Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Osteoporosis is defined as a skeletal disorder that is characterized by compromised bone strength and that predisposes to an increased risk for fracture. The pertinent clinical outcomes of this disease include fractures, bone pain, loss of height, and physical deformity. The concept of bone strength is central to understanding the disorder because patients who suffer an osteoporotic or fragility fracture may or may not have osteoporosis by bone mineral density criteria. The World Health Organization defines osteoporosis as a bone mineral density that is equal to or greater than 2.5 standard deviations below that of an average individual at peak bone mass (generally ages 20 to 30 years, depending on the measured skeletal site). Osteopenia, or low bone density, is defined as a bone mineral density between −1.1 and −2.4 standard deviations, inclusive, below young average normal.
Approximately half of White women will develop an osteoporosis-related fracture in their lifetime, which is greater than their risk for breast cancer, heart attack, and stroke combined. In addition, one in five men will also have an osteoporosis-related fracture. Nearly three fourths of these fractures occur in women, with most occurring in White women.
The rate of osteoporotic fractures varies significantly across ethnicities and geography. Blacks have a lower lifetime risk for osteoporotic fracture, approximately half that of Whites. Differences in bone size, bone microarchitecture (thicker trabeculae in Blacks), body composition, calcium absorption in youth, and life expectancy are potential reasons for this phenomenon. Asian Americans and Hispanics have risks of fracture that are intermediate between that of Whites and Blacks, even though Asian Americans have bone mineral densities that generally approximates that of Whites. Indeed, the risk for hip fracture in U.S. Asian men and women is actually equal to or lower than that of U.S. Blacks. These U.S. data are consistent with the global experience as well, because rates of hip fracture in China are lower than those observed in the United States, despite similar bone density at the hip. Differences in hip geometry, physical activity, and diet have been proposed as possible explanations. For unknown reasons, however, rates of hip fracture have actually been increasing in the Far East, whereas they have declined somewhat in the United States. Changing patterns of nutrition and physical activity could be responsible for the former, although the latter observation remains heretofore unexplained.
The most common site of osteoporotic fracture is the spine, which accounts for more than 750,000 fractures annually. Fractures of the proximal femur, which disproportionately confer a greater cost than other osteoporotic fractures, account for approximately 300,000 fractures annually in the United States and nearly 75% of the costs. Additional sites of fracture include the distal forearm, proximal humerus, and pelvis, with the latter two more commonly occurring in elderly people.
The risk for fracture increases markedly with age, , although the pattern of fracture risk differs by skeletal site. The risk for Colles fractures increases until the mid-60s and then plateaus, whereas the risk for hip fractures increases exponentially in woman after the age of 65 years. The risk of vertebral fracture rises earlier than that of hip, but many spine fractures are not clinically apparent and are identified only by radiographic assessment. Although clinically silent, such fractures confer a significant independent risk for future fractures, particularly if they are recent.
