Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Osteosarcoma is the most common primary bone tumor. There are approximately 600 cases/year in the United States. The term osteosarcoma was first used in the early 1800s by Alexis Boyer, the imperial family surgeon for Napoleon. Although low-grade forms of osteosarcoma do exist, more than 90% of osteosarcomas are high-grade malignant lesions. Low-grade osteosarcomas will be discussed, but most of what follows pertains to high-grade osteosarcomas.
Osteosarcoma usually occurs in adolescents and young adults. Presenting symptoms can include tumor-related pain with or without a mass, a painless mass, and a pathologic fracture. The distal femur is the most frequent site of primary disease, followed by the proximal tibia and then the proximal femur, hip, and proximal humerus. As early as 1818 it was recognized that untreated patients with osteosarcoma often developed metastatic disease. The most common site of metastasis is the lungs, followed by bone. Metastatic disease is present in approximately 15% of patients at the time of diagnosis.
Prior to the 1970s, when chemotherapy was first used to treat osteosarcoma, overall survival (OS) was approximately 20%. With modern chemotherapy and surgical control, the 10-year OS of patients with localized osteosarcoma is around 70%. The OS is much worse for patients with metastases at diagnosis and for those with relapsed disease. In both groups, the 5-year OS rate is approximately 25%. The most active chemotherapy agents in osteosarcoma are doxorubicin (Doxo), cisplatin (CP), and high-dose methotrexate (HDMTX). Ifosfamide and etoposide are also active against osteosarcoma and are typically used in addition to Doxo, CP, and HDMTX in patients with metastatic disease and in patients with recurrence.
In the past three decades, a number of discoveries have led to a better understanding of osteosarcoma biology. For example, the tumor suppressor gene TP53 and the retinoblastoma gene RB1 are altered in osteosarcoma. Oncogenes, including MYC, MET, and FOS, have also been implicated in osteosarcomagenesis. These tumor suppressors, oncogenes, and other biologic factors will be discussed in detail. Unlike most childhood cancers, there has been minimal improvement in OS over the past three decades. Consequently, one of the research priorities in osteosarcoma is the identification of additional active therapies.
Osteosarcoma is the most common primary bone tumor in children. The average annual incidence of osteosarcoma in children younger than 20 years in the United States is 4.8/million. Approximately 3% of all malignancies in this age group are osteosarcomas. In 2000, the most recent year for which data are available, there were 440 cases of osteosarcoma in children from birth to 19 years of age in the United States. There were an additional 135 cases in young adults aged 20 to 29. Internationally, incidence rates approximate the U.S. rate, with the exception of a higher incidence in some African countries. In Sudan and Uganda, the osteosarcoma incidence in children younger than 14 years is 5.3 and 6.4/million respectively, compared with an incidence of between 2 and 3/million in this age group in the United States, Europe, and Asia.
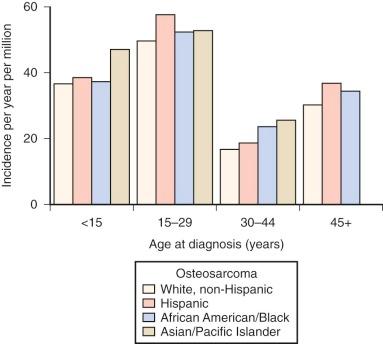
Osteosarcoma occurs rarely in children younger than 5 years. After age 5, the incidence increases steadily, reaching a peak at age 15 years. After the adolescent peak, the rate of osteosarcoma steadily declines, leveling off at a rate of 1 to 2/million. A second peak in incidence, approximately half the magnitude of the adolescent peak, occurs in the sixth to seventh decade. Osteosarcoma in older patients is associated with Paget disease and prior radiation therapy, although approximately half of older patients with osteosarcoma have neither condition. The adolescent peak occurs at age 13 years in girls and between ages 15 and 17 years in boys ( Fig. 62-1 ). The age at peak incidence corresponds to the age of greatest growth velocity in each gender. This association contributes to the evidence supporting a role for growth in the cause of osteosarcoma (see “ Etiology ”). Osteosarcoma is slightly more common in males, particularly in the 15- to 19-year-old age group.
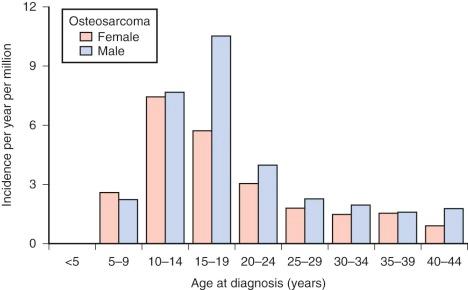
In the United States, osteosarcoma is slightly more common in African Americans, Hispanics, and Asians and Pacific Islanders ( Fig. 62-2 ). Several studies have reported that rates of osteosarcoma in black children younger than 14 years are about twice those in white children of the same age. In the most recent report on osteosarcoma from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute, U.S. blacks younger than 20 years continue to have a slightly higher rate of osteosarcoma. As shown in Figure 62-2 , for the age group 15 to 29 years, the racial group with the highest rate of osteosarcoma is Hispanic. The relative risk of developing osteosarcoma for Hispanic children younger than 14 years of age, based on data collected between 1975 and 2003, is 1.3. Asians and Pacific Islanders have the highest rate of osteosarcoma in the age groups younger than 15 years and 30 to 44 years. However, the reported incidence in Hispanics and in Asians and Pacific Islanders is likely to be inaccurate given the small absolute number of affected individuals and uncertainty in the denominator (i.e., the number of Hispanics and Asians and Pacific Islanders within each age group living in the United States). In summary, there is evidence for a slightly increased incidence of osteosarcoma among U.S. blacks and Hispanics. Recent SEER data show an increased rate in Asians and Pacific Islanders younger than 15 years, but this finding needs further investigation.
