Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Metabolic liver diseases may manifest as acute, life-threatening illnesses in the neonatal period or as chronic liver disease in adolescence or adulthood, with progression to liver failure, cirrhosis, or HCC. In a 2015 report of the Scientific Registry of Transplant Recipients, 13% of all liver transplants in the USA were performed because of complications resulting from metabolic liver disease. When the pediatric population alone was analyzed, approximately 22% of the liver transplants performed in children from 2011 to 2013 were for complications of metabolic or genetic liver disease. Nontransplant treatment options have become available that may, in certain cases, obviate the need for LT.
The diverse presenting features of metabolic liver disease are listed in Box 77.1 . Certain metabolic liver diseases in young patients may mimic other illnesses, such as acute infections and intoxications. By contrast, the older patient with metabolic liver disease may present with symptoms and signs of chronic liver disease. Because metabolic diseases can resemble multiple other disorders, a high index of suspicion is required. Evaluation of any infant presenting with cholestasis should include a consideration of metabolic liver disease. Any patient with progressive neuromuscular disease, developmental delay, or regression of developmental milestones also requires evaluation. Metabolic liver disease should be an immediate consideration in patients of all ages with elevated serum aminotransferase levels and one or more of the presenting features in Box 77.1 .
| Symptoms |
|
| Signs |
|
| Other findings |
|
A detailed history can often raise the possibility of metabolic liver disease. A family history of consanguinity, multiple miscarriages, or early infant deaths may suggest a metabolic derangement. Close relatives with undiagnosed liver disease, progressive neurologic or muscle disease, or undiagnosed developmental delays should also raise suspicion. Introduction of certain foods may correlate with the onset of symptoms, as in patients with urea cycle defects (UCDs), galactosemia, or fructosemia. A history of specific dietary aversions may be revealing.
Recommended initial screening tests are listed in Box 77.2 . Because patients with metabolic liver disease often present with acute and recurrent symptoms and because the characteristic laboratory abnormalities for many of these illnesses may normalize between acute episodes, diagnostic studies should be obtained when the patient is experiencing symptoms. In enigmatic cases, serum and urine samples should be obtained during the acute illness and saved (frozen) for definitive studies, if available. A liver biopsy can be valuable. In addition to biopsy specimens for standard histology, a frozen specimen should be saved for biochemical assessment and a sample prepared for later electron microscopic study to look at the subcellular organelles, which may exhibit characteristic changes in some metabolic disorders. The increasing availability of molecular diagnostic testing allows genotypic evaluation for some diseases to complement phenotypic diagnosis.
∗ Specimens of serum and urine during acute episodes should be saved for later studies.
† Obtain if the patient is acidotic or has neurologic symptoms.
Deficiency of α 1 -antitrypsin (α 1 -AT) is transmitted in an autosomal recessive fashion and leads to an increased risk of lung and liver disease. This deficiency is one of the most common genetic diseases in the world and the second most common metabolic disease affecting the liver (after hereditary hemochromatosis [see Chapter 75 ]).
The prototypical member of the serpin family of protease inhibitors (Pis), α 1 -AT binds with and promotes the degradation of serine proteases in the serum and tissues. The most important serine protease is pulmonary neutrophil elastase, which is inhibited by α 1 -AT through formation of a tight 1:1 α 1 -AT-to-elastase complex. Therefore, the primary role of α 1 -AT is to prevent extracellular matrix degradation by neutrophil elastase in the lungs. The pathogenesis of α 1 -AT lung disease reflects a loss of function mechanism. In persons with the phenotype PiZZ, serum α 1 -AT levels are reduced to less than 15% of the normal serum concentration of 85 to 250 mg/dL. Consequently, there is relative uninhibited neutrophil elastase activity and resulting emphysema. The accumulation of small amounts of abnormal α 1 -AT Z polymers in the lungs has also been shown to promote inflammation and contribute to interstitial lung disease. ,
Allelic α 1 -AT mutant variants produce Pi gene products that can be distinguished from the normal product by electrophoretic methods; the normal allelic representation is designated PiM. The PiZ variant produces a mutant α 1 -AT Z protein that contains a single amino acid replacement of glutamine with a lysine residue due to a mutation at position 342 of the α 1 -AT (SERPINA1) gene. Homozygosity at the PiZ allele is the most common and classic pathologic form of α 1 -AT deficiency and is capable of leading to liver and lung disease. Approximately 125 naturally occurring variants of α 1 -AT have been described. Although most of these variants are either of no clinical significance or are extremely rare, some variants—PiS(Iiyama), PiM(Duarte), and PiM(Malton)—have been reported to be associated with liver injury and even cirrhosis. , Additional variants that have demonstrated liver abnormalities include the PiP(Brescia), PiM(Wurzburg), PiKing’s, and PiS alleles, often with compound heterozygosity. , ,
Under normal circumstances, α 1 -AT is produced in the rough endoplasmic reticulum (ER) of hepatocytes and is targeted to the secretory pathway via the Golgi apparatus. Structural misfolding and polymerization of the mutant α 1 -AT Z protein causes its aberrant retention in the hepatocyte ER with a resulting gain of function defect that leads to cirrhosis. Several mechanisms of liver injury in α 1 -AT deficiency have been proposed.
