nearly 90% of patients with LEMS will have P/Q-type VGCC antibodies, 10%–15% will remain seronegative. Up to 40% of SCLC patients will have P/Q-type VGCC antibodies without clinical or electrophysiologic evidence of LEMS. An evaluation for underlying malignancy is necessary with postcontrast computed tomography (CT) or magnetic resonance imaging (MRI) of the chest. Should these be negative, then an 18 F-fluorodeoxyglucose (FDG) positron emission tomography (PET) is recommended. Follow-up chest imaging is recommended for cancer surveillance in those patients with LEMS and a smoking history. Antibodies to SOX-1, which is a transcription factor in the SRY family of high-mobility group box proteins, has high specificity for SCLC. SOX-1 antibodies have been described in 67% of patients with paraneoplastic LEMS and only 5% of nonparaneoplastic LEMS.
Electrophysiologic testing is critical in the diagnosis of LEMS. There are three key findings: low-amplitude compound muscle action potentials (CMAPs) at rest, decrement of CMAP amplitude on slow (2–3 Hz) repetitive nerve stimulation, and postactivation facilitation with an increase in CMAP amplitude by more than 100% following either high-frequency repetitive nerve stimulation or 10 seconds of voluntary isometric contraction. The electrophysiologic difference between a presynaptic and postsynaptic neuromuscular junction transmission disorder is the lack of facilitation of CMAP amplitude in those with postsynaptic disease (myasthenia gravis). The low-amplitude CMAPs on routine nerve conduction studies would also not typically be seen in myasthenia gravis. In both LEMS and myasthenia gravis, one would expect to see a decrement of the CMAP amplitude with low-frequency repetitive nerve stimulation testing. In myasthenia gravis, the decremental pattern decreases during a train of CMAPs, whereas in LEMS, the decremental pattern becomes progressively greater. Two or three muscles may be screened for facilitation, including abductor pollicis brevis, abductor digiti minimi, and extensor digitorum brevis muscles. Single-fiber EMG demonstrates increased jitter and blocking. Symptomatic management should be withdrawn 12 hours before electrophysiologic studies.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here