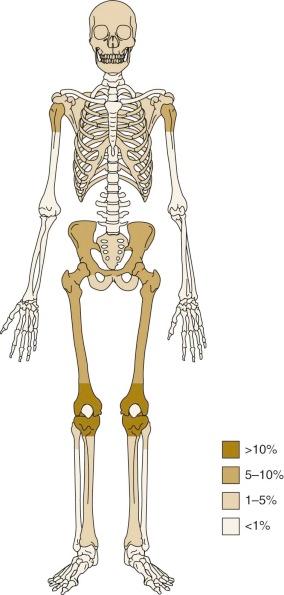
The most common anatomic sites of osteosarcoma at initial presentation are the long bones of the lower limbs. Fifty percent of osteosarcomas are located around the knee, in the distal femur, proximal tibia, or proximal fibula ( Fig. 62-3 ). Approximately 10% of tumors occur in the mid or proximal femur, and 9% occur in the proximal humerus. Of patients with osteosarcoma, 10% to 20% have metastatic disease at initial presentation. The most common site of metastasis is the lungs. Isolated pulmonary metastases are present in 61% of patients with metastatic disease at presentation. Isolated metastases to bone are present in 16% of patients, and 14% have both pulmonary and bone metastases. Seven percent of patients have metastases to other rare sites plus bone and/or pulmonary metastases, and 2% of patients have isolated metastases to rare sites. Rare sites of metastasis in patients with primary metastatic disease, as opposed to metastatic disease in patients with recurrent disease, are lymph nodes, central nervous system, liver, adrenal gland, and soft tissues.
When a patient presents with osteosarcoma, it is often difficult to identify a cause. However, recognition of causative factors has led to the discovery of biologic mechanisms of transformation, as in the case of predisposition to osteosarcoma in Li-Fraumeni syndrome, or the identification of exposures to be avoided, as in the case of radiation-induced osteosarcoma. Inherited syndromes in which there is an increased occurrence of osteosarcoma are hereditary retinoblastoma and Li-Fraumeni, Rothmund-Thomson, Bloom, and Werner syndromes. There is a clear association between the risk for osteosarcoma and prior radiation therapy or treatment with alkylating chemotherapy. Epidemiologic studies and clinical observation have suggested that increased height may be a risk factor for the development of osteosarcoma. Finally, environmental exposures to radium and beryllium likely increase the risk for osteosarcoma, but exposure to high doses of these agents is rare.
As noted, peak rates of osteosarcoma coincide with the period of maximal linear growth during puberty. Most osteosarcomas occurring in the adolescent age group are in the long bones of the axial skeleton, the bones in which the most active growth occurs. These clinical observations have led to the hypothesis that the hormonal milieu during maximal linear growth encourages the development of osteosarcoma and that, by extension, osteosarcoma would be more common in taller adolescents. Multiple epidemiologic studies have addressed this question, but results are contradictory. Approximately 50% of the studies have found that osteosarcoma patients are taller at diagnosis than age-matched controls or the normal population. The remaining studies have not supported an association between height and the occurrence of osteosarcoma. The larger studies and those with more reliable sources of height data have shown a positive association between tall stature and osteosarcoma. These data, in part, have led to the investigation of the insulin-like growth factor axis in osteosarcoma (see “ Biology ”).
Approximately 1% of patients develop secondary bone tumors after treatment of a primary pediatric malignancy ; of these secondary bone tumors, 50% to 80% are osteosarcomas. On the other hand, secondary Ewing sarcoma is rare. The relative risk of secondary osteosarcoma increases greatly with radiation doses to the bone higher than 1000 cGy. In patients who received between 3000 and 5000 cGy to the bone during treatment of their primary pediatric malignancy, the relative risk of secondary osteosarcoma was variably reported, but it may be as high as 100. A study in patients with primary Ewing sarcoma has concluded that only higher radiation doses increase the risk for secondary sarcomas, with no tumors developing in patients who had received less than 4800 cGy. The highest risk, 130 cases/10,000 person-years of observation, was observed among patients who had received 6000 cGy or more. In the 92 published cases of secondary osteosarcoma for whom radiation dose was reported, the median radiation dose delivered was 4500 to 5400 cGy. The average time between diagnosis of the primary malignancy and development of secondary osteosarcoma is 10 years. Another factor that increases the likelihood that survivors of a primary cancer will develop secondary osteosarcoma is treatment with alkylating agents. This class of chemotherapy increases the relative risk of secondary osteosarcoma by a factor of 5.
Data regarding an increased risk for cancer in people exposed to low levels of radiation from the atomic bombs in Hiroshima and Nagasaki have led to increased concern about the risk of diagnostic imaging with x-rays and computed tomographic (CT) scans, particularly in pediatric patients. However, current evidence does not suggest that osteosarcoma risk would be increased from commonly used imaging modalities. In particular, the radiation dose of a CT scan is approximately 6 cGy and the risk for bone cancer in pediatric cancer survivors who received less than 1000 cGy to the bone is not increased.
Osteosarcoma is seen in a number of inherited cancer predisposition syndromes. These syndromes have often illuminated mechanisms of transformation in sporadic osteosarcoma. Children with hereditary retinoblastoma have a germline heterozygous, inactivating deletion, and/or a frameshift or point mutation of the tumor suppressor gene retinoblastoma 1 (RB1). Affected children develop retinoblastoma usually in one or both eyes. The cumulative incidence of a nonretinoblastoma malignancy in hereditary retinoblastoma is 51%, and approximately 40% of these second malignancies are osteosarcomas. Both osteosarcomas and retinoblastomas in these patients have loss of heterozygosity of the RB1 allele, resulting in a complete loss of RB1 function. As discussed later (see “ Biology ”), loss of RB1 function also occurs in sporadic osteosarcomas.