The failure of proteins to properly exit the ER results in many diseases. When misfolding occurs, several physiologic responses normally ensue. Often, unfolded or misfolded proteins are tagged for degradation via ER-associated degradation (ERAD), in which chaperones and associated factors recognize and target substrates for retrotranslocation to the cytoplasm, where they are degraded by the ubiquitin-proteasome machinery. If continued accumulation of incorrectly folded proteins occurs, a complementary ER quality control mechanism, the unfolded protein response, is usually triggered. , Autophagy is a third pathway that reduces ER stress from abnormal protein aggregation. If ERAD efficiency is compromised, autophagy-mediated destruction occurs whereby portions of the ER along with protein aggregates are engulfed in double-membrane structures called autophagosomes and delivered to the lysosome for degradation. Indeed, each of these pathways has been mechanistically linked to the development of α 1 -AT-associated liver disease. Studies have demonstrated impaired interaction between abnormal Z-type protein and its ERAD-associated molecular chaperone calnexin, thereby resulting in retention of polymers in the ER. , Additionally, ERAD defects confounded with other misfolding protein variants, such as the HFE variant H63D, have been reported to increase the risk of liver damage in α 1 -AT deficiency. Others have shown that PiZ polymers within the ER are not associated with normal unfolded protein response activation, , but rather with a secondary ER overload response pathway that results in the proinflammatory release of interleukin-6 and interleukin-8, which are thought to contribute to the development of liver injury in α 1 -AT deficiency. Autophagy is activated by the intracellular accumulation of PiZ, and the use of autophagy enhancer drugs has been shown to mitigate the accumulation and proteotoxicity of misfolded PiZ polymers in a Caenorhabditis elegans model of α 1 -AT deficiency.
Although the prevalence of the classic α 1 -AT deficiency allele, PiZ, is highest in populations derived from northern European ancestry, many racial subgroups are affected worldwide, and millions of persons have combinations of deficiency alleles (i.e., PiSS, PiSZ, or PiZZ). In the USA, the overall prevalence of deficiency allele combinations is approximately 1 in 4126 for PiZZ and 1 in 1018 for PiSZ. , Mounting evidence suggests that heterozygous α 1 -AT deficiency states in adults can contribute to the development of cirrhosis, chronic liver failure, and HCC. Furthermore, heterozygosity may exacerbate the chronic liver disease caused by obesity and alcohol abuse in adults, as well as cholestatic liver diseases in children. ,
In the most unbiased epidemiologic study of α 1 -AT deficiency to date, reported by Sveger, 200,000 Swedish infants were screened for α 1 -AT deficiency, 184 were found to have abnormal allelic forms of α 1 -AT (127 PiZZ, 2 PiZnull, 54 PiSZ, and 1 PiSnull), and 6 (5 PiZZ and 1 PiSZ) died in early childhood, but only 2 of cirrhosis. About 10% of newborns with α 1 -AT deficiency (PiZZ) present with cholestasis, and as many as 50% continue to have elevated serum aminotransferase levels at age 3 months; most are clinically asymptomatic. , Liver disease does not develop in patients with null α 1 -AT phenotypes, whereas early-onset emphysema develops in all of them. Nevertheless, investigations using the C. elegans model have demonstrated proteotoxic effects from null variants. The investigators concluded that because the mechanism of degradation of null variants is different from that of PiZ polymers, the mechanism of proteotoxicity is likely to be different as well.
Wide variation exists in the severity of liver disease among patients with the classical form of α 1 -AT (PiZZ) deficiency, and little is known about the factors that predispose affected persons to a severe, progressive decline in hepatic function. Even children in whom cirrhosis develops can have highly variable progression to end-stage liver disease (ESLD). Moreover, siblings with PiZZ have variable degrees of liver involvement; in a study reported by Hinds and colleagues, 5 of 7 children with PiZZ-associated α 1 -AT deficiency who required LT had siblings with PiZZ who lacked persistent liver involvement. Therefore, additional factors must be involved in determining the severity of liver disease in α 1 -AT deficiency. ER mannosidase I and sortilin have been identified as possible genetic modifier candidates that contribute to the development of liver disease in PiZZ homozygotes. ,
Of 150 patients with α 1 -AT deficiency from Sveger’s original study who subsequently underwent evaluation at age 16 and 18 years, none had physical examination findings of liver disease. Elevated serum aminotransferase or GGTP levels were found in fewer than 20% of patients with a PiZZ phenotype and in fewer than 15% of those with a PiSZ phenotype. By the third decade of life, analysis of this same cohort showed that 6% of PiZZ and 9% of PiSZ patients had a marginal increase in serum ALT levels. A separate analysis of 647 patients with a PiZZ phenotype found that 49% had slight increases in aminotransferase levels when a stricter cutoff for normal levels was used, suggesting small amounts of ongoing liver injury.