Men also have an age-independent increase in the risk of fracture, although the increase in incidence generally lags at least 5 to 10 years behind that of women ( Fig 225-1 ). This sex-based difference in the incidence of fracture is likely related to anatomic differences. Specifically, although men and woman have similar volumetric bone density at a given skeletal site, men’s larger bone size confers an independent mechanical protection against fracture.
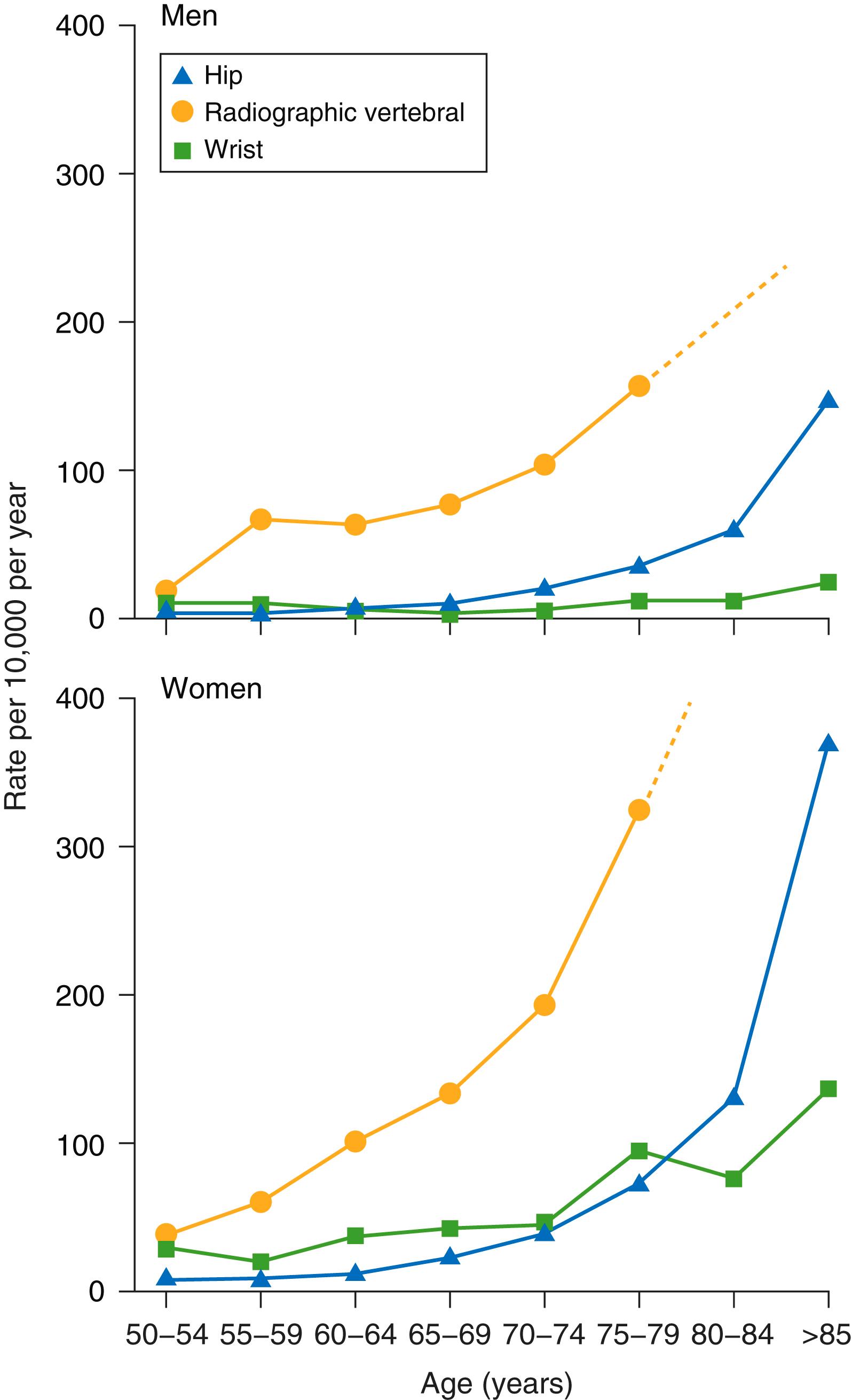
The aging of the U.S. population and the independent contribution of age to the risk of fracture predict that the morbidity and cost related to osteoporosis will increase in the United States alone to greater than 3.2 million fractures, with an associated annual cost of $95 billion by the year 2040. Despite these ominous predictions, less than one third of women who incur a low-trauma hip fracture typically undergo bone density measurement or initiate pharmacologic antifracture therapy, thereby underscoring the need for significant improvement in the delivery of osteoporosis care.
Bone mineral density in adults is determined by the magnitude of bone acquisition during adolescence and young adulthood compared with the rate of bone loss that ensues thereafter. These processes are generally referred to as bone modeling and remodeling, respectively. Heritable factors, including sex and ethnicity, account for 60 to 80% of the variability in skeletal development, including peak bone mass, bone size, and bone geometry, although nutrition, lifestyle, and other factors also have a significant impact. Peak bone mass is achieved in most individuals by the early to late 20s and differs in timing by skeletal site (ages 18 to 20 years for proximal femur, 25 to 30 years for spine). Modeling of the skeleton during this time represents a true increase in bone mass and bone size through endochondral ossification of the axial skeleton and periosteal apposition of the appendicular skeleton.
Skeletal remodeling is a finely orchestrated process of bone resorption and subsequent formation. It is a necessary physiologic function that results in repair of damaged bone and redistribution of the skeleton to adapt to changes in mechanical stress and to provide calcium to the systemic circulation for critical cellular processes. The osteocyte, which accounts for 90 to 95% of all bone cells, is the critical cell that regulates both resorption and formation. Osteocytes are derived from osteoblasts that are embedded within the bone matrix. During this maturation phase, this “osteoid-osteocyte” cell actively secretes and calcifies bone matrix material. In addition, mature osteocytes within bone contain dendritic processes that may directly regulate the recruitment of osteoblasts and formation of the bone. The protein sclerostin, which directly downregulates bone formation through the Wnt pathway, is an important part of this process. Mature osteocytes also may form new bone within their lacunae. In addition, osteocytes produce proteins that regulate mineralization, including positive (PHEX, DMP-1) and negative (FGF-23) factors.