In the Li-Fraumeni syndrome, patients carry a heterozygous inactivating mutation of the tumor suppressor gene TP53. Affected individuals have a greatly increased cancer risk at a young age. The most common malignancies are breast cancer and bone and soft tissue sarcomas, brain tumors, and leukemia. Slightly more than 10% of the malignancies in patients with Li-Fraumeni syndrome are osteosarcomas. Among patients with Li-Fraumeni syndrome, mutations in the TP53 DNA-binding domain (as compared with mutations outside the DNA-binding domain or null mutations) are associated with early sarcomas. Patients with osteosarcomas and other cancers that develop in Li-Fraumeni syndrome have loss of the normal TP53 allele consistent with the tumor suppressor function of the TP53 gene product. Loss of TP53 function by TP53 mutation or amplification of MDM2 or COPS3 is also present in most sporadic osteosarcomas (see “ Biology ”).
Rothmund-Thomson, Bloom, and Werner syndromes are members of a family of autosomal recessive disorders caused by mutations in one of the RECQ DNA helicases. Rothmund-Thomson syndrome, caused by mutations in the RECQL4 gene, is characterized by rash, skeletal dysplasias, and sparse hair. Up to 30% of patients with Rothmund-Thomson syndrome develop osteosarcoma at a slightly younger age than in sporadic osteosarcoma. Bloom syndrome, caused by mutations in the BLM (RECQL2) gene, is characterized by short stature, telangiectasia, and an increased risk for developing a wide variety of cancers at a young age. Unlike in Rothmund-Thomson syndrome, only a small percentage of cancers occurring in affected individuals are osteosarcomas. Werner syndrome is caused by a mutation in the WRN (RECQL3) gene. Patients have scleroderma-like skin changes, premature aging, and a typical body habitus with short stature, long spindly limbs, and a stocky trunk. Like in Bloom syndrome, the risk for developing cancer at a young age is increased in Werner syndrome. Also like in Bloom syndrome, osteosarcoma constitutes fewer than 10% of the malignancies occurring in patients with Werner syndrome. For a further discussion of the role of RECQ DNA helicases in osteosarcoma, see “ Biology ”.
The risk for developing osteosarcoma is increased in adults with Paget disease. In Paget disease, which affects up to 1% of adults older than the age of 40 years, bone resorption by osteoclasts is increased. As a result, there is a compensatory increase in osteoblast activity and bone formation. Osteosarcoma develops in less than 1% of patients with Paget disease, but this represents a higher rate than that seen in the general population. The average age of patients with Paget disease–related osteosarcoma is 70 years.
A single genome-wide association study has been conducted in osteosarcoma. There are two susceptibility loci achieving statistical significance in osteosarcoma. One locus occurs in a gene desert and the other, at 6p21.3, implicates GRM4, a glutamate receptor gene involved in inhibition of the cyclic adenosine monophosphate (AMP) cascade.
Previously, some believed that trauma may cause osteosarcoma. However, modern epidemiologic studies have not supported a relationship between trauma and osteosarcoma. Prior observations linking trauma and osteosarcoma were likely the result of the fact that a traumatic event may result in a previously existing osteosarcoma being brought to medical attention.
Environmental exposures, other than ionizing radiation, for which there is evidence of an association between exposure and osteosarcoma risk, include exposure to radium and beryllium. Radium is a radioactive element that is variably present in well water. When ingested, radium retained in the body is deposited in bone. Studies of those exposed to high levels of radium while painting dials on clocks in the 1920s strongly indicated that exposure to high doses of radium causes osteosarcoma. The risk for osteosarcoma from exposure to low levels of radium in drinking water is less clear. Two studies have shown a slight increase in risk for bone sarcoma or osteosarcoma in those born in areas with drinking water containing relatively high concentrations of radium. Conversely, two studies have shown no link between mean levels of radium in water and osteosarcoma. Beryllium is a metallic element with limited use in the production of aerospace structural material, x-ray tubes, and nuclear reactors. Although intravenous injection of beryllium salts causes osteosarcoma in rabbits, there is limited evidence of an increased risk for osteosarcoma caused by beryllium exposure in humans. Case-control studies have failed to identify other environmental or occupational exposures that increase the risk for osteosarcoma. Because fluoride can stimulate bone formation there has been concern about a possible relationship between fluoride in drinking water and osteosarcoma. This is a very difficult question to study adequately given that multiple factors beyond water fluoridation may impact fluoride intake. So far, there is no substantial evidence to support a causative association between fluoride exposure and osteosarcoma.
Clinical, laboratory, and radiologic findings are often highly suggestive of osteosarcoma but are not pathognomonic. Biopsy is always required for diagnosis. Atypical imaging characteristics, including a benign appearance in high-grade osteosarcoma, have been described. The differential diagnosis of osteosarcoma includes benign and malignant bone tumors ( Box 62-1 ; Fig. 62-4 ).
Ewing sarcoma
Chondrosarcoma
Fibrosarcoma
Langerhans cell histiocytosis
Lymphoma
Neuroblastoma
Metastatic rhabdomyosarcoma
Metastatic melanoma
Aneurysmal bone cyst
Osteoblastoma
Osteoid osteoma
Giant cell tumor
Unicameral bone cyst
Osteomyelitis
Clinical symptoms of osteosarcoma are nonspecific; 25% of patients have pain and a palpable mass at presentation, and 70% of patients present with pain as their only complaint. Pain tends to be intermittent and exacerbated by activity. The remaining patients present with a painless mass. The duration of symptoms before diagnosis ranges from 1 day to 5 years, with a median of about ![]() months. In approximately 10% of patients, symptom duration is longer than 6 months. A pathologic fracture is present in 8% of patients at the time of diagnosis.
months. In approximately 10% of patients, symptom duration is longer than 6 months. A pathologic fracture is present in 8% of patients at the time of diagnosis.
