Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
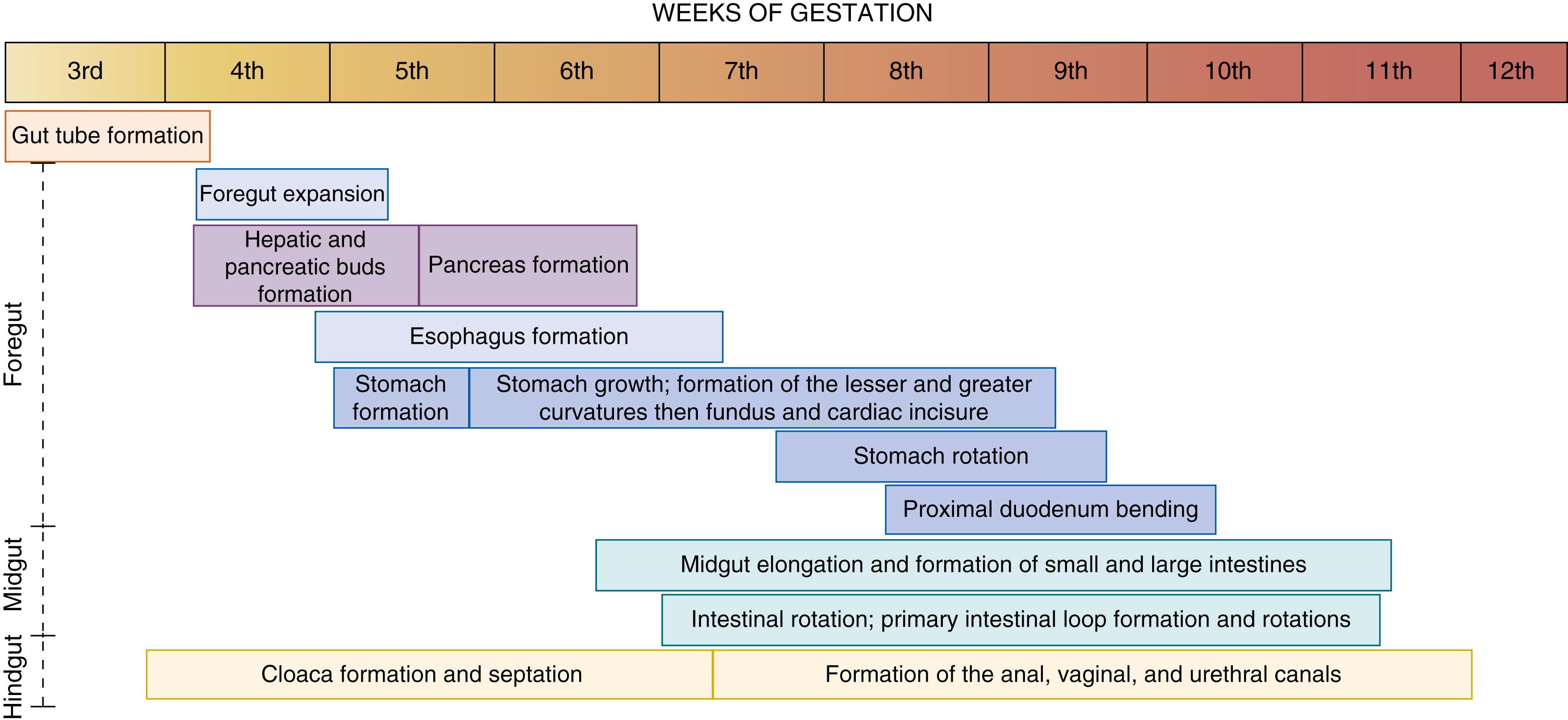
Development of the gastrointestinal tract involves crucial processes, including endoderm formation and patterning along the anterior-to-posterior, dorsoventral, and left-right axis; gut tube morphogenesis into the foregut, midgut, and hindgut domains; assembly of mesenchyme, epithelial morphogenesis, and cytodifferentiation. The organogenesis of the gut tube and its derivatives starts around the third week of gestation from a primitive abdominal tube. From the initiation of gut tube formation until approximately the 12th week of gestation, the gut undergoes a series of events that will define the specific segments and organs of the digestive tract ( Fig. 82.1 ). During the third trimester, organogenesis of the digestive tract is complete, but the intestines undergo rapid growth, resulting in a doubling of intestinal length that will continue over the first several years of life. In this chapter, we will detail the events occurring during the organogenesis of the digestive tract. We will emphasize the embryonic mechanisms, give a brief overview of the molecular pathways involved in the orchestration of the digestive tract organ formation, and describe the associated organogenesis defects.
Around the second week of gestation, gastrulation starts in the epiblast with the formation of the primitive streak (Carnegie stage 6b). Two primitive germ layers arise by ingression and migration through the primitive streak to form endodermal and mesodermal layers below the epiblast; cells that do not migrate through the streak become the ectoderm, the third and last primitive germ layer.
During the third and fourth weeks, the three germ layers begin to fold into an elongated cylinder. The gut tube is first seen as pockets in the anterior (rostral) and posterior (caudal) aspect of the embryo, termed the embryonic intestinal portals, which are the primordial anlage of the foregut and hindgut. At this stage (Carnegie stage 9) the midgut has not yet formed a tube and is open to the yolk sac. As the embryo grows, the anterior and posterior intestinal portals deepen and extend toward the center of the embryo, and the lateral portions of the midgut fold toward the midline. This primitive gut consists of a blind-ending tube with the foregut terminating cranially in the buccopharyngeal membrane and the hindgut terminating caudally in the cloacal membrane. , , The embryonic gut tube is lined by endoderm and surrounded by splanchnic mesoderm; the ectodermal contributions to the gut are derived from migrating neural crest cells that delaminate primarily from the vagal neural crest, with a smaller contribution from the sacral neural crest.