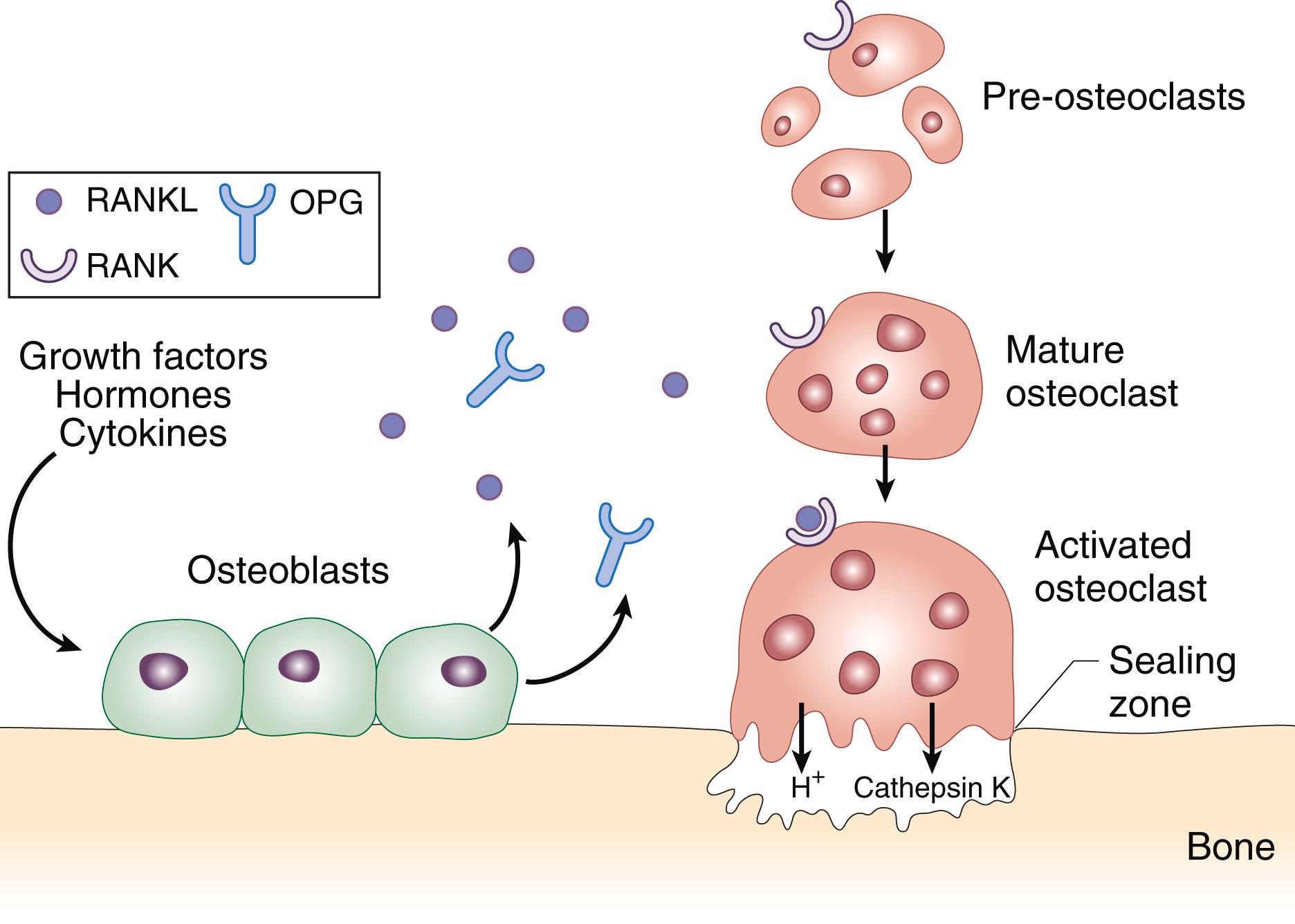
Osteocytes also regulate bone resorption, both directly through apoptosis proximate to skeletal microcracks or fatigue damage in need of repair as well as indirectly through enhancement of pre-osteoblast or mesenchymal stromal cell development. These events result in the production, expression, and release of cytokines that are critical to the recruitment and development of osteoclasts, including interleukin-1 (IL-1), IL-6, osteoprotegerin (OPG), and receptor activator of nuclear factor-κB ligand (RANKL). The cognate receptor for RANKL, RANK, is expressed on the surface of the developing and mature osteoclast, which itself is a derivative of cells of the monocyte-macrophage lineage. RANKL is a critical determinant of osteoclast recruitment, development, and survival, such that disruption of RANKL signaling results in high bone density fragility disorders (e.g., osteopetrosis). OPG, which is also produced by pre-osteoblasts, is a decoy receptor for RANK and binds to RANKL, thereby preventing RANKL binding to RANK serving as an endogenous suppressor of osteoclast function. In essence, RANKL/OPG represent a “yin/yang” paradigm, in which osteoclast biology and bone resorption are intricately regulated and controlled ( E-Fig. 225-1 ).
Peak bone mass is achieved by the third decade. Longitudinal studies in children and adolescents suggest that hormonal, physical activity, nutritional, and genetic factors are important in this process. Hereditary influence is the most important determinant and least well understood. Growth hormone and sex hormones play critical roles in growth of the appendicular (long bones) and axial (vertebrae) skeleton, with the former maturing earlier than the latter (end of puberty vs. young adulthood). Males have larger bones as a result of greater periosteal (outer surface of bone) expansion of bone than occurs in females. Current evidence supports a positive effect of exercise and loading on bone size and mineral density, although a subsequent potential antifracture benefit later in adult life is not proven. Poor nutrition and concomitant disease may also affect bone accrual, primarily by delaying the onset and progression of puberty, although “catch-up” growth, which depends on the degree of insult and timing of resolution, also can occur.