Radiologic evaluation in osteosarcoma informs the initial diagnostic impression, aids in the determination of the best approach for biopsy, facilitates surgical resection, and identifies sites of metastatic disease, if present. The recommended imaging studies for osteosarcoma at the time of diagnosis are plain radiography and magnetic resonance imaging (MRI) of the primary tumor site to evaluate the extent of local disease, a bone scan to screen for bony metastases, and a CT scan of the chest to determine whether pulmonary metastases are present. Whenever possible, these imaging studies should be performed before diagnostic biopsy because postsurgical atelectasis interferes with evaluation of the lung parenchyma. MRI of the primary tumor should include the entire bone from which the tumor arises and the closest adjacent joint to screen for skip metastases properly. An additional MR image should be obtained after neoadjuvant chemotherapy has been given but before definitive resection to determine the best plan for the surgical approach.
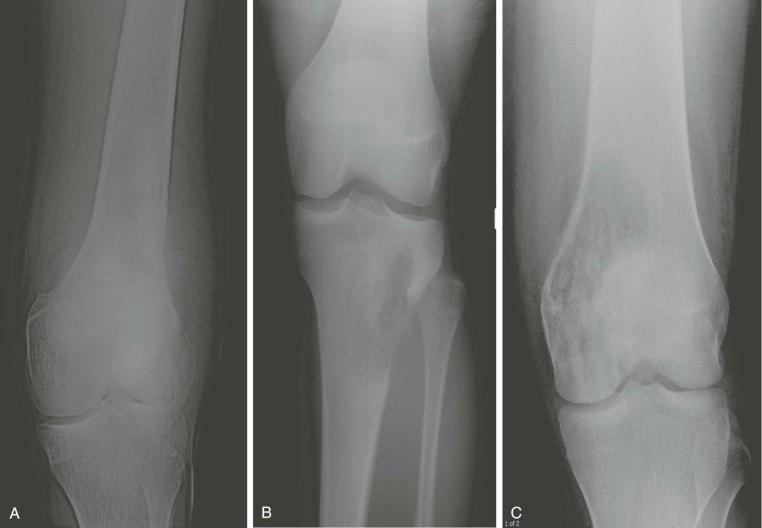
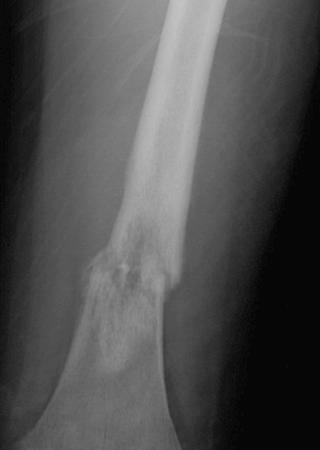
A plain radiograph of the primary tumor has usually been obtained before referral to an oncologist or orthopedist. Most appendicular osteosarcomas are located in the metaphyseal portion of the long bone. Both lytic destruction of bone and sclerotic irregular new bone formation can occur. Osteosarcoma usually has a mixed pattern of bone lysis and sclerosis, but one of these patterns can predominate. Regardless of the predominant pattern, the boundary between tumor and normal bone is usually irregular. The bone cortex is often disrupted by tumor growth into the surrounding soft tissue. Osteoid formation within the tumor results in areas of calcification visible in the bony and soft tissue components of the tumor. Periosteal new bone formation in osteosarcoma can be irregular or can occur in a sunburst pattern. “Sunburst” refers to linear new bone growth that is perpendicular to the bony cortex and arranged in a fanlike pattern ( Fig. 62-5 ). In contrast, in Ewing sarcoma, periosteal new bone growth typically occurs in an onionskin pattern in which linear new bone is oriented parallel to the bony cortex. Periosteal new bone formation in osteosarcoma can also be present at the edge of the preserved cortex, resulting in the Codman triangle. Plain radiographs also assist in the diagnosis of pathologic fractures ( Fig. 62-6 ).
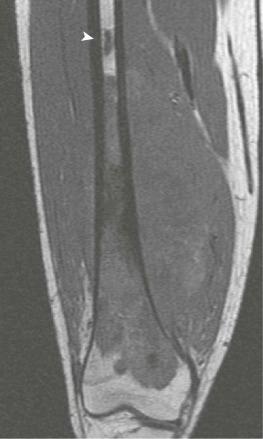
Although plain radiographs of the primary tumor are informative, MRI is preferable for local staging, presurgical planning, and identification of skip metastases. CT is an acceptable alternative to MRI, but most orthopedic surgeons and oncologists prefer MRI. In two studies directly comparing preoperative CT and MRI results with pathologic findings in patients with osteosarcoma, MRI was found to be superior to CT in defining the longitudinal extent of intraosseous tumor, extension into adjacent muscle compartments, and involvement of the neurovascular bundle. The two modalities were equivalent in their ability to define cortical bone and joint involvement. However, a more recent study in a larger patient population did not find a statistically significant difference between CT and MRI in their ability to predict the involvement of bone, muscle, joints, and the neurovascular bundle. One possible explanation for the contradictory results is improvements in CT quality during the interval between the studies. In addition, the later study grouped all primary bone tumors together for analysis but, given that 60% of the bone tumors were osteosarcoma, results would likely be similar for osteosarcomas alone. In comparison to CT and technetium-99 m ( 99m Tc) bone scanning, MRI more accurately predicts the intraosseous extent of osteosarcoma. The presence of significant edema has been associated with inaccurate assessment of the margins of tumor on MRI. Because edema can resolve with chemotherapy, imaging performed for the purpose of surgical planning should be obtained after the administration of neoadjuvant chemotherapy.
