Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The various abnormal ventriculoarterial connections, such as transposition in its regular or congenitally corrected variants, double-outlet ventricles, and common arterial trunk, are all congenital malformations involving the ventricular outflow tract. They are often described in terms of “conotruncal malformations,” but this term is poorly defined. As currently used, it rarely includes lesions of the arterial valves, which are also components of the ventricular outflow tracts. All these lesions, along with pulmonary and aortic atresia, have been discussed at length in this text. However, several additional lesions, all of which involve different parts of the outflow tracts, are best described in a separate chapter. Therefore this chapter discusses aortopulmonary (AP) windows, anomalous origin of one pulmonary artery (PA) from the intrapericardial aorta, aortoventricular tunnels, and aneurysms of the aortic sinuses of Valsalva. Also discussed is the rare situation of either duplication of an outflow tract or the even more rare variant of duplication of the aortic root. This chapter begins with a reprise of the normal anatomy of the outflow tracts. It is axiomatic that the abnormal arrangements cannot properly be appreciated without full understanding of the normal situation. Until recently, it was difficult to provide rational explanations for the development of all the normal components, apart from seeking to explain the morphogenesis of the malformations. The ability to illustrate with accuracy the temporal development of the outflow tracts, as already described in Chapter 3 , now makes it possible to suggest reasons why the outflow tracts should develop abnormally in such a way as to produce the various lesions. Therefore the chapter includes a recapitulation of the steps involved in normal separation of the initially common outflow tract into the separate outlets for the right and left ventricles, the arterial roots, and the intrapericardial arterial trunks. These changes are related to the postnatal anatomy of the various lesions as a prelude to describing their clinical features.
As discussed earlier, it has been conventional to describe both the development and the morphology of the outflow tracts in terms of the truncus and conus. This approach has proven less than satisfactory, largely because it ignores the arterial roots, which occupy a significant length within the middle of both outflow tracts ( Fig. 51.1A and D ).
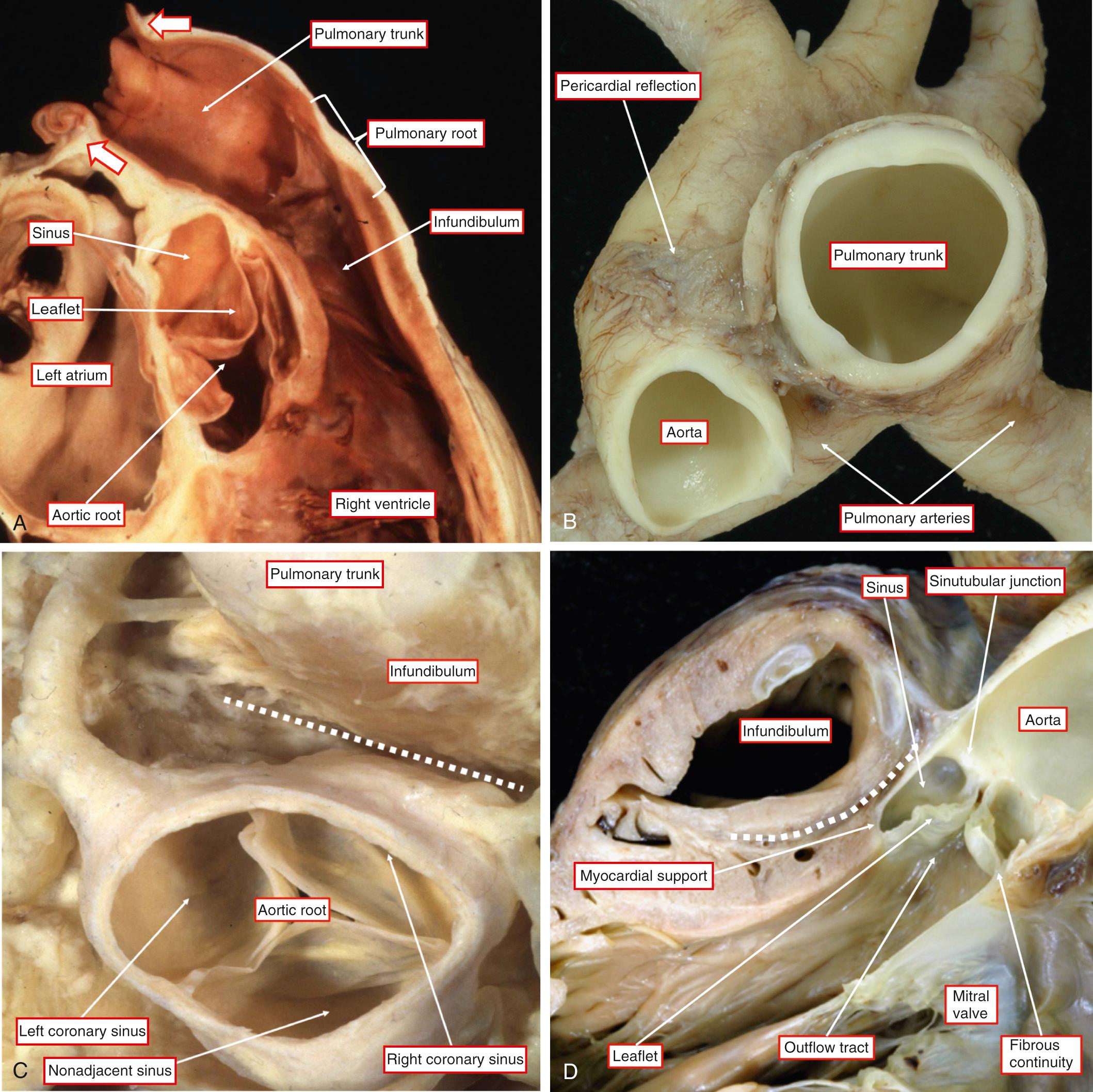
The proximal parts of the outflow tracts are bounded by the semilunar leaflets of the arterial valves. The outflow tract of the right ventricle is exclusively muscular. Only two sinuses of the aortic root, in contrast, have myocardial support within the left ventricle. These sinuses give rise to the coronary arteries. They are also sinuses that are adjacent to the pulmonary trunk (see Fig. 51.1C and D ). On rare occasion, the nonadjacent sinus of the aortic root can give rise to one or other of the coronary arteries. That is why we prefer to describe it as the nonadjacent rather than the noncoronary sinus. Fig. 51.1B shows how extracavitary fibroadipose tissue interposes between the right ventricular infundibulum and the aortic root. For quite some time, this area was described as being an “outlet” or “conal” septum. This is clearly not the case because the subpulmonary infundibulum is a freestanding myocardial sleeve. The arterial roots themselves are made up of the semilunar valvar leaflets supported by the sinuses of Valsalva. The myocardium of the proximal part of the outflow tracts is found supporting the bases of all the pulmonary valvar sinuses. Myocardium is also found supporting the bases of the two aortic sinuses giving rise to the coronary arteries. The presence of the myocardium within the sinusal bases reflects the fact that the semilunar hinges of the arterial valvar leaflets cross the anatomic ventriculoarterial junction. As described in Chapter 2 , the semilunar hinges of the leaflets, when reconstructed in three dimensions, have a crownlike configuration. This is why it is best not to describe the hinges in terms of an “annulus.” In addition, echocardiographers describe the virtual ring made by joining together the basal attachments of the leaflets as the “annulus.” The structure of the hinges of the leaflets and the nature of their support are key to understanding the morphology and etiology of both aortoventricular tunnels and aneurysms of the sinuses of Valsalva. We also prefer to avoid using the word “cusp” when describing the components of the arterial roots. This word is frequently used interchangeably to describe both the valvar sinuses and the valvar leaflets. As we will show, to appreciate the morphology of the malformations discussed in this chapter, it is important to distinguish between the leaflets and their supporting sinuses. The third, and distal, parts of the outflow tracts are occupied by the intrapericardial arterial trunks. Each of the trunks also has its own discrete walls, so that there is no septal structure interposing between them (see Fig. 51.1B ). The pericardial reflections divide the ascending aorta into intrapericardial and extrapericardial components. In contrast, the right and left PAs branch from the pulmonary trunk at the margins of the pericardial cavity. Therefore the origin of the arterial duct is extrapericardial.