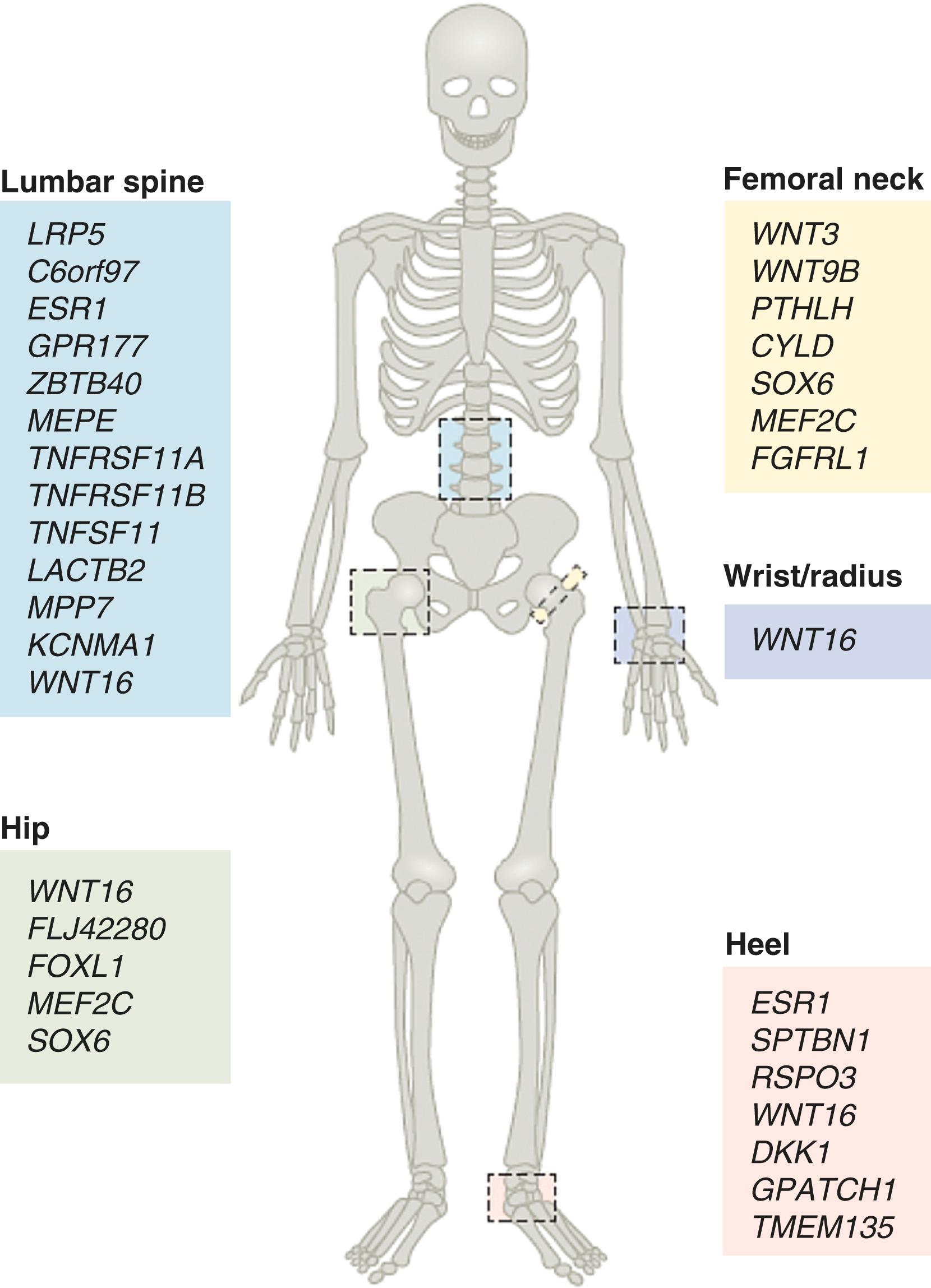
Although these factors are all important, genetic factors appear to account for 60 to 80% of the variance in peak bone mineral density. Genome-wide association studies have identified 90 distinct loci that are significantly associated with bone mineral density. Additionally, the genetic determination of bone mineral density is relatively skeletal site-specific ( E-Fig. 225-2 ). Three obligatory pathways of bone metabolism were initially identified by genome-wide association studies, (i.e., Wnt, RANK-RANKL-OPG, and endochondral ossification), and two additionally pathways (NOTCH and Indian Hedgehog) have also recently been identified, although they may interact through the Wnt pathway. Wnt signaling pathway is obligatory for bone formation, owing to its role in the proliferation and differentiation of osteoblasts. Endochondral ossification, a process that involves the cartilage growth plate and subsequent ossification of the cartilaginous skeleton, is dependent on transcription factors (SOX6, RUNX2) and proteins (parathyroid hormone–related peptide, bone sialoprotein 2, and osteopontin) that are necessary for the development of the cartilage growth plate, for bone matrix mineralization, and for osteoblast differentiation. These three initially identified pathways are now the target of existing and developing therapies for osteoporosis.