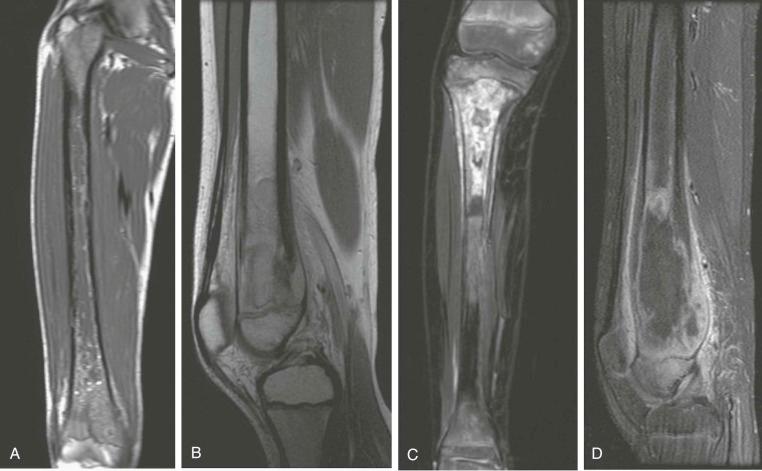
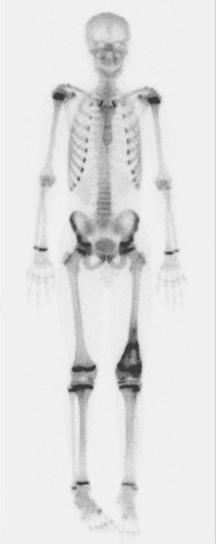
A skip metastasis is defined as a smaller distinct focus of osteosarcoma that is either within the same bone as the primary tumor or on the other side of the joint adjacent to the primary tumor. There must be normal structures not involved with tumor present between the primary tumor and skip metastasis. Skip metastases are synchronous with the primary tumor and thus are present at initial presentation. Diagnosis of skip metastases is of critical importance for prognostication and because complete surgical remission in osteosarcoma with skip metastases requires complete resection of both the primary tumor and skip metastases. Over 80% of skip metastases are visible on MR images ( Fig. 62-7 ). In contrast, skip metastases are visible on bone scan, CT scans, and plain radiographs in only 46%, 45%, and 36% of cases, respectively. Interestingly, a common reason for failure to identify skip metastases by bone scanning is merging of the signal from the primary tumor with the signal from the skip metastasis. To ensure that skip metastases are identified, MRI of the primary tumor should include the entire bone and adjacent joint. Bony metastases that are not skip metastases are best identified with a 99m Tc bone scan ( Fig. 62-8 ). The usefulness of 18 F-labeled fluorodeoxyglucose positron emission tomography (FDG-PET) in osteosarcoma has not yet been adequately evaluated. Results of preliminary studies have suggested that FDG-PET is more useful for assessing tumor response to chemotherapy and for posttherapeutic monitoring for recurrence than for assessing the initial tumor. Because edema can resolve with chemotherapy, imaging performed for the purpose of surgical planning should be performed after the administration of neoadjuvant chemotherapy.
CT of the chest is the most sensitive radiologic study for detection of pulmonary metastases. A generally accepted definition of definitive pulmonary metastatic disease is three or more lesions 5 mm or larger or one lesion 1 cm or larger. The interpretation of very small lesions (<5 mm) and solitary lesions between 5 mm and 1 cm detected by high-resolution CT can be difficult. In some cases, it is not possible to determine whether such CT findings represent metastatic disease or a nonmalignant lung process, and biopsy may be needed. Compared with a CT scan, a plain radiograph of the chest has a sensitivity of 57% and a 99m Tc bone scan has a sensitivity of 41%. Although CT is highly sensitive, evaluation at the time of thoracotomy usually reveals more pulmonary metastases than are appreciated on the CT scan. Importantly, chest CT scans should be obtained before biopsy of the primary tumor because postanesthesia atelectasis makes chest CT scans more difficult to interpret.
Whole-body MRI screening as a method of surveillance in patients with cancer predisposition is currently being evaluated. In a small group or individuals (n = 25) with hereditary retinoblastoma, whole-body MRI detected two cases of osteosarcoma. An additional individual was diagnosed with osteosarcoma due to symptoms. A screening protocol including whole-body MRI utilized in individuals with Li-Fraumeni syndrome detected no cases of osteosarcoma but did detect one case of malignant fibrous histiocytoma.
Traditionally, bone tumor tissue for pathologic examination was obtained by open biopsy. However, percutaneous core needle biopsies have become more common as the availability and sophistication of interventional radiology services have increased. A number of retrospective series have reported on the diagnostic yield and accuracy of core needle biopsies in osteosarcoma. Although none specifically studies pediatric osteosarcoma, all series that provide patient age included children. In these published series, core needle biopsy resulted in a definitive, correct diagnosis in 78% to 94% of cases. The more recently published series have reported a diagnostic accuracy of 90% or greater. However, even those percutaneous biopsies that yield sufficient tumor material for a diagnosis often do not yield sufficient tumor specimen for other biologic studies.
The outcome of percutaneous biopsy varies depending on the provider, technique, and patient population. In the published series reporting a high diagnostic yield, biopsies were performed by experienced interventional radiologists in consultation with orthopedic surgeons in tertiary or referral centers with considerable expertise in the diagnosis and management of bone tumors. In the more recent series, patients underwent conscious sedation or were given general anesthesia. Specific techniques included the use of fluoroscopic, CT, or ultrasound guidance and use of at least a 14-gauge needle. The tumor component targeted for biopsy was the soft tissue mass, if present, followed by the lytic aspect of an intraosseous lesion.
The most common reason for failure to obtain diagnostic tissue by core needle biopsy is the presence of a highly sclerotic tumor. Of the various types of osteosarcoma, telangiectatic osteosarcoma most often results in a nondiagnostic specimen or is misdiagnosed. Development of osteosarcoma in a percutaneous biopsy tract has been reported. Therefore, it is important that the biopsy site be located so that it can be resected en bloc with the primary tumor. Because fine-needle aspiration has a diagnostic yield lower than core needle biopsy and does not obviate the need for sedation in the pediatric population, it is not a recommended approach for diagnosis.