Although liver disease is often mild during infancy and childhood, patients with α 1 -AT deficiency have an increased risk of developing cirrhosis and HCC. A study of 57 PiZZ adults with lung disease revealed that 63% had findings of chronic liver disease by conventional liver biochemical testing and liver US. A further analysis of 3 large U.S. LT databases revealed that the vast majority, 77.2%, of liver transplants performed in patients with a diagnosis of α 1 -AT deficiency, occurred in adults. The authors concluded that α 1 -AT deficiency-associated liver disease is predominantly an age-dependent degenerative disease and that pediatric cases represent outliers with particularly powerful, as yet unidentified, modifying factors. Therefore, the diagnosis of α 1 -AT deficiency should be considered in any patient presenting with noninfectious chronic hepatitis, hepatosplenomegaly, cirrhosis, portal hypertension, or HCC.
Histopathologic features of α 1 -AT deficiency change as the patient ages. In infancy, liver biopsy specimens may show bile duct paucity, bile duct proliferation, intracellular cholestasis with or without giant cell transformation, mild inflammatory changes, or steatosis, with few of the characteristic periodic acid–Schiff–positive, diastase-resistant globules. These inclusions, which result from polymerized α 1 -AT Z protein, are most prominent in periportal hepatocytes and may also be seen in Kupffer cells. Immunohistochemistry with monoclonal antibody to α 1 -AT Z can also be performed to verify the diagnosis. As the patient ages, these changes may resolve completely or progress to chronic hepatitis or cirrhosis.
α 1 -AT is considered a hepatic acute-phase reactant, and its release may be stimulated by stress, injury, pregnancy, or neoplasia. Because these factors can influence α 1 -AT production, even in patients with the PiZZ phenotype, the diagnosis of α 1 -AT deficiency should be based on phenotype analysis and not solely on the serum α 1 -AT level. A liver biopsy specimen, although not universally recommended, can confirm the diagnosis ( Fig. 77.1 ). Commercial tests are available to detect the most common mutant alleles by PCR analysis of genomic DNA. In addition, molecular genetic testing, sequence analysis, and deletion/duplication analysis can be performed quickly to identify common and rare disease variants.
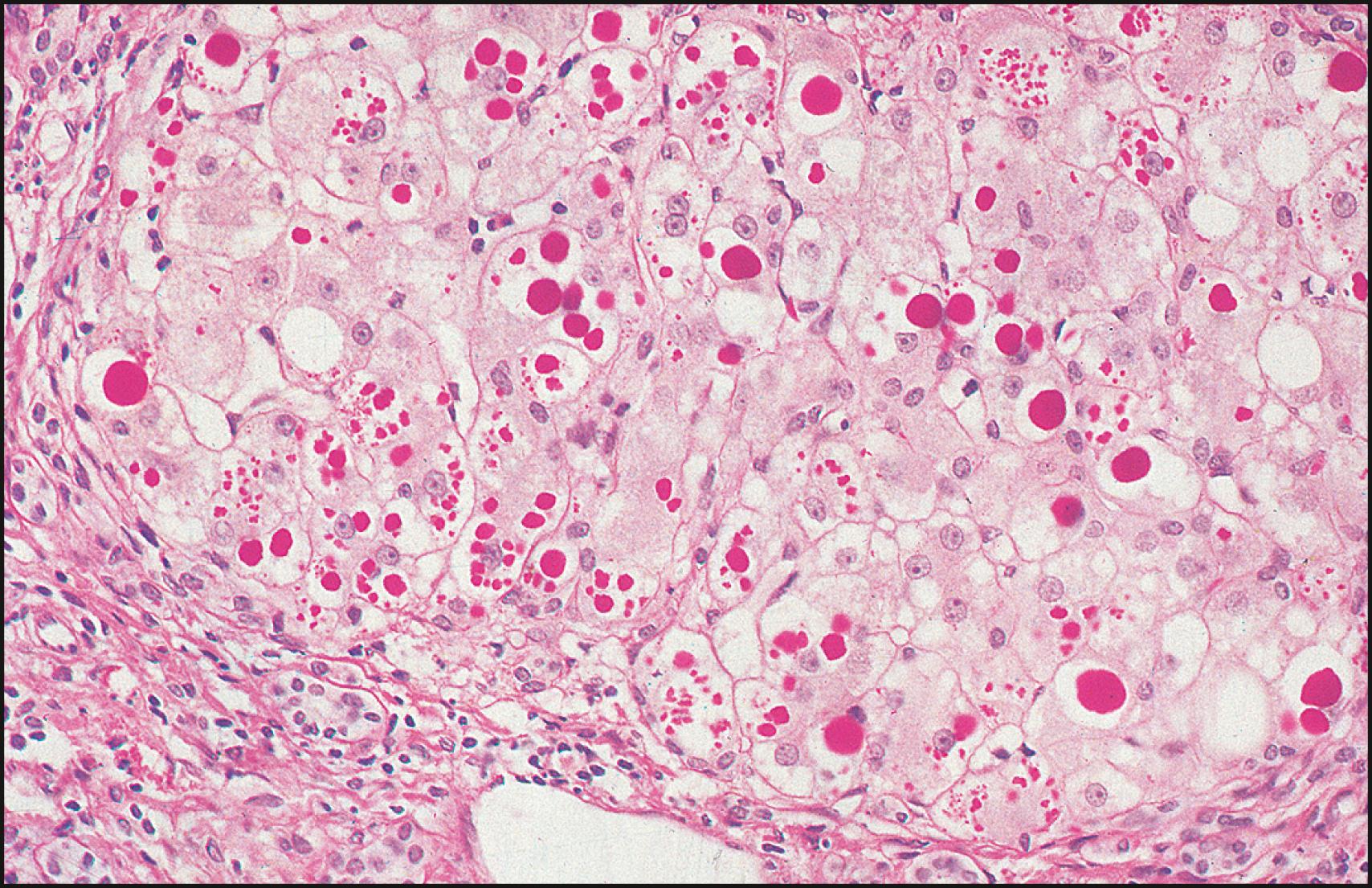
Generalized recommendations to enhance the detection α 1 -AT deficiency are available , ; however, targeted approaches have achieved a much higher rate of detection. Adults with chronic lung disease and siblings of affected patients with lung or liver disease should be targeted for screening, and appropriate education and genetic counseling should be offered to patients with α 1 -AT deficiency identified by screening.