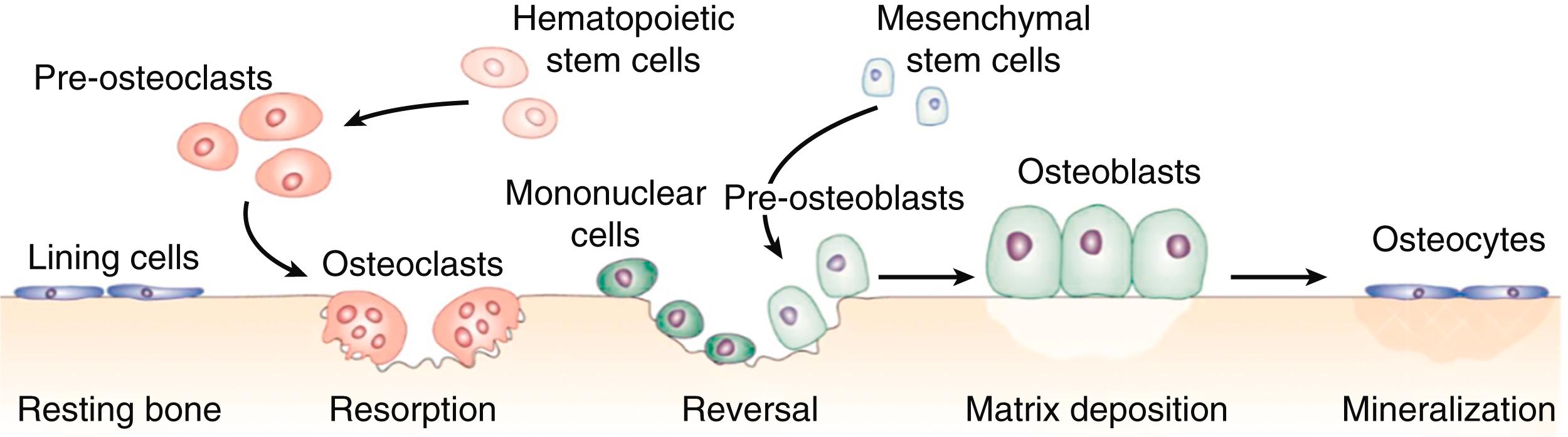
In adults, bone remodeling is a physiologic process through which skeletal repair and adaption to changes in biomechanical stress occur. The bone remodeling cycle occurs in the basic multicellular unit, which itself is composed of bone-resorbing osteoclasts, bone-forming osteoblasts, bone lining cells, and embedded osteocytes and is the apparatus that facilitates remodeling ( E-Fig. 225-3 ). After the age of 30 years, this process is reasonably matched, thereby resulting in rates of bone loss of only 0.3 to 0.5% per year from the third through fifth decades of life. As individuals age, mismatches in bone remodeling (as a result of reduced formation, increased resorption, or a combination of both) result in greater rates of bone loss. Biologic changes, such as menopause ( Chapter 222 ) and aging, systemic disease, personal vices, and medications (most notably glucocorticoids), may contribute to this imbalance. In addition, genetic factors influence the rates of bone loss and fracture.
The rate of net bone loss accelerates during the latter decades of life because of a number of factors. Perhaps most important for women, rates of bone loss increase substantially in the perimenopausal and early postmenopausal years ( Chapter 222 ), amounting to losses of 1 to 5% per year. Unfortunately, no biologic markers currently can determine which women a priori are “rapid losers.” Bone loss is slower in obese women, likely because of higher estrogen levels afforded by production through aromatization in adipose tissue. Following both natural and surgical menopause, a decline in circulating estrogen is primarily responsible for bone loss, which is mediated primarily through upregulation of cytokines (most notably RANKL) and a resultant increase in the number, activity, and depth of osteoclast-mediated bone resorption sites. In addition, the production of osteoprotegerin is diminished, thereby further amplifying bone resorption, although estrogen replacement can restore the production of osteoprotegerin while reducing RANKL expression and thereby help mitigate bone loss during this period. Although bone resorption and formation occur sequentially during this period, resorption outpaces formation because of potentiation of the former aided by the release of soluble cytokines, thereby resulting in a significant uncoupling of bone remodeling and accelerated bone loss. Fortunately, this phase of rapid bone loss is typically limited to 2 to 3 years in most women.
Evidence also supports a key role for reduced estrogen levels in men, perhaps because reduced aromatase activity in fat tissue leads to loss of bone and an increased risk of fracture. With aging, declines in circulating insulin-like growth factor-I levels also may inhibit the production of mesenchymal stromal cells and pre-osteoblasts, in addition to limiting periosteal bone expansion. Bone loss in later decades also is caused by lower 25-hydroxyvitamin D (25[OH]D) levels, often related to restricted sun exposure and a reduced capacity to generate previtamin D in the skin with aging, as well as limited dietary intake of vitamin D and calcium. Low vitamin D results in secondary hyperparathyroidism ( Chapter 227 ), which accelerates cortical bone loss, as well as likely increasing the risk for falls. Increase in bone marrow adiposity is also inversely and significantly correlated with aging-related bone loss, in part because fewer osteoblast precursors are formed. Finally, loss of weight and muscle mass (sarcopenia) is associated with bone loss, with evidence suggesting that a common factor (myostatin) may provide insights on this muscle-bone interaction.