Surgical or open biopsy of bone tumors can be incisional or excisional. Excisional biopsy is almost never performed in osteosarcoma because neoadjuvant chemotherapy can decrease tumor edema and, in doing so, simplify resection. In addition, time is required for adequate surgical planning, especially for younger children in whom placement of a growing prosthesis is being considered. Complications of open biopsy include anesthesia-related events, infection, bleeding, and tumor seeding in the biopsy tract. In addition, disruption of anatomic compartments and incorrect localization of the biopsy incision can lead to a more complex or more extensive tumor resection and can occasionally result in an amputation that would not have otherwise been necessary. As with percutaneous biopsy, the incision for an open biopsy should be placed and oriented in a way that allows it to be excised en bloc with the primary tumor. Complications of open biopsy, including those that result in more extensive resections, are unusual when the biopsy is performed at a center specializing in the diagnosis and treatment of malignant bone tumors.
In summary, the percutaneous and open biopsy routes are both acceptable. Given the implications for ultimate surgical resection of a poorly performed biopsy, biopsy should be performed or planned by an orthopedic surgeon with bone tumor expertise, ideally by the same orthopedic surgeon who will perform the ultimate resection. Percutaneous biopsies should only be performed by experienced interventional radiology staff working in conjunction with an experienced orthopedic surgeon. If the percutaneous route is recommended, it is important to counsel patients and families about the possible risks of core needle biopsy, including the potential for an inconclusive specimen. For a further discussion of the debate regarding the correct route for bone tumor biopsy, see Box 62-2 .
There are two possible approaches to obtaining a diagnostic specimen in suspected osteosarcoma—open biopsy and core needle biopsy. Considerable controversy exists regarding which diagnostic approach is best. An open biopsy is performed in the operating room by an orthopedic surgeon. As discussed in more detail in the text, an open biopsy should be performed by orthopedic surgeon with experience treating bone tumors and ideally by the same orthopedic surgeon who will ultimately perform the tumor resection. In a core needle biopsy, while imaging the tumor with CT, an interventional radiologist obtains several needle cores of tumor material. As with an open biopsy, a core needle biopsy should be performed by an interventional radiologist with expertise in the diagnostic evaluation of bone tumors. Planning for core needle biopsy should include a discussion with the orthopedic surgeon who will ultimately perform the resection. The relative merits of core needle and open biopsy as they relate to several core issues are discussed below:
Because orthopedic surgeons directly visualize a tumor during an open biopsy and because pathologists often view frozen sections during open biopsies, nondiagnostic open biopsies are uncommon. In retrospective series, core needle biopsy results in a definitive, correct diagnosis in 78% to 94% of cases. The more recently published series report a diagnostic accuracy of 90% or more. The interventional radiology-guided core needle biopsies in these studies were performed in centers with considerable experience with the diagnosis of bone tumors and similar diagnostic accuracy would not be expected in inexperienced centers. Less tumor material is obtained from core needle biopsies, which can compromise the pathologist's ability to perform additional testing, such as cytogenetics, in some cases.
Both core needle and open biopsies require general anesthesia in children. In general, open biopsies are longer procedures, requiring a greater length of general anesthesia. Open biopsies are also more invasive and result in a longer postprocedure recovery.
Whether the biopsy approach is core needle or open, definitive surgical treatment of osteosarcoma, which is essential for cure, requires excision of the biopsy tract or site en bloc with the primary tumor. A poorly placed biopsy can convert a tumor amenable to limb-sparing surgery into one that requires amputation. This impact on ultimate resectability is one of the greatest potential negative aspects of core needle biopsy. Because interventional radiologists are less knowledgeable about surgical approaches for definitive tumor resection in osteosarcoma, the risk of a poorly placed biopsy is potentially higher with a core needle biopsy. However, supporters of core needle biopsy argue that this risk can be reduced through discussion of the biopsy with the patient's treating orthopedic surgeon.
Although the availability of tumor material for the study of osteosarcoma biology is not an important issue from the perspective of the individual patient with osteosarcoma, it is an important issue from the perspective of researchers and future patients who could benefit from research discoveries. Because tumor resection occurs after the administration of chemotherapy, osteosarcoma resection specimens are often not suitable for laboratory investigation. After open biopsy there is usually sufficient prechemotherapy tumor material available for biologic studies after the diagnosis is made. This is not the case with core needle biopsies. Thus, progress in osteosarcoma research would likely be negatively affected if the majority of centers were to perform core needle biopsies.
Laboratory test results are often normal in osteosarcoma. When laboratory abnormalities are present, they are nonspecific, having many other potential causes. The serum alkaline phosphatase level is elevated in approximately 50% of patients presenting with osteosarcoma. A higher percentage of patients with metastatic disease have a high alkaline phosphatase level, but this is not a reliable test of metastatic disease, because some patients with metastatic disease will have a normal result. As will be discussed later, alkaline phosphatase elevations are associated with a poorer prognosis. Lactate dehydrogenase (LDH) is elevated in approximately 30% of osteosarcoma patients. The erythrocyte sedimentation rate is generally not elevated in patients with osteosarcoma, whereas it is often elevated in those with Ewing sarcoma.