When first formed during Carnegie stages 12 through 14 in humans and during embryonic days 9.5 and 10.5 in the mouse, the outflow tract, derived by additional migration of cells into the heart tube from the second heart field, is a tube with exclusively myocardial walls that extends from the outlet of the developing right ventricle to the margins of the pericardial cavity. It has an obvious dogleg bend in its middle part (see Fig. 51.2A ).
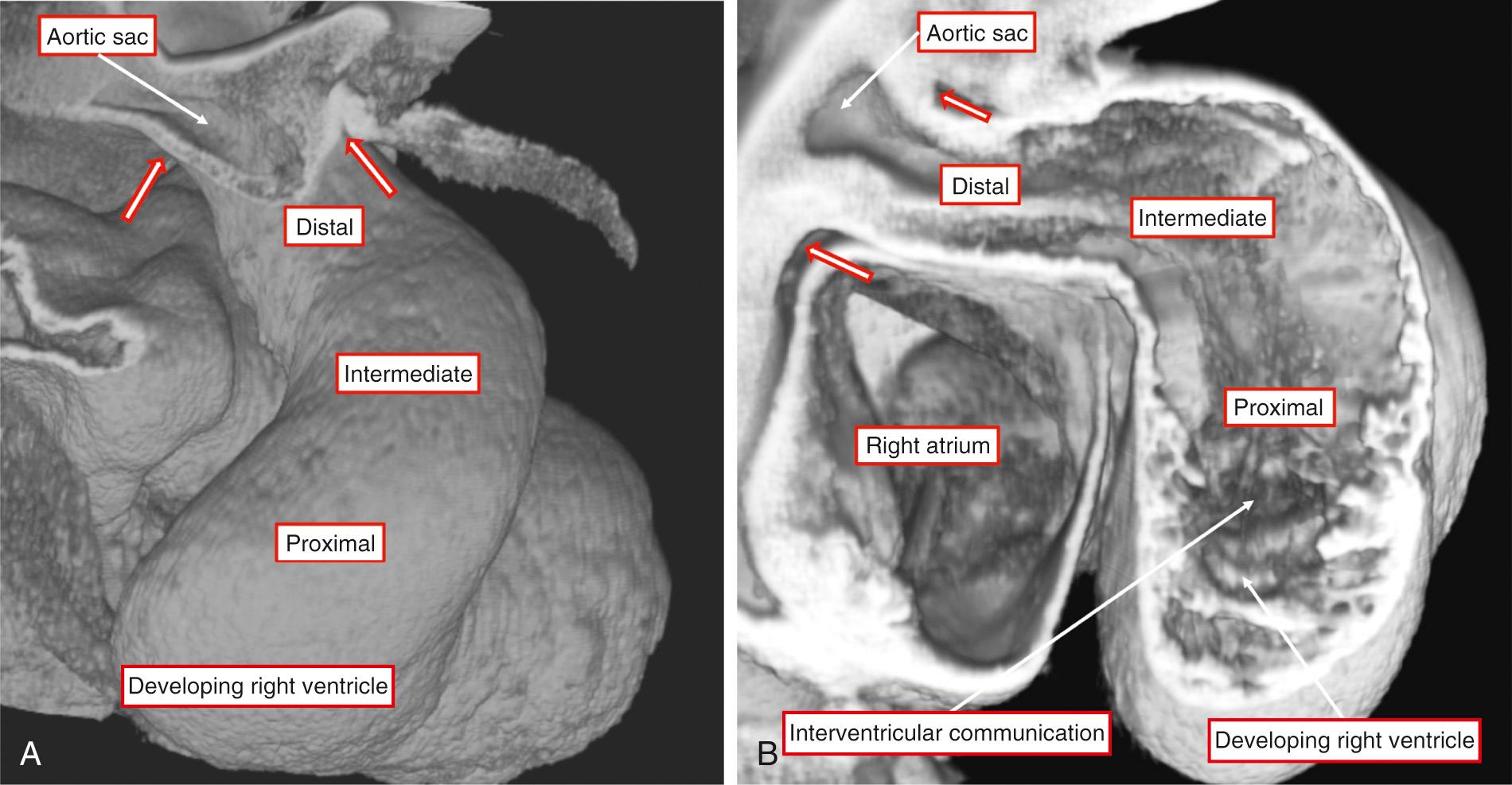
When first formed, the lumen of the outflow tract is lined in circumferential fashion throughout its length by a layer of cardiac jelly. The initial stages of its conversion into separate outflow tracts, which eventually have predominantly nonmyocardial walls, involve the addition of further material to the tube from the second heart field. The newly added tissues are nonmyocardial (see Fig. 51.2B ). These nonmyocardial walls extend initially as a ring with two parietal tongues. The addition of the nonmyocardial tissues distally serves to produce an effective regression of the distal myocardial border away from the margins of the pericardial cavity. Concomitant with this proximal movement of the distal myocardial border of the tube, the jelly that initially surrounded the common lumen is changed, by the process of endothelial-to-mesenchymal transformation, into paired cushions. They spiral when traced toward the ventricular origin of the tube. When initially formed, their distal margins are confluent with the myocardial border, which has a marked “fish mouth” appearance. As development proceeds, the cushions begin to fuse in distal to proximal direction. This divides the parts of the tube with myocardial walls into separate channels that eventually exit from the right and left ventricles. However, at the start of fusion of the cushions, the proximal border of the tube is supported exclusively by the right ventricle (see Fig. 51.2B ). With continuing fusion of the cushions, there is ongoing proximal regression of the distal myocardial border. This is accompanied by addition of still further nonmyocardial tissues to the distal part of the outflow tract. Consequent to these changes, by Carnegie stage 15 in humans and during embryonic day 11.5 in the mouse, it becomes possible to recognize a discrete nonmyocardial distal component of the tube. The component that has retained its myocardial walls can itself now be identified as having distal and proximal parts, with formation of two additional cushions, known as the intercalated cushions, in its distal part ( Fig. 51.3A ).