Bone loss also occurs as a result of secondary processes, such as diseases and medications (see Table 225-1 ), which can be identified in more than one fourth of individuals with osteoporosis. Endocrine disorders, including hypogonadism ( Chapters 216 and 217 ) and Cushing syndrome ( Chapter 208 ), predominate, but malabsorption ( Chapter 126 ), chronic liver disease ( Chapter 139 ), and certain medications (e.g., antiepileptic drugs such as phenytoin) are also implicated. Glucocorticoids, whether via exogenous administration or endogenous overproduction, reduce bone formation by reducing the function and survival of osteoblasts and osteocytes. Rarely, malignancies can be the principal cause of osteoporosis, with the best example being multiple myeloma ( Chapter 173 ), which causes bone loss through uncoupling of bone resorption and formation. Anorexia nervosa ( Chapter 200 ) interferes with the anabolic effect of insulin-like growth factor-I on bone. Alcohol intake ( Chapter 364 ) of only 1 or 2 drinks per day increases the risk for osteoporosis, likely through suppression of osteoblast function, both directly and indirectly through associated malnutrition, as well as possibly through hypogonadism-associated bone loss from accelerated bone turnover. Tobacco use ( Chapter 363 ) may also accelerate bone loss, owing to global effects that include reduced sex hormones, altered calcium metabolism, and weight loss and frailty, resulting in uncoupled bone remodeling. Finally, relatively rare connective tissue disorders, such as osteogenesis imperfecta ( Chapter 229 ) and Marfan syndrome ( Chapter 239 ), increase skeletal fragility by disturbing the skeletal matrix and mechanical integrity rather than altered bone remodeling.
| ENDOCRINE DISORDERS |
| Hypogonadism: female and male Hypercortisolism: endogenous and exogenous Hyperthyroidism Hyperparathyroidism Idiopathic hypercalciuria Diabetes mellitus: type 1 and type 2 |
| NUTRITIONAL AND GASTROINTESTINAL DISORDERS |
| Malabsorption: celiac disease, gastrointestinal bypass Vitamin D deficiency Cirrhosis (including primary biliary cirrhosis) Pancreatic insufficiency Inflammatory bowel disease Cystic fibrosis Anorexia nervosa, bulimia |
| HEMATOLOGIC AND ONCOLOGIC DISORDERS |
| Multiple myeloma Hemolytic anemia Hemoglobinopathies: thalassemia, sickle cell Myeloproliferative neoplasms Skeletal metastases Mastocytosis |
| CONNECTIVE TISSUE AND METABOLIC DISORDERS |
| Osteogenesis imperfecta Ehlers-Danlos syndrome Marfan syndrome Homocystinuria Gaucher disease Pompe disease |
| MEDICATIONS AND EXPOSURES |
| Glucocorticoids Aromatase inhibitors Thyroxine (excessive) Antiepileptics Heparin Gonadotropin-releasing hormone agonists Depo-Provera Immunosuppressants: tacrolimus, cyclosporine Chemotherapy Selective serotonin reuptake inhibitors ∗ Proton pump inhibitors ∗ Thiazolidinediones ∗ Sodium glucose cotransporter 2 inhibitors ∗ Alcohol |
| MISCELLANEOUS |
| Rheumatoid arthritis Immobilization Juvenile osteoporosis Pregnancy-associated osteoporosis |
The age at which bone loss occurs (e.g., young adult vs. an 80-year-old) affects the ability to recover bone mineral density with resolution or treatment of the condition (younger people have more robust recovery than elderly people) and also appears to hold for conditions of accelerated bone resorption (e.g., immobilization) or reduced bone formation (e.g., glucocorticoids). Predicated on and paralleling this observation, the response of bone mineral density to treatment is generally more pronounced in individuals with higher rates of bone turnover, likely reflecting to some extent a “filling in” of bone remodeling space. Menopausal stage in women also influences the rate of bone loss, with greater rates of decline occurring within the first 2 to 3 years of cessation of menses ( Chapter 222 ). A faster rate of bone mineral density decline in the lumbar spine in early menopause also predicts a higher risk of fracture. Concomitant vitamin D deficiency and resulting secondary hyperparathyroidism also potentiate rates of bone loss, particularly in elderly people.
Most fragility fractures, which are defined as fractures occurring from the energy imparted from a fall from a standing height or less, occur in individuals who have low (osteopenia) or even normal bone mineral density. Given these observations, it is clear that other factors (e.g., falls and traumatic injuries) must also significantly influence the risk of fracture. Excluding falls and trauma, however, studies to date have also identified qualitative factors that are integral to bone strength, including skeletal microarchitecture, bone turnover, the accumulation of damage (e.g., microfractures), and the pattern or degree of mineralization. Newer technologies (including high-resolution peripheral quantitative computed tomography and high-resolution magnetic resonance imaging [MRI]) may improve the understanding of how these qualitative changes in bone compromise skeletal strength ( Fig. 225-2 ).