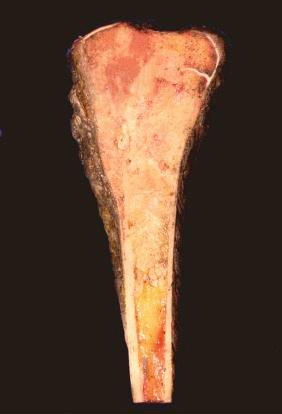
On gross examination, osteosarcoma usually presents as a large mass located in the metaphysis ( Fig. 62-9 ) arising in the medullary cavity and crossing the bony cortex, with invasion into the adjacent soft tissues. Microscopically, the malignant cells are pleomorphic but spindle cells typically predominate. Epithelioid, small round cells, multinucleated giant cells, and plasmacytoid cells may also be present and occasionally are the predominant morphology. Osteoid, which can be plentiful or sparse, is currently essential for the diagnosis of osteosarcoma. Sometimes, it is not present in the initial biopsy sample but is seen in the definitive resection specimen. If not present in either, the diagnosis of osteosarcoma cannot be made with certainty. The histologic appearance of osteoid is dense, pink, and amorphous. Classic or conventional osteosarcoma comprises 70% to 80% of cases of osteosarcoma. A number of other types of osteosarcoma are distinguished from classic osteosarcoma based on clinical or pathologic features ( Table 62-1 ).
| Type | Pathology | Approximate % (of all Osteosarcoma) |
|---|---|---|
| Classic | Anaplastic, numerous mitoses | 70-80 |
| Osteoblastic | Predominant matrix osteoid | 35 |
| Chondroblastic | Predominant matrix chondroid | 18 |
| Fibroblastic | Spindle cells in herringbone pattern | 18 |
| Jaw | Similar to classic osteosarcoma | 3-7 |
| Secondary | Similar to classic osteosarcoma | 7 |
| Telangiectatic | Cysts and septa, scant osteoid, giant cells present | 4-10 |
| Small cell | Small round malignant cells, osteoid present but quantity variable | 1-4 |
| Low-grade intramedullary | Minimal atypia, few mitoses | 1-2 |
| Parosteal | Located on bone surface, low grade, matrix osteoid and, in 50%, cartilage | 4 |
| Periosteal | Located on bone surface, intermediate grade, predominant matrix cartilage | 1-2 |
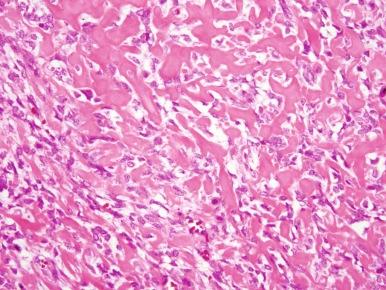
Classic osteosarcoma has a highly malignant appearance, with prominent anaplasia and frequent mitoses. There is variability in the histologic appearance of conventional osteosarcoma. To facilitate pathologic diagnosis and best describe this variability in appearance, classic osteosarcoma is divided into three subtypes based on the predominant matrix produced by the malignant cells. In the first, 50% of typical osteosarcoma is osteoblastic osteosarcoma, in which osteoid is the predominant matrix ( Fig. 62-10 ). Next, 25% of typical osteosarcoma is chondroblastic osteosarcoma in which cartilaginous islands are the predominant matrix. This subtype is differentiated from chondrosarcoma by the presence of osteoid. Because osteoid can be sparse in chondroblastic osteosarcoma, distinguishing chondroblastic osteosarcoma from chondrosarcoma can require extensive biopsy material, which may necessitate rebiopsy. Sixty-one percent of chondrosarcomas have mutations in the isocitrate dehydrogenase 1 and 2 genes ( IDH1 and IDH2 ). Mutations in these genes have not been seen in osteosarcoma. Therefore, the identification of an IDH1 or IDH2 mutation in a malignant bone tumor with a chondroid matrix would strongly suggest a diagnosis of chondrosarcoma. The remaining 25% of typical osteosarcoma is fibroblastic. In fibroblastic osteosarcoma, osteoid is minimal and spindle cells grow in a herringbone pattern. Many osteosarcomas have a mixture of different histologies, but in mixed histology cases one histology usually predominates. Of note, the subtypes of typical osteosarcoma were originally designated for the purpose of diagnosis, and their prognostic significance is uncertain. A better histologic response to chemotherapy in fibroblastic osteosarcoma and a poorer histologic response to chemotherapy in chondroblastic osteosarcoma have been consistent findings in several studies. Telangiectatic osteosarcoma also has a better histologic response to chemotherapy (see later). Whether these differences in histologic response to therapy are correlated with differences in OS is not entirely clear. In the largest study to evaluate this question, there was a slight, statistically significant 5-year OS advantage in patients with fibroblastic osteosarcoma. Unlike other types of osteosarcoma, histologic response to chemotherapy may not be correlated with outcome in chondroblastic osteosarcoma.
The remaining 20% to 30% of osteosarcomas that are not classic osteosarcomas are divided into secondary (occurring in Paget disease or after irradiation), jaw, telangiectatic, small cell, parosteal, periosteal, and low-grade intramedullary osteosarcoma. Telangiectatic osteosarcoma accounts for 4% to 11% of all osteosarcoma cases. The age and gender distribution are the same as in conventional osteosarcoma. Pathologic fracture is more common, occurring in 15% to 25% of cases. Radiographically, a lytic pattern is present because of the presence of large or small cystic areas and minimal osteoid formation. Microscopically, cysts are divided by septa that are lined with malignant cells producing scant osteoid. Cysts are also lined by benign, multinucleated giant cells. The presence of cysts and multinucleated giant cells can lead to misdiagnosis as an aneurysmal bone cyst or giant cell tumor, both benign bone tumors. Although case series published prior to the modern era of multiagent chemotherapy had reported that telangiectatic osteosarcoma has an adverse prognostic implication, more recent studies have reported a better histologic response to therapy and improved survival as compared with typical osteosarcoma.
Small cell osteosarcoma is a rare but histologically distinct variant representing 1% to 4% of osteosarcomas. The clinical and epidemiologic characteristics are the same as conventional osteosarcoma. Microscopically, tumors are composed of small round cells that produce variable amounts of osteoid. Differentiation from Ewing sarcoma and lymphoma can be difficult.