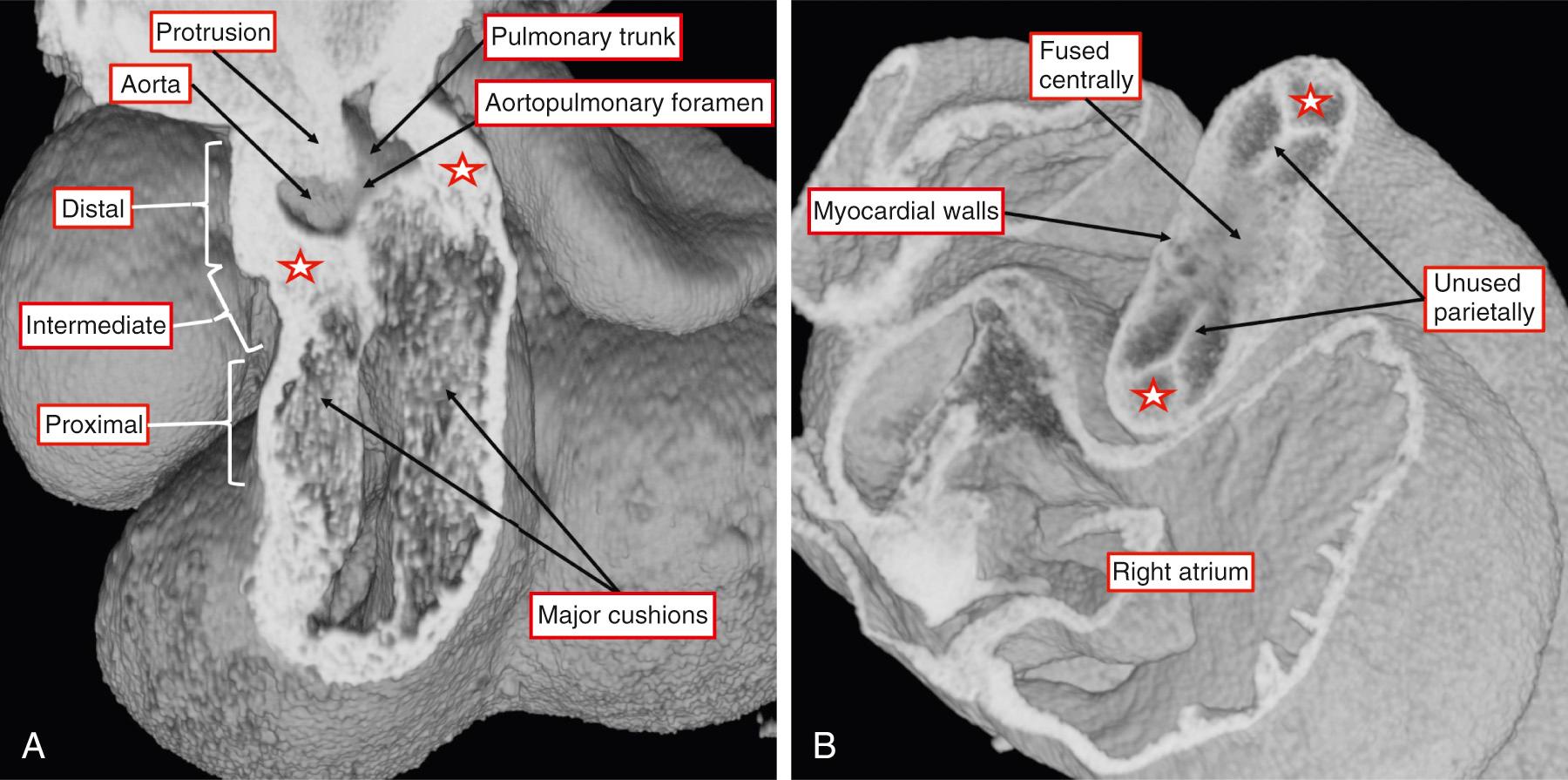
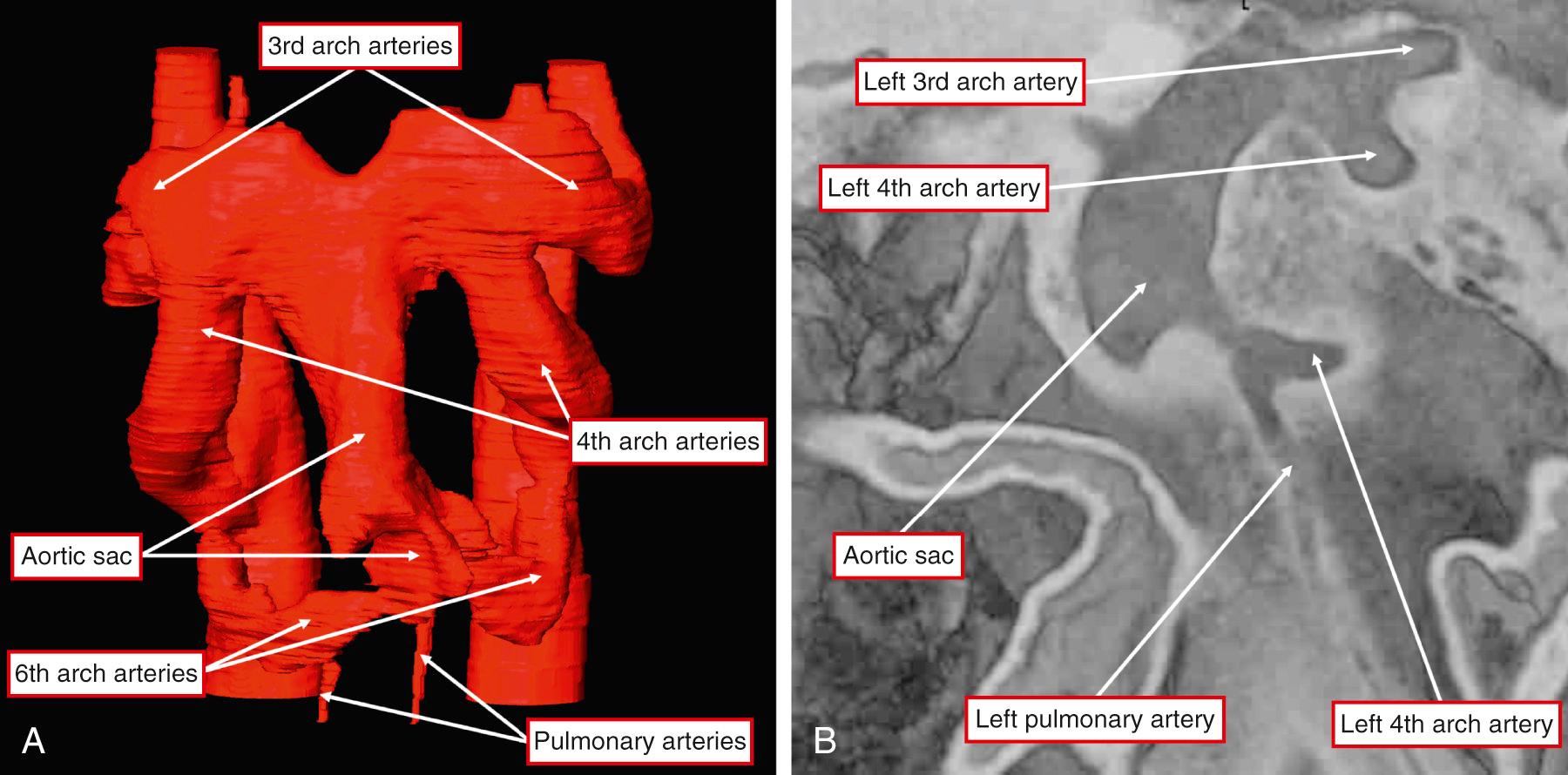
The distal part that has retained its myocardial walls now occupies the intermediate component of the overall outflow tract (see Fig. 51.3A ). It is the nonmyocardial distal part of the overall outflow tract that now becomes separated into the intrapericardial arterial trunks. This is achieved by growth into the outflow tract of an oblique protrusion from the dorsal wall of the aortic sac. The protrusion grows from between the origins of the arteries of the fourth and sixth pharyngeal arches, which will become the systemic and PAs, respectively. Therefore, as it grows into the pericardial cavity, the protrusion is an embryonic AP septum. Concomitant with its growth toward the distal margins of the major outflow cushions, the cushions themselves have fused to separate the intermediate part of the outflow tract into the arterial roots. The space between the leading edge of the protrusion and the distal margins of the fused outflow cushions is an embryonic AP foramen (see Fig. 51.3A ). If development proceeds normally, the protrusion fuses with the cushions, obliterating the AP foramen, and the intrapericardial arterial trunks rapidly develop their own walls. It is then no longer possible to recognize any septal tissues between them. Failure to close the embryonic foramen accounts for persistence of AP windows. However, it is a mistake to consider the lesions as aortopulmonary septal defects. Abnormal development of the distal outflow tract also provides a rational explanation for direct aortic origin of one of the PAs. As the protrusion from the dorsal wall of the aortic sac grows into the cavity of the distal outflow tract, it does so in markedly oblique fashion. It is frequently stated that the PAs are derived from the sixth arch arteries; this is not the case. The right and left PAs develop within the pharyngeal mesenchyme, taking origin from the sixth arch arteries close to their own origin from the aortic sac ( Fig. 51.4 ). Therefore it is easy to envisage that increasing or decreasing obliquity of the protrusion could leave one or other, usually the right, PA in continuity with the systemic component of the aortic sac.