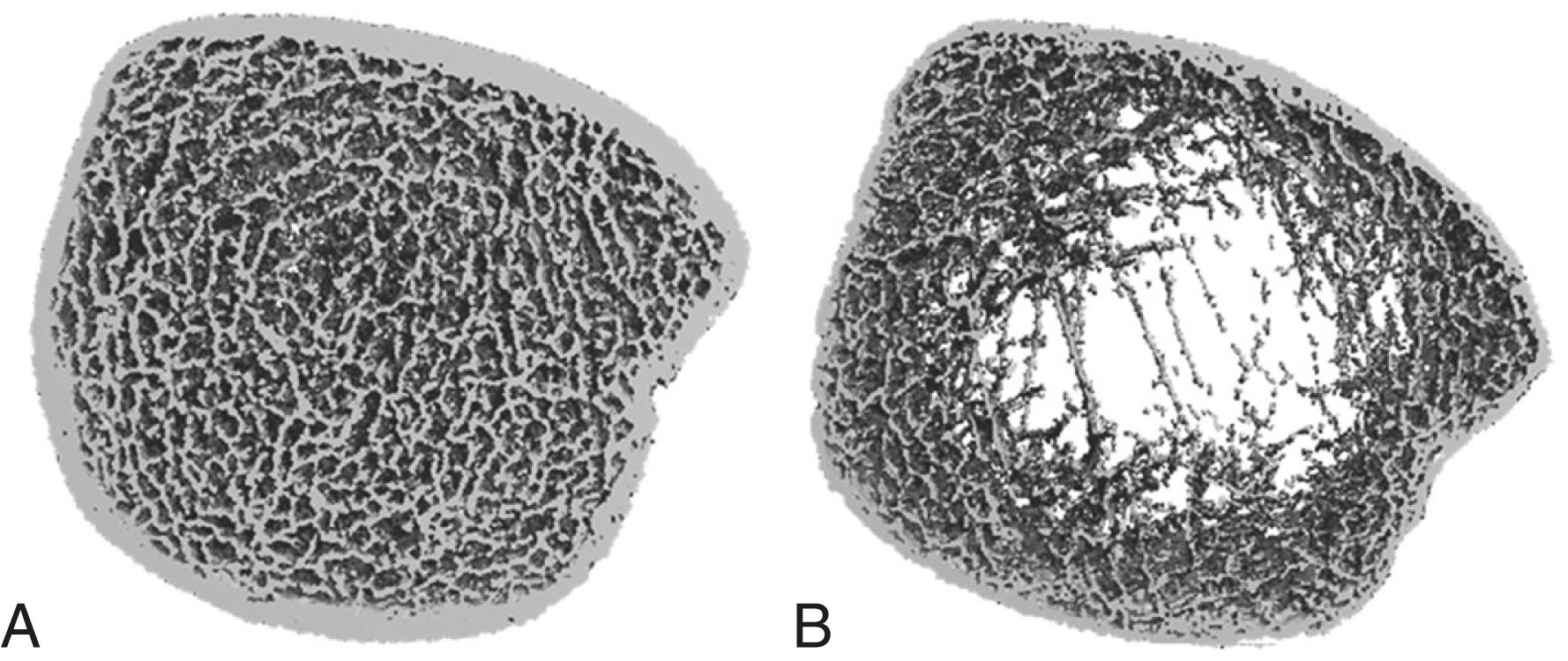
Low bone mass in and of itself does not cause pain, unless it is due to osteomalacia ( Chapter 226 ). Because osteoporosis typically antedates clinical symptoms by many years, most osteoporotic individuals are asymptomatic.
Osteoporosis may be diagnosed in an individual who presents with a fragility fracture, typically of the vertebrae, proximal femur, proximal humerus, or distal forearm. Other sites that are subject to fragility fractures include the pelvis, ribs, and proximal tibia. Nevertheless, even a high-trauma fracture in a postmenopausal woman or elderly man should cause suspicion for osteoporosis.
Fractures of the spine, which are often asymptomatic, are generally from the midthoracic region through the lower lumbar region, with the greatest frequency at T11 through L2. Patients often present after a fall or a spinal flexion-loading event, in which they may hear a “pop” and complain of sharp midline back pain that may radiate to the flanks. Patients may also present with complaints of back “tiredness,” which is improved with sitting or lying down; this symptom is likely related to paraspinal weakness or spasm from abnormal spinal curvature that occurs with chronic vertebral compression. Back pain may commonly be related to other pathology ( Chapter 369 ), such as degenerative disc and spine disease that is concomitantly present.
Vertebral fractures often occur without an obvious acute precipitant. In contrast, nonvertebral fracture events are always clinically evident. Hip fractures generally occur with falls, although they may rarely occur with limited force such as twisting.
Physical examination may indicate the presence of osteoporosis and associated fractures, as well as potentially identifying underlying secondary processes contributing to the disease. Measured height loss, best confirmed using a calibrated device such as a stadiometer, of greater than 4 cm since young adult maximum height is suggestive of prior vertebral fractures. Height loss also occurs with scoliosis and aging (approximately 1⁄3 inch of height is lost per decade after age 50 years). A kyphotic deformity ( Chapter 86 ) of the upper thoracic spine may be present, although it is important to distinguish it from accentuated cervical lordosis with associated prominence of T1. Spinal tenderness to palpation and percussion can occur with an acute vertebral compression fracture. Palpable tenderness of the long bones may suggest underlying osteomalacia ( Chapter 226 ) with periosteal expansion and nerve irritation. Reduced rib-pelvis and increased wall-occiput distances (<2 and >0 fingerbreadths, respectively) also are correlated with vertebral fractures as well.