Osteosarcomas of the jaw, located in the maxilla or mandible, deserve specific mention. Even when secondary osteosarcomas are excluded, the mean age of patients with osteosarcoma in this location is the mid-30s, which is older than in classic osteosarcoma. These tumors are more often low grade; approximately 50% are low grade in most series. Histologically, the appearance is similar to that of classic osteosarcoma but a larger proportion of tumors are chondroblastic. In concordance with the greater proportion of low-grade tumors, prognosis is better for osteosarcoma of the jaw. Although local recurrence does occur, especially when the initial resection is inadequate, metastasis is unusual.
Low-grade intramedullary osteosarcoma is relatively rare, making up 1% to 2% of all osteosarcomas. Patients developing this form of osteosarcoma tend to be older than those with conventional osteosarcoma, with 30 years being the mean age at diagnosis. Microscopic examination reveals spindle cells permeating bony trabeculae, with minimal atypia and few mitoses. A variable amount of osteoid is present. Recurrence and metastasis are rare. With adequate resection, chemotherapy is generally not indicated.
Parosteal osteosarcoma is another low-grade osteosarcoma distinguished from low-grade intramedullary osteosarcoma by its location on the cortical surface of the bone. It is generally a sclerotic tumor attached to the bone by a broad base. Microscopically, the stroma is sparsely populated by minimally atypical spindle cells. The matrix contains osteoid and, in 50% of cases, cartilage. A limited degree of medullary involvement is seen in 25% of cases. Areas of dedifferentiation can be present at diagnosis or at the time of recurrence and portend a poorer prognosis. Of patients with dedifferentiation, 31% will die of disease, usually with pulmonary metastasis. Parosteal osteosarcoma makes up approximately 4% of all osteosarcomas. Like intramedullary low-grade osteosarcoma, parosteal osteosarcoma occurs in slightly older patients than conventional osteosarcoma and recurrence and metastasis are rare in adequately resected cases without dedifferentiation.
Parosteal osteosarcoma must be distinguished from periosteal osteosarcoma. Periosteal osteosarcoma also arises from the surface of the bone but is an intermediate- or high-grade lesion. Periosteal osteosarcoma has an age distribution similar to that of typical osteosarcoma but almost all tumors arise in the proximal tibia. The appearance on plain radiography is highly characteristic and suggests the diagnosis. Histology is of a spindle cell neoplasm with osteoid and cartilaginous islands present, usually in large amounts. The prognosis is better than in conventional osteosarcoma but probably not as good as in parosteal osteosarcoma without dedifferentiation or intermedullary low-grade osteosarcoma. There is controversy regarding the need for chemotherapy for periosteal osteosarcoma. The entity is too rare to permit conduct of prospective randomized controlled trials. Interpretation of retrospective series is difficult because variability in chemotherapy administration likely correlates with variability in clinicopathologic features related to outcome.
Secondary osteosarcoma arises in previously irradiated bone or in patients with Paget disease. In both cases, patients are older than those with conventional osteosarcoma. Pathology is similar to conventional osteosarcoma. Prognosis is poorer in Paget disease than in conventional osteosarcoma. There is some evidence that radiation-induced osteosarcomas have similar outcomes to de novo osteosarcoma if standard osteosarcoma chemotherapy is given.
Staging of osteosarcoma follows the Enneking or musculoskeletal society staging system ( Table 62-2 ). The TNM system is not used for several reasons. The TNM system lacks biologic relevance in that osteosarcoma seldom metastasizes to lymph nodes. In addition, it is more complex than needed for predicting outcome, because patients in different TNM stages have overlapping prognoses.
| Stage | Grade | Site | Metastasis | % of Patients | Surgical Margin | Chemotherapy | Representative Subtype |
|---|---|---|---|---|---|---|---|
| IA | G1 | T1 | M0 | 5 | Wide | No | Parosteal |
| IB | G1 | T2 | M0 | 3 | Wide | No | |
| IIA | G2 | T1 | M0 | 5 | Wide | Yes | Periosteal |
| IIB | G2 | T2 | M0 | 74 | Wide | Yes | Classic |
| III | G1, G2 | T1, T2 | M1 | 13 | Variable | Yes | Classic |
The Enneking staging system is based on tumor grade (G), site (T), and metastases (M). Low-grade (G1) tumors are well differentiated and have few mitoses, corresponding to Broders grades I and II. Osteosarcoma is high grade (G2) if it is poorly differentiated and has numerous mitoses, corresponding to Broders grades III and IV. As noted, most osteosarcomas are high grade (G2). Surgical site (T) is determined by whether the tumor is contained within its anatomic compartment of origin (T1) or extends beyond its compartment of origin (T2). For example, a typical osteosarcoma that arises in the medullary space and extends through the cortex into the soft tissue is T2. A parosteal osteosarcoma that does not extend from the bone surface, its site of origin, into the medullary space and does not invade the surrounding soft tissue is T1. Most classic osteosarcomas are T2. Metastases are absent (M1) or present (M2). Stages IA and IB are low-grade (G1) tumors. Stages IIA and IIB are high-grade tumors. Stage IA tumors are low-grade osteosarcomas that are T1 or lack extension beyond the compartment of origin. Stage IB tumors are low-grade osteosarcomas that are T2 or demonstrate extension beyond the compartment of origin. Similarly, stage IIA tumors do not have extracompartmental extension, whereas IIB tumors do. Stage III osteosarcomas are tumors of any grade and any site that have distant metastases. The vast majority of osteosarcomas are stage IIB. The next most common stage for osteosarcoma at presentation is stage III. Stages IA, IB, and IIA are uncommon, with 3% to 5% of osteosarcomas falling into each of these categories.
The most significant predictor of survival in osteosarcoma is the presence of residual disease caused by incomplete resection of primary tumor in localized osteosarcoma, local recurrence, or the presence of metastases. Consequently, the natural history, prognosis, and prognostic factors of localized, relapsed, and metastatic osteosarcoma differ significantly.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here