As explained earlier, by the time the embryonic AP foramen has closed, producing the separate intrapericardial arterial trunks, the distal ends of the major outflow cushions have themselves separated the intermediate part of the outflow tract into the putative aortic and pulmonary roots. The interdigitation of the major and the newly formed intercalated cushions within this part of the outflow tract provides the scaffold for formation of the arterial valves (see Fig. 51.3B ). However, at this stage, which occurs during embryonic days 11.5 and 12.5 in the mouse and by Carnegie stage 15 in humans, there has been no formation of the arterial valvar sinuses.
The walls of the arterial valvar sinuses are formed by still further proximal growth of the nonmyocardial tissues derived from the second heart field. This additional growth is accompanied by excavation of the distal margins of the cushions themselves to form the leaflets and their semilunar hinges. During the process of endothelial-to-mesenchymal transformation, the cushions themselves have been invaded by cells migrating from the neural crest. The neural crest cells then form columns of condensed mesenchyme in the central parts of the unfused proximal cushions. Some investigators have described these structures as producing an “aortopulmonary septal complex.” However, as described in Chapter 3 , the columns cannot be identified until after the formation of the intrapericardial arterial trunks. The neural crest cells, nonetheless, do play an important role in separating the arterial trunks from each other. The protrusion from the dorsal wall of the aortic sac is itself covered by cells derived from the crest, although the core of the protrusion is derived from the second heart field. It is the ongoing growth of the tissues from the second heart field that form the walls of the arterial valvar sinuses, with the cells derived from the neural crest being confined to the valvar leaflets and their hinges, albeit with differential contributions to the intercalated cushions. Subsequent to fusion of the central parts of the major outflow cushions, the core of the cushion mass attenuates. This area becomes converted into the fibroadipose tissue that, in the postnatal heart, separates the aortic and pulmonary roots. A similar process takes place in the proximal part of the outflow tract ( Fig. 51.5 ).