No current disease-specific therapies are available for the hepatic manifestations of α 1 -AT deficiency, and the initial treatment remains symptomatic care. The importance of providing fat-soluble vitamin supplementation, when indicated, adequate nutrition, and counseling to avoid obesity, alcohol, smoking, and second-hand smoke cannot be overemphasized. As the pathophysiology is increasingly elucidated, more therapeutic targets are being identified.
Understanding the role of autophagy and other mechanisms involved in the clearance of misfolded proteins has led to investigations to assess the role of autophagy-enhancer medications (e.g., carbamazepine, rapamycin) , and the use of viral vectors that can deliver transcription factors that lead to increased autophagic activity. These approaches have demonstrated the ability to reduce intracellular inclusions and fibrosis in animals, and future human-based studies are warranted. Alternatively, targeting pathways to increase the secretion of the misfolded protein products may also prove beneficial. Suberoylanilide hydroxamic acid has been shown to promote secretion of the Z protein from epithelial cell lines.
The use of RNA interference molecules has demonstrated the ability to target the mRNA that encodes Z variant and prevent its production. This approach has been shown to decrease circulating levels of α 1 -AT in both mice and non-human primates, , with reduction, reversal, and prevention of liver disease in mice. Monoclonal antibody technology has been shown to enable small molecule intervention that potentially can both block the formation of aberrant polymers (thereby decreasing the accumulation of intracellular misfolded proteins) and increase the secretion of Z protein that retains inhibitory activity against neutrophil elastase.
Collectively, these approaches have the potential to decrease hepatocellular accumulation of the misfolded α 1 -AT variants and ameliorate the injurious stimuli. Additional technologies including the use of stem cell technology, likely in combination with gene editing techniques such as CRISPR (clustered regularly interspaced short palindromic repeats), will surely play a role in future efforts to better understand and treat α 1 -AT deficiency.
α 1 -AT deficiency remains the most common genetic liver disease for which LT is performed. Severe progressive liver disease due to α 1 -AT deficiency is most common in adult males, thereby suggesting that the mechanisms of liver disease injury are similar to those for other age-dependent degenerative diseases, with severe pediatric disease suggested to be influenced by powerful modifiers. LT not only replaces the injured organ, but also corrects the metabolic defect, thereby preventing further progression of systemic disease. LT has proved to be a highly effective intervention for those with the most severe phenotypes, with 5-year survival rates ranging between 83% and 90% for children and adults. , Although data are lacking on the risk of HCC in patients with liver disease due to α 1 -AT deficiency, a prudent approach is to follow the AASLD guidelines for surveillance: liver US every 6 months in patients with cirrhosis, with or without an accompanying serum AFP determination.