By convention, the boundaries of the foregut, midgut, and hindgut correspond to the three arteries that supply the abdominal digestive tube: the celiac axis supplies the foregut, the superior mesenteric artery supplies the midgut, and the inferior mesenteric artery supplies the hindgut. The foregut gives rise to the anterior aspect of the digestive tube, including the pharynx and oral cavity, the esophagus, the stomach, and the upper duodenum. The foregut also gives rise to the endoderm-derived organs, including the thyroid, lungs, liver and biliary system, and pancreas. The midgut develops into the distal portion of the duodenum, jejunum, ileum, cecum, appendix, and proximal portion of the transverse colon. The hindgut gives rise to the distal portion of the transverse colon, descending colon, sigmoid colon, and rectum. The hindgut also gives rise to urogenital sinus derivatives, including the vaginal and urethral tracts. In addition, the gut tube regions are also defined by their specific structure, function, cell types, and gene expression. The establishment of the gastrointestinal territories is dependent on the rostrocaudal gradients of morphogen signals—Wnt, bone morphogenetic protein (BMP), and fibroblast growth factor (FGF)—provided by the mesoderm, as well as the expression of regional transcription factors (e.g., Hox-family and Caudal-family homeoproteins) in both the mesoderm and the endoderm. ,
During the fourth week of gestation the anterior intestinal portal of the primitive gut grows and forms an invagination that will constitute the foregut. The definitive endoderm is patterned along an anterior-posterior axis maintained by region-specific transcription factors—that is, the anterior portion of the foregut expresses hematopoietically expressed homeobox (HHEX), sex determining region Y (SRY)-box 2 (SOX2), and forkhead box A2 (FOXA2) proteins, whereas the posterior portion of the foregut expresses caudal-type homeobox 1, 2, and 4 (CDX1, CDX2, and CDX4), and pancreatic and duodenal homeobox 1 (PDX1) proteins. From the foregut arise portions of the digestive tract: the pharynx, the esophagus, the stomach, the pylorus, and the anterior half of the duodenum. Also, the thyroid, the liver, the tracheal apparatus and lungs and the pancreas originate from the foregut. The precursors of both thyroid and lung buds are localized to the ventral midline of the foregut. Precursor cells of the liver and pancreas are localized at multiple sites in the foregut endoderm. Endodermal buds form the liver parenchyma and hepatic ducts, gallbladder and common bile ducts, and dorsal and ventral pancreatic buds.
The esophagus connects, cranially, to the posterior end of the pharynx at the pharyngoesophageal junction and, caudally, to the stomach at the orifice of the cardia of the stomach. The average length of the esophagus ranges from 8 to 10 cm in the newborn, increasing to 18 to 26 cm in the adult. The esophagus lies ventrally to the vertebral column and dorsally to the trachea. ,
Both the esophagus and the trachea are derived from the anterior portion of the foregut. At around 4 weeks (22 to 23 days of gestation), a median ventral diverticulum, the tracheal diverticulum, appears in the posterior region of the foregut. The tracheal diverticulum elongates with the proliferation of endodermal cells. Lateral ridges are formed, creating a separation between the trachea and the esophagus, the esophagotracheal septum, occurring at 34 to 36 days of gestation. The tracheal diverticulum ultimately becomes the primitive respiratory tract. As these tracheoesophageal folds continue to develop, the caudal part of the foregut forms a spindle-shaped dilation that becomes the stomach. The developing esophagus is localized between the caudal foregut dilation and the tracheal diverticulum. Elongation of the developing esophagus occurs first cranially and then caudally and reaches its final relative length by 7 weeks of gestation.
The tracheoesophageal separation of the early foregut is controlled by signaling molecules and transcription factors that follow a specific dorsoventral patterning. Studies in the mouse reported several transcription factors involved in the anterior foregut separation. SOX2 is expressed within the epithelial cells of the dorsal foregut, whereas the NK2 homeobox 1 (NKX2-1, also known as thyroid transcription factor [TTF1] ) is expressed in the ventral foregut, thus demarcating the separation between the presumptive ventral tracheal and esophageal diverticula. Also, the separation of the esophagus from the early foregut is governed by several signaling pathways. The Sonic hedgehog (Shh) pathway induces foregut separation. In humans, Shh mutation leads to an abnormal foregut separation as seen in the VACTERL association. The temporal Shh expression, from the ventral to the dorsal foregut endoderm, orchestrates the foregut separation. , Genetic deletion of Shh pathway effectors such as GLI family zinc finger 1-3 (GLI1-GLI3) or forkhead box F1 (FOXF1) causes abnormal foregut separation and esophageal atresia. , Whereas Shh is expressed by the epithelium, GLI1, GLI2, GLI3, and FOXF1 are expressed by the mesenchyme, highlighting an example of the continuous endoderm-mesoderm crosstalk that occurs during organogenesis of the digestive system. Wnt signaling is also essential to the generation of the esophagus. Several Wnt ligands have been identified both at the ventral side of the mesenchymal and at the endodermal foregut. For example, the ligands WNT2 and WNT2b are expressed in the mesenchyme and their simultaneous inhibition blocks the tracheoesophageal separation. Importantly, epithelial inhibition of the Wnt pathway increased the expression of SOX2 at the ventral foregut despite NKX2-1 expression, resulting in an unseparated foregut. , Similarly to Wnt the BMP pathway forms a dorsoventral gradient where BMP4 and BMP7 are enriched at the ventral side of the foregut and the BMP inhibitor Noggin is enriched at the dorsal side. The disruption of this morphogenetic gradient