In symptomatic patients, the diagnosis of osteoporosis is commonly made by radiographs of the involved site. Radiologic findings also may identify the presence of osteoporosis, serendipitously when obtained for other reasons in asymptomatic patients with osteoporosis.
Plain films can detect bone loss by means of accentuation of vertical striations on spine radiographs that represent loss of horizontal trabeculae, although this generally indicates bone density loss of at least 25% or more. Kyphosis and compression fractures may be present, and patients often are unaware of the deformities because nearly three fourths of such fractures occur without acute pain. Lateral chest radiographs and computed tomographic (CT) scans often identify such fractures ( Fig. 225-3 ). The degree of compression fracture is also important because more severe fractures (>25% vertebral height loss) especially appear to predict future vertebral fractures.
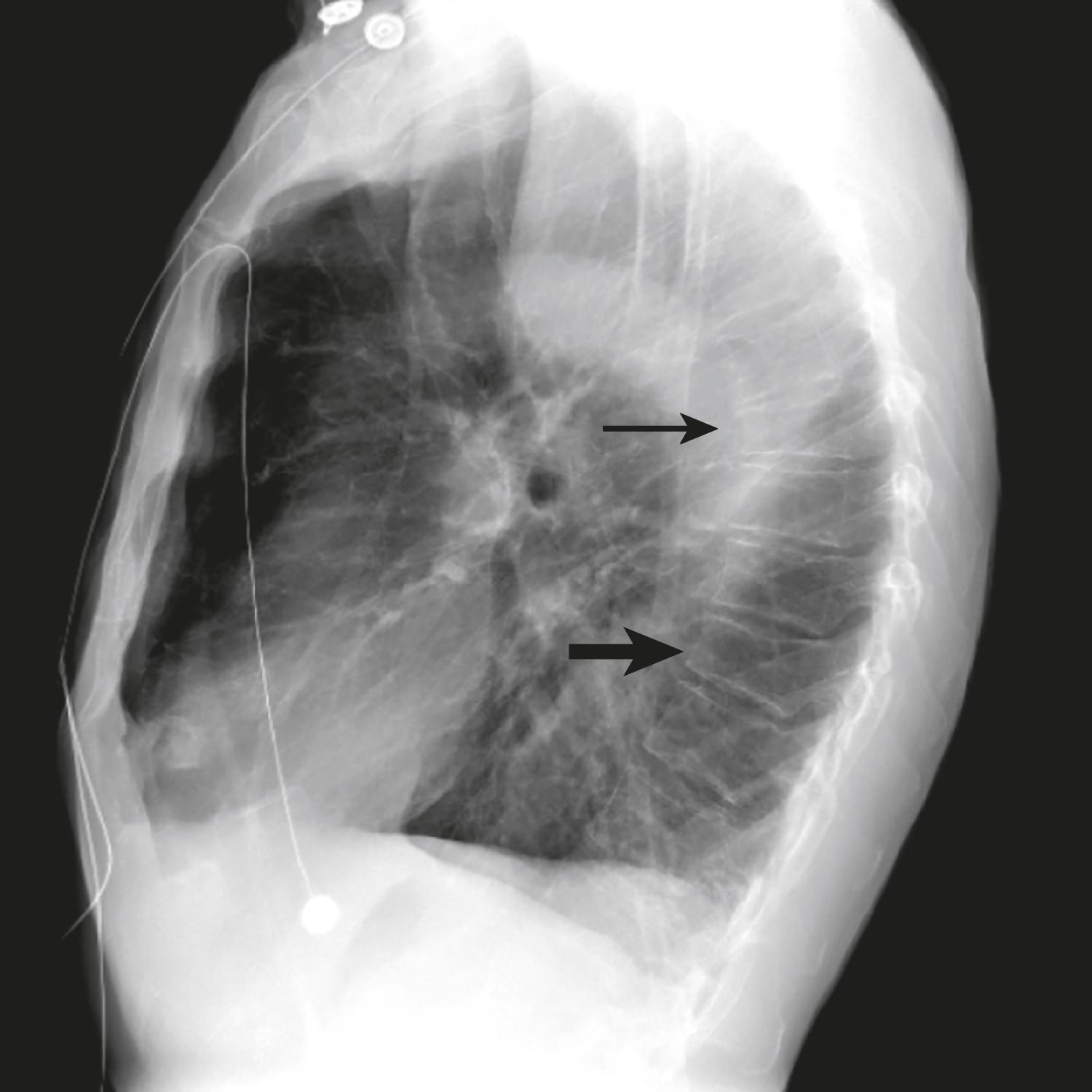
When recent fractures are suspected, CT and MRI may be used, given that plain radiographs have a lower sensitivity acutely and for detecting stress fractures. MRI also can be used to define a vertebral fracture with persistent swelling and edema based on T2 characteristics, thereby potentially identifying patients who could benefit from vertebroplasty or kyphoplasty. Finally, whole-body bone scintigraphy is the most sensitive test for detecting fracture within the past 6 to 12 months but can be falsely positive because of inflammation, infection, or tumor.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here