Replacement therapy with purified α 1 -AT is the only treatment option approved by the FDA for lung disease associated with α 1 -AT deficiency. Patients who receive replacement therapy have a slower rate of decline in lung tissue and function than untreated patients. This therapy does not benefit α 1 -AT deficiency–associated liver disease.
More than 12 distinct inborn disorders of glycogen metabolism have been described, but only 3 are associated with serious liver disease: glycogen storage disease (GSD) types I, III, and IV. Other GSDs may cause hepatomegaly or liver histologic changes but generally do not cause clinically important liver disease. The overall incidence of GSD types I, III, and IV is estimated to range from 1 in 50,000 to 1 in 100,000 in the general population.
Glycogen metabolism occurs in many tissues, but the areas of clinical importance are the muscle, liver, and polymorphonuclear neutrophils. The body uses glycogen to store glucose and as a ready reserve when systemic glucose is required (see Chapter 72 ). Glycogen is composed of long-chain glucose molecules arranged in a linear 1,4 linkage. Eight to 10% of the glucose molecules are attached in a 1,6 linkage to form branching chains, which permit efficient storage of glucose while minimizing the impact on intracellular osmolality. The substrates for glycogen synthesis, glucose-6-phosphate (Glu-6-P) and glucose-1-phosphate, are derived from several pathways, including fructose and galactose metabolic cycles, as well as gluconeogenesis and glycogenolysis ( Fig. 77.2 ).
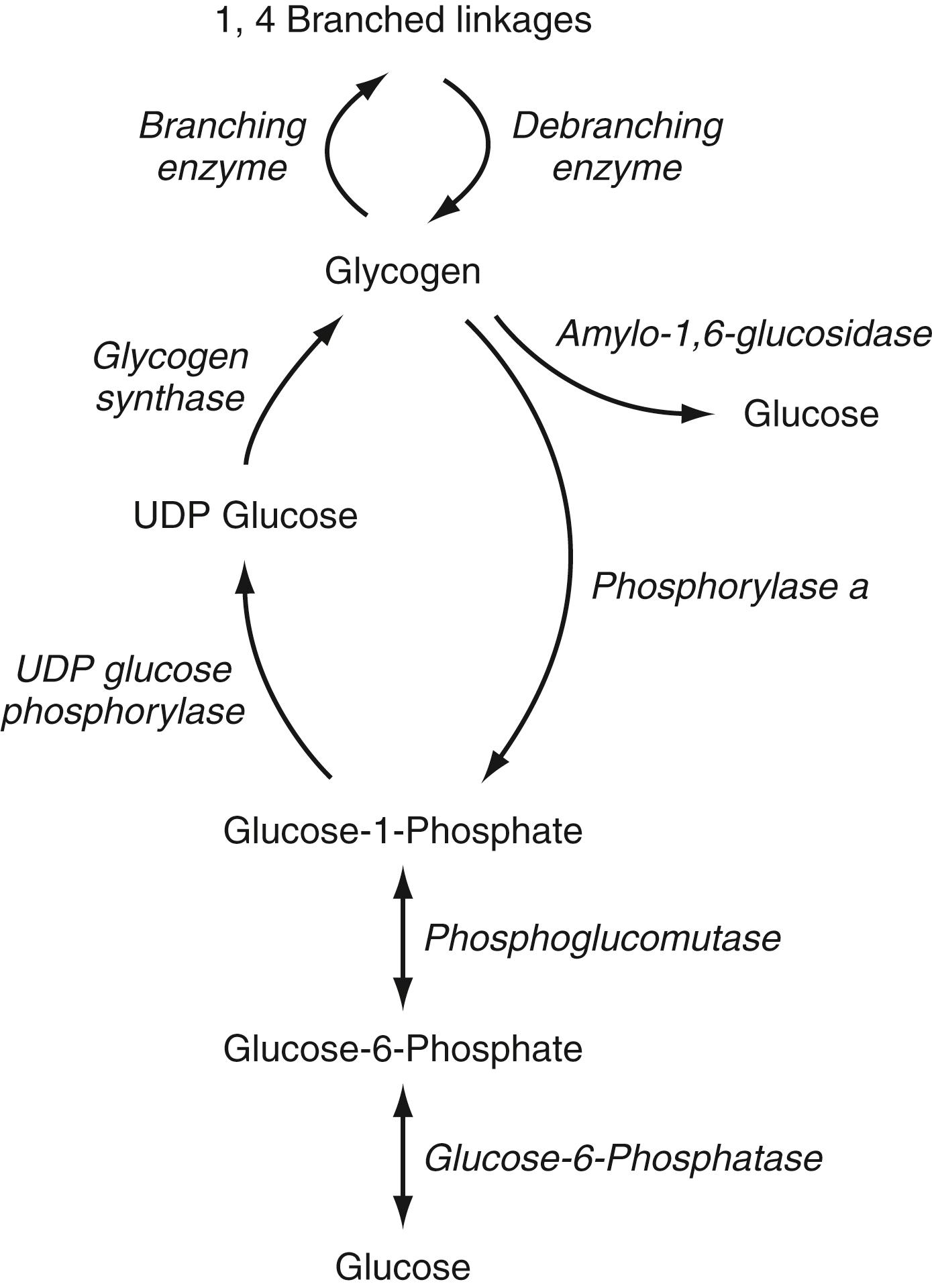
GSD type I, the most common inborn error of glycogen metabolism, results from deficiency of a 2-component enzyme system involved in the transport of Glu-6-P from the cytosol into the ER by Glu-6-P translocase (encoded by the ubiquitously expressed SLC37A4 gene, GSD type Ib) and subsequent cleavage of Glu-6-P by glucose-6-phosphatase (Glu-6-Pase, encoded by the G6PC1 gene, GSD type Ia), located on the luminal side of the ER. Clinical and molecular genetic observations have disclosed 2 subtypes of GSD type I, Ia, and Ib, that account for virtually all cases. The clinical phenotype with respect to liver disease is similar in the 2 forms; however, patients with GSD type Ib often have intermittent severe neutropenia and polymorphonuclear leukocyte (neutrophil) dysfunction, making them prone to recurrent episodes of severe bacterial infections and Crohn-like intestinal disease. Loss of function of an isoform of Glu-6-Pase encoded by the G6PC3 gene, although also ubiquitously expressed, leads to severe congenital neutropenia type 4, an autosomal recessive condition.