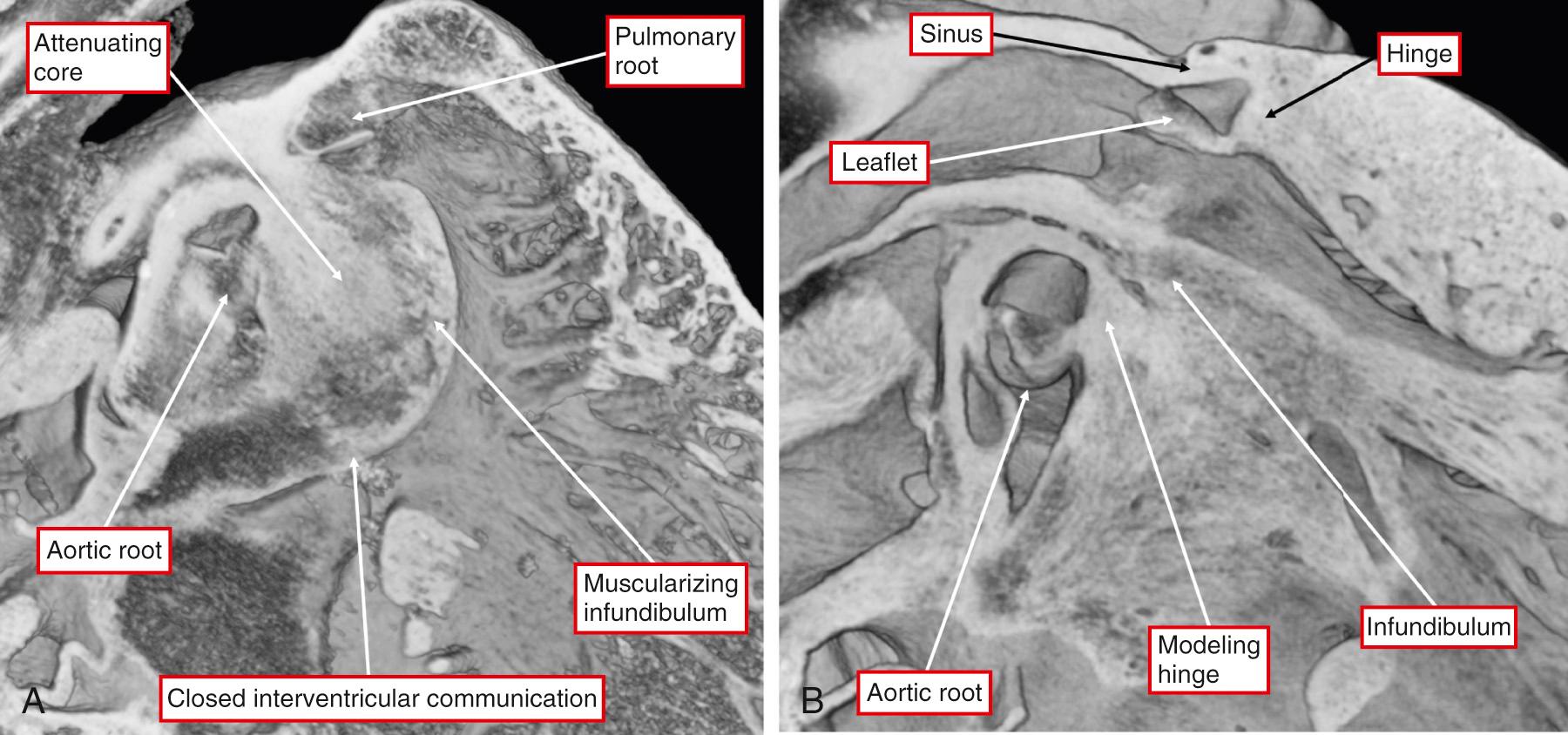
When the major cushions began their fusion, the outflow tract was supported exclusively by the developing right ventricle. As the fusion continues, the caudal part of the proximal outflow tract is transferred across the crest of the muscular ventricular septum to arise from the developing left ventricle. This brings the fused cushions themselves into line with the ventricular septal crest. At the same time, the surface of the fused cushions begins to muscularize, while the core of the cushions mass begins to attenuate (see Fig. 51.5A ). By the beginning of embryonic day 14.5 in the mouse and by Carnegie stage 18 in humans, the persisting part of the embryonic interventricular communication has been closed by growth of the so-called tubercles derived from the ventricular surfaces of the major atrioventricular cushions (see Fig. 51.5A ). During these stages the distal parts of the cushions remodel to form the arterial valvar leaflets, with myocardium derived from the proximal outflow tract incorporated into the basal parts of the developing left ventricular myocardial cone. Abnormal development of the cushion mass and its myocardial support gives the potential for abnormal communications around the hinges of the developing aortic valvar leaflets (see Fig. 51.5B ). Such communications bypassing the valvar leaflets are the essence of the various forms of aortoventricular tunnel. It is likely that abnormal development of the junction between the myocardial and nonmyocardial components of the intermediate part of the outflow tract also sets the scene for eventual aneurysmal formation of the valvar sinuses. It is unlikely to be a coincidence that such aneurysmal formation is associated both with formation of the tunnels and with doubly committed and juxtaarterial ventricular septal defects (VSDs), the latter defects formed consequent to failure of muscularization of the proximal outflow cushions.
It is currently much harder to provide a rational explanation as to why there should be formation of three arterial roots or why the right and left PAs should have separate ventricular outlets. It should be remembered, nonetheless, that it is a normal finding in reptiles and crocodilians to find three arterial trunks arising from the heart. Further comparisons between development in mammals and these other phyla may well cast further light on these problems.
AP windows provide communications between the cavities of the intrapericardial arterial trunks but in the presence of separate aortic and pulmonary roots ( Fig. 51.6 ). They can be found with atresia of one or other arterial valve, then providing access to the otherwise blind-ending circulation. The presence of separate aortic and pulmonary roots serves to distinguish the windows from common arterial trunk, as it does from solitary aortic trunk. The latter entity is found when there is complete absence of the intrapericardial PAs. The separate nature of the walls of the intrapericardial arterial trunks means that it is incorrect to describe the lesions as “aortopulmonary septal defects.” As we have shown, the lesions are due to failure of closure of the embryonic AP foramen. Small windows can be found adjacent to the sinutubular junctions ( Fig. 51.7A ).