Disruption of the function of Glu-6-Pase (type Ia) or Glu-6-P translocase (type Ib) inhibits the use of glucose in gluconeogenesis, glycogenolysis, and the metabolism of fructose or galactose. This inability to release stored glucose leads to hypoglycemia within 90 to 180 minutes of the last orally ingested glucose. Lactate and fatty acid metabolism and glycolytic pathways are then used as sources of energy.
Most patients with GSD type I present in infancy with symptoms of metabolic derangement, such as lethargy, seizures, or coma as a result of profound hypoglycemia or metabolic acidosis, a protruding abdomen caused by hepatomegaly, muscular hypotonia, and delayed psychomotor development.
Physical signs invariably include hepatomegaly, usually with a normal-sized spleen. Patients in whom the disease is poorly controlled for a long time exhibit short stature and growth failure and may be prone to adiposity. Delayed bone age and reduced postpubertal bone mineral density are common. Xanthomas can appear after puberty and localize to the elbows, knees, buttocks, or nasal septum, the last leading to epistaxis. Patients with GSD type I are susceptible to a wide spectrum of neurologic injury that may result in epilepsy, hearing loss, and abnormal neuroimaging findings, likely a result of recurrent episodes of hypoglycemia.
Other metabolic and extrahepatic comorbidities can be seen. Lactic acid levels can reach 4 to 8 times normal; the accompanying metabolic acidosis may manifest as muscle weakness, hyperventilation, malaise, headache, or recurrent fever. Hyperuricemia is common and may lead to gout, arthritis, or progressive nephropathy. Nephromegaly secondary to increased glycogen deposition is common, and with advancing age, progressive renal disease, hypertension, and renal failure requiring dialysis and transplantation may develop. Because of hypoglycemia, patients have chronically high serum levels of glucagon with depressed levels of insulin. Hypertriglyceridemia and hypercholesterolemia are present in both GSD Ia and GSD Ib (but more prominently in GSD Ia) and may account for the greater frequency of xanthoma formation. Hypercalcemia during acute metabolic decompensation has been observed. Bleeding dysfunction, manifest as recurrent epistaxis, easy bruising, oozing after dental surgeries, and menorrhagia, can be seen secondary to impaired platelet function or acquired von Willebrand–like disease. ,
Patients with GSD type Ib often also have severe intermittent neutropenia and neutrophil dysfunction as well as high platelet counts. Crohn-like IBD often occurs in patients with GSD type Ib at the time of severe neutropenia, and patients are prone to severe bacterial infections, with abscess formation throughout the body. No correlation has been found between the severity of bacterial infections or degree of neutropenia and the molecular defect in Glu-6-P transport activity in humans with GSD type Ib, suggesting that other as yet unknown factors, such as modifying genes, define the ultimate disease phenotype.