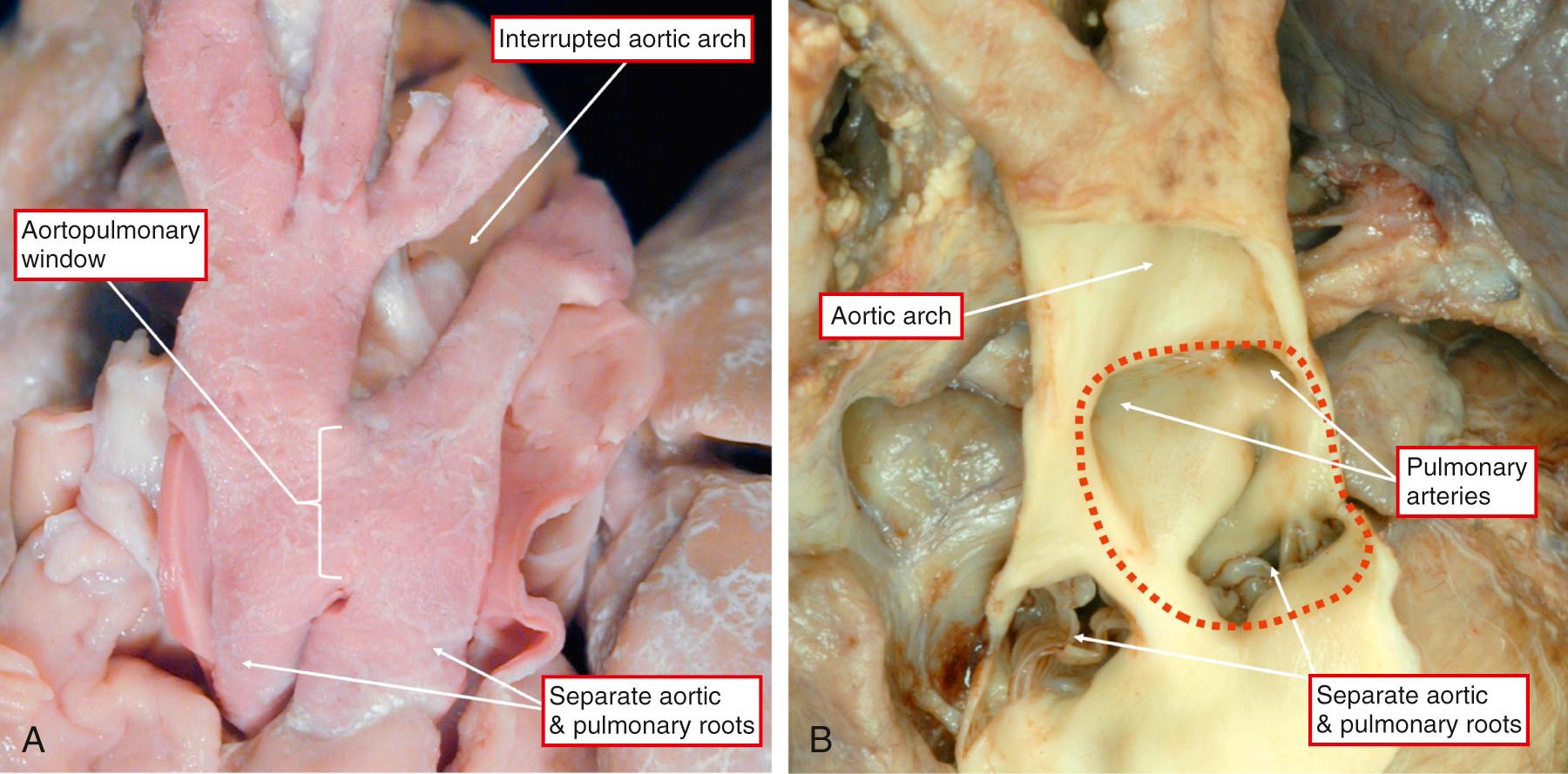
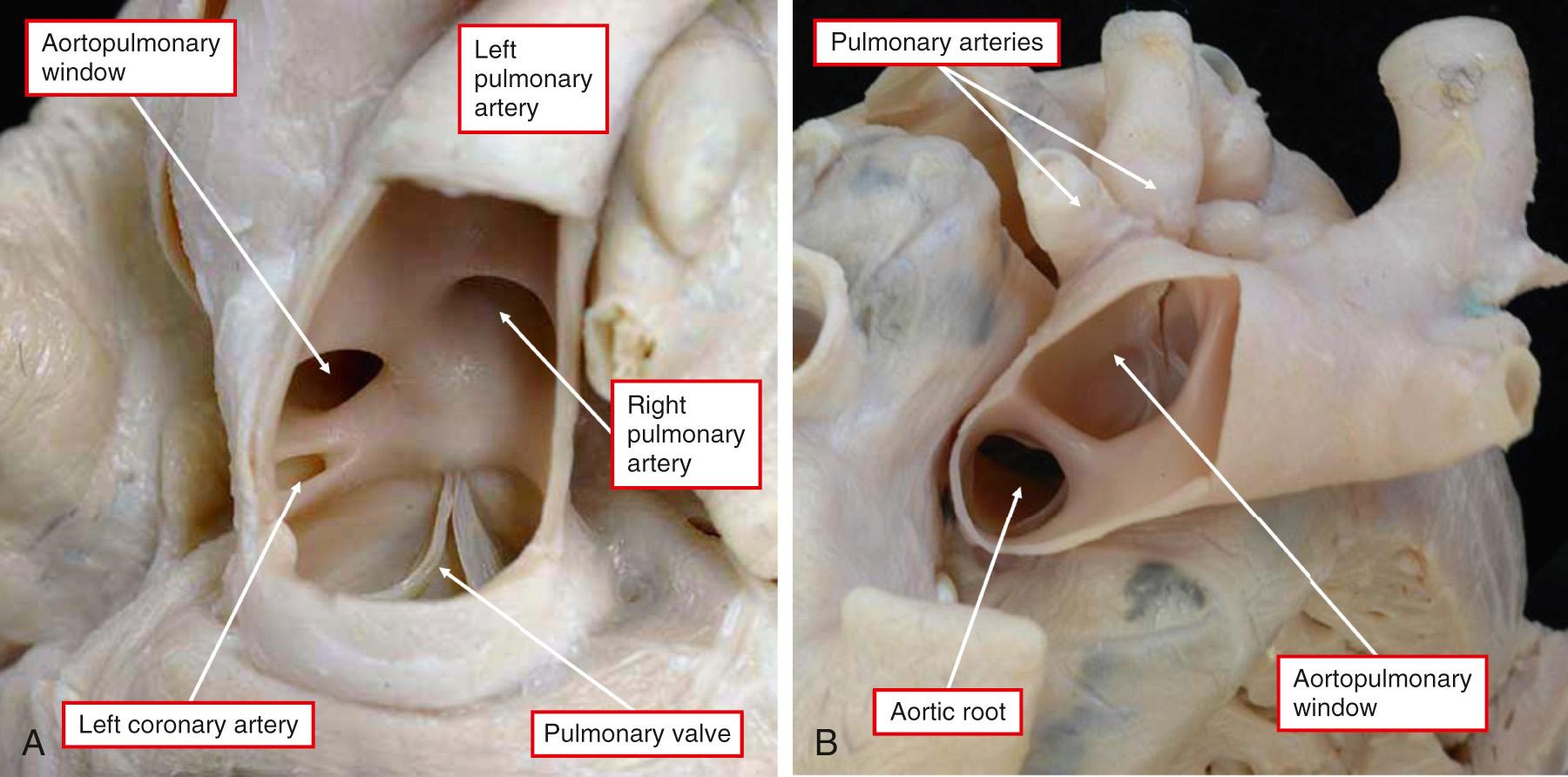
When larger, the defects extend more distally toward the margins of the pericardial cavity. The largest windows can occupy most of the adjacent area between the intrapericardial arterial trunks (see Figs. 51.6B and 51.7B ). When the deficient area reaches to the margins of the pericardial cavity, it is frequent to find aortic origin of the right PA, oftentimes also in association with interruption of the aortic arch (see Fig. 51.6 ). Association with origin of the left coronary artery from the pulmonary trunk is also frequent (see Fig. 51.7A ).
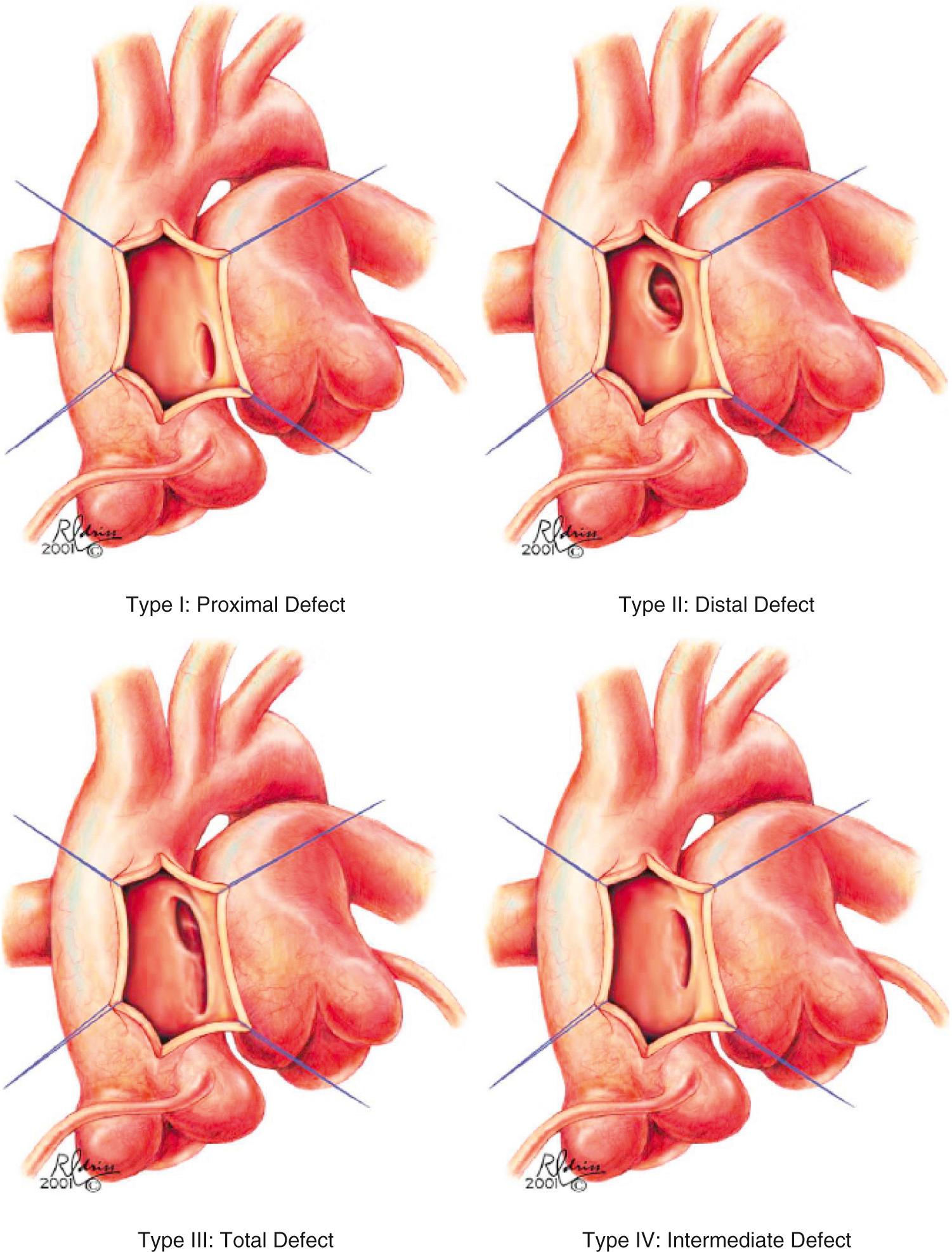
The prevalence of AP window in patients with congenital heart disease is 0.1% to 0.2%. AP window can occur as an isolated lesion, or it can be associated with other cardiac abnormalities in 30% to 50% of cases, the most common of which are arch abnormalities, specifically interrupted aortic arch (commonly type A) and coarctation of the aorta, and rarely VSD, tetralogy of Fallot and transposition of great arteries. Mori and colleagues classified AP window into three types: proximal (type I), distal (type II), and total (type III). This classification was recently modified with the addition of an intermediate category (type IV) ( Fig. 51.8 ). Interrupted aortic arch in the setting of AP window is not usually associated with DiGeorge syndrome, suggesting a distinct malformation unrelated to “conotruncal” abnormalities. Abnormal origin of the coronary arteries is common, with the coronary arteries arising frequently from the edge of the defect. The size of the communication in an AP window is variable, but they are generally large, unrestrictive, and hemodynamically significant. In rare cases the AP window can be small and pressure restrictive. Iatrogenic/traumatic AP window has been reported following balloon angioplasty of the PAs, after PA stent placement for supravalvar pulmonary stenosis in post–arterial switch patients.