Hepatomegaly in GSD type I results from increased glycogen storage in the liver, as well as a large degree of fatty infiltration; the latter likely develops because of a wide array of perturbations in lipid metabolism, including increased free fatty acid flux into the liver. Patients demonstrate mild elevations in serum aminotransferase levels but generally do not develop cirrhosis or liver failure.
Hepatocellular adenomas develop in 22% to 75% of patients, as early as 3 years of age but most commonly in the second decade of life, and tend to increase in both size and number as the patient ages (see Chapter 96 ). In hepatocellular adenomas in GSD type I, β-catenin mutations have been reported in 28%, but differ from sporadic adenomas in that hepatic nuclear factor 1α inactivation has not been observed; inflammatory-type adenomas may also occur (see Chapter 96 ). In rare instances, adenomas can transform to HCC; unfortunately, serum AFP and carcinoembryonic antigen levels, as well as features of the lesions on hepatic imaging, are not predictive of malignant transformation. Whether poor metabolic control increases the risk of hepatocellular adenoma formation in patients with GSD type Ia is controversial. In some patients, hepatocellular adenomas have been demonstrated to regress and disappear after adequate nutritional therapy, but in general, the course is unpredictable, especially in nonadherent patients. Because imaging and serum marker levels are unreliable in predicting malignant transformation in this patient population, it is uncertain whether resection of an adenoma or LT is preferable.
In suspected cases of GSD type I, mutation analysis is the first choice for diagnosis. Complete G6PC sequencing is usually performed first; unless neutropenia is present, in which case SLC37A4 sequencing should be pursued. Although liver biopsy is no longer necessary when GSD type 1 is suspected, Glu-6-Pase activity can be analyzed on snap-frozen liver tissue. Deficient enzyme activity confirms the diagnosis of GSD type Ia. Targeted mutation analysis is useful for prenatal diagnosis and carrier testing for patients with a known family mutation and may be useful when knowledge of common mutations for specific ethnic groups is available. Multigene panels that include G6PC , SLC37A4 , and other genes of interest are available.
GSD type I is a multisystem disorder best managed by a team of health care professionals with experience in managing the multiple aspects of the disease. Management centers on preventing the acute metabolic derangements and potential long-term complications and enabling the patient to attain normal psychological development and a good quality of life. In general, a primary physician with expertise in metabolic disorders should coordinate the patient’s care with other specialists, including a metabolic dietician, nephrologist, geneticist, endocrinologist, hepatologist, genetic counselor, neurologist, and cardiologist, depending on the clinical manifestations.
Consensus guidelines for the management of GSD type I have been proposed. Maintaining blood glucose levels at 70 mg/dL or greater is important to achieve good metabolic control. Levels should be kept consistent to avoid hypoglycemia and fluctuations in the blood glucose levels. In infants and children, the avoidance of fasting for more than 3 to 4 hours is critical, because hypoglycemia and its accompanying complications often can develop. In older children and adolescents, fasting up to 5 to 6 hours may be safe. Regular blood glucose monitoring is critical to establish an optimal regimen. Offering smaller, more frequent meals and avoiding sucrose, fructose, and galactose are generally recommended. Access via nasogastrostomy or a surgically placed gastrostomy tube is recommended for emergencies and overnight gastric feeds. Raw, uncooked cornstarch is often introduced between 6 and 12 months of age for management. General guidelines for dosing are 1.6 g of cornstarch per kilogram of body weight every 3 to 4 hours for young children and 1.7 to 2.5 g of cornstarch per kilogram every 4 to 5 hours for older children, adolescents, and adults. Some adults may require only one dose of cornstarch at bedtime to maintain their glucose levels. Overnight administration of modified cornstarch has demonstrated the ability to prevent hypoglycemia more effectively than uncooked cornstarch. Because optimal glycemic control is not always possible and the risk of severe hypoglycemia is high if delivery of glucose is interrupted inadvertently, serum lactate levels should be kept at the high end of normal, because lactate is an alternative fuel for the brain. Multivitamins, calcium, and vitamin D supplementation are necessary because of the restricted nature of the diet. Importantly, both undertreatment (resulting in hypoglycemia) and overtreatment (resulting in insulin resistance) can be harmful.