The presentation usually is in the neonatal period or early infancy, similar to that of patients with large left-to-right shunts such as large arterial ducts or VSDs. Clinical signs and symptoms appear in the first few weeks or months of life and include tachypnea, sweating with feedings, poor feeding, failure to gain weight, and increased respiratory infections secondary to pulmonary overcirculation as the pulmonary vascular resistance falls. Cyanosis is unusual in early infancy and suggests elevated pulmonary vascular resistance with bidirectional shunting. Physical examination demonstrates tachypnea, bounding pulses, and a systolic murmur heard along the left sternal border. A mitral rumble is common in the setting of a large left-to-right shunt. When an AP window is associated with interrupted aortic arch, neonatal presentation with weak femoral pulses and shock can occur due to ductal constriction. Pulmonary hypertension is a frequent late complication in uncorrected large AP window, in which there is reversal of flow from the PA to the aorta with features of Eisenmenger syndrome, including cyanosis and clubbing.
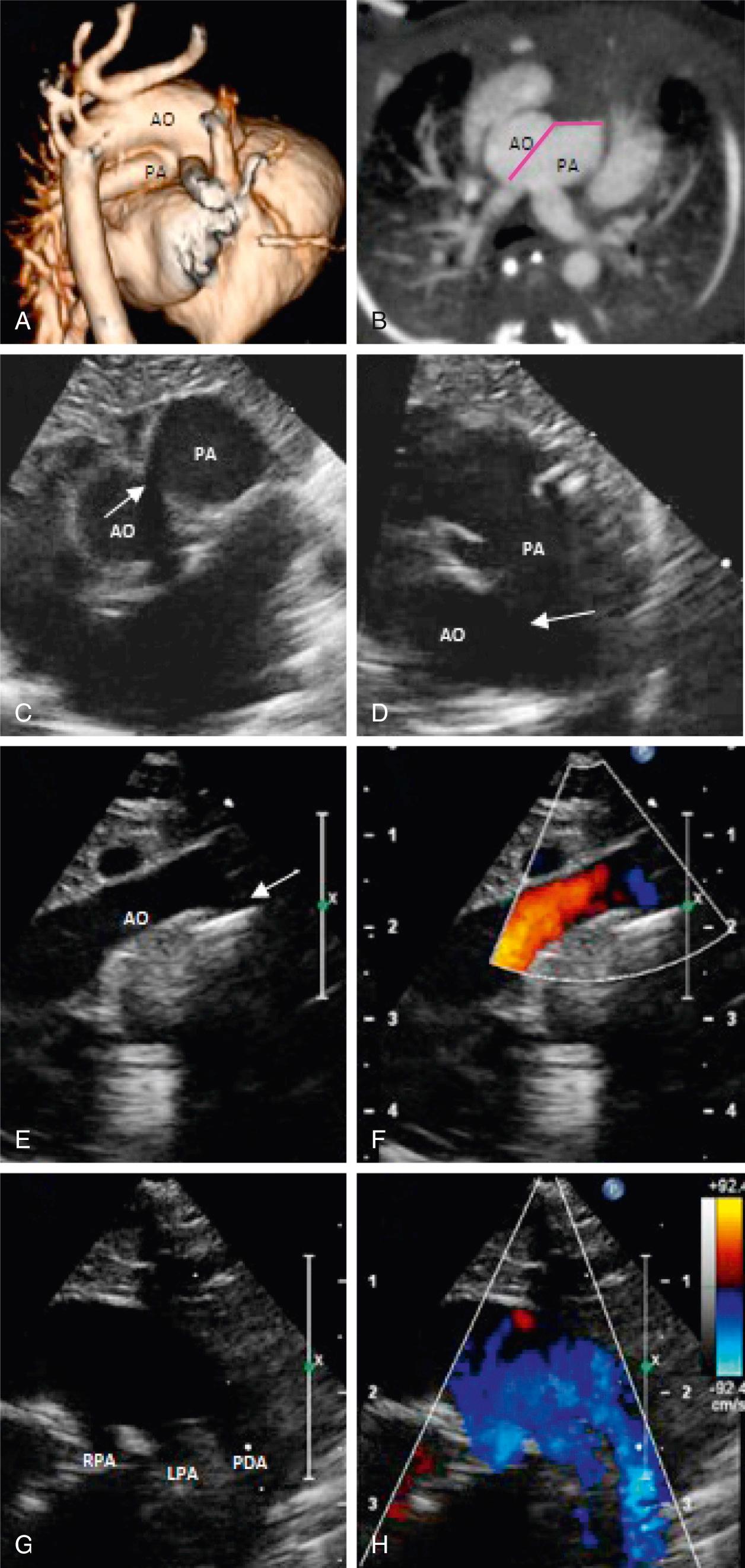
Antenatal diagnosis is uncommon because there may be minimal identifiable flow across the defect due to equal pressures in the ascending aorta and PA in the fetal life. Still, in the current era, several cases of prenatal diagnosis of AP window have been reported. Recent reports have demonstrated increased accuracy of prenatal diagnosis. The AP window may be best visualized in the three-vessel view located below the bifurcation of the right PA and the high short-axis view of the great arteries (doughnut view) with cephalad angulation. After birth, the electrocardiogram may show features of right and left ventricular hypertrophy and tachycardia, and the chest x-ray can show cardiomegaly and increased pulmonary vascular markings in patients with pulmonary overcirculation. The diagnosis is easily confirmed with two-dimensional echocardiography; identifying two separate semilunar valves in parasternal long and short-axis views excludes common arterial trunk. The AP window is best viewed in high parasternal short-axis view with the transducer tilted superiorly ( Fig. 51.9 ). Complete evaluation to rule out additional anomalies (e.g., presence of an arterial duct in association with interrupted aortic arch, or tetralogy of Fallot) is essential. Color Doppler can be helpful in confirming flow through the AP window. Cardiac catheterization is currently rarely required for the diagnosis of an AP window in infancy. Catheterization is reserved for patients who present in later infancy and childhood with pulmonary vascular disease, in which case pulmonary vasodilator testing is performed to determine reactivity of the pulmonary vascular bed. Aortography in the anteroposterior and lateral projections can usually demonstrate the window. It is essential to evaluate coronary arterial anatomy preoperatively and avoid missing anomalous origin of either coronary artery from the pulmonary trunk because this is associated with poor outcomes. Magnetic resonance imaging (MRI) or multislice computed tomography (CT) may be useful in this regard.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here