Patients should undergo regular monitoring for the development of liver adenomas, especially after the onset of puberty. Although good metabolic control may lead to regression of the adenomas, there remains the risk of transformation to HCC, particularly when the size or number of lesions increase rapidly or the vascularity increases. An abdominal US should be performed at baseline and then every 12 to 24 months. Additional imaging modalities such as CT, MRI, or contrast-enhanced US can be considered to characterize suspicious lesions. Percutaneous ethanol injection, radiofrequency ablation, and partial liver resection are treatment options for liver adenomas (see Chapter 96 ). Monitoring for the development of renal comorbidities in patients with GSD type I should include US assessment to determine kidney size and growth, as well as the presence of nephrolithiasis or nephrocalcinosis. Urinalysis, urine electrolyte determination, and glomerular filtration rate (GFR) determinations should be assessed regularly. Angiotensin-converting enzyme inhibitors can be used in the setting of hyperfiltration (sustained estimated GFR >140 mL/min/1.73 m 2 ) and when either microalbuminemia or proteinuria occur.
The presence of anemia should prompt investigation for nutritional causes, adenomas, enterocolitis, and occult blood loss. Severe anemia should trigger an evaluation for adenomas in GSD type Ia and enterocolitis in GSD type Ib. Neutropenic patients with GSD type Ib should be treated with granulocyte colony-stimulating factor, particularly if there is associated fever, infections, or enterocolitis.
Adenoviral-mediated gene replacement therapy of recombinant Glu-6-Pase in both murine and canine models of GSD type Ia deficiency has led to encouraging results and may be an option in humans in the future ; however, the adenoviral vector genomes have been shown to be gradually lost over the weeks following vector administration. Hepatocyte transplantation has been performed successfully in patients with GSD type I, but long-term outcomes are lacking. , LT has corrected the metabolic error in patients with GSD type I and permitted normalization of fasting tolerance and catch-up growth; however, other extrahepatic comorbidities such as renal disease and neutropenia persist and progress.
GSD type III results from mutations in the AGL gene with resulting deficiency in the glycogen-debranching enzyme (GDE) and leads to the accumulation of limit dextrin units, which restrict subsequent glucose release by phosphorylase. Because deficiency of GDE does not interfere with metabolism of Glu-6-P, patients with GSD type III retain effective mechanisms for gluconeogenesis. Therefore, the clinical course is milder than in patients with GSD type I, and patients can fast for longer periods; most survive into adulthood. In infancy, however, GSD type III may be indistinguishable from GSD type I.
GDE possesses 2 independent catalytic activities, an amylo-1,6-glucosidase and oligo-1,4→1,4 glucan transferase. Both activities are deficient in the 2 main clinical subtypes of GSD type III, types IIIa and IIIb. Differential expression of 4 major GDE mRNA isoforms in liver and muscle tissue distinguishes the 2 types: type IIIa affects liver and muscle and accounts for 80% of patients, and type IIIb affects the liver only and accounts for 15% of patients. Rare isolated loss of 1 of the 2 GDE activities has been observed (i.e., glucosidase activity in type IIIc and transferase activity in type IIId). ,
Persons with GSD type III typically exhibit hypoglycemia, hepatomegaly, and growth failure. Liver enlargement results from increased glycogen deposition, not fatty infiltration. The liver may show fibrotic septa that rarely lead to frank cirrhosis and ESLD. Serum lactate and uric acid levels are normal, and aminotransferase levels are increased only moderately until advanced liver disease occurs. Hyperlipidemia may be present but is not as pronounced as in GSD type I. Patients have normal responses to fructose and galactose loading.
Patients with GSD type III may also display progressive skeletal, cardiac, and bulbar muscle weakness, which worsens with activity, and muscle wasting. , Nephromegaly is not seen, but ventricular hypertrophy or cardiac arrhythmias may occur; frequent cardiac evaluation and monitoring are recommended. Hepatomegaly and ketotic hypoglycemia accompanied by elevations in serum aminotransferase and creatinine kinase (representing muscle injury) levels are suggestive of GSD type III. Identifying pathogenic AGL variants confirms the diagnosis with both targeted single gene testing and multigene panels available. If genetic testing does not confirm the diagnosis, direct enzyme analysis of peripheral leukocytes, muscle, or liver tissue can be performed. Comorbidities in GSD type III are common, including cardiac complications in 58%, neuromuscular complications in 34%, and hepatic complications in 11% of patients.
A high-protein, low-carbohydrate diet has been suggested to normalize metabolic activity, ensure normal growth, restore muscle function, and minimize hepatomegaly. This diet provides adequate substrates for gluconeogenesis while reducing the need for glycogen storage. Unlike GSD type I, patients with GSD type III need not avoid fructose and galactose, because these sugars can be used for energy production. Patients with refractory hypoglycemia or persistent hepatomegaly may require a nighttime continuous infusion or cornstarch, as is used for GSD type I. LT has been successful but is usually not necessary for patients with GSD type III, even in those with evidence of cirrhosis, if liver synthetic function remains well preserved. Following transplantation, extrahepatic complications such as heart and muscle dysfunction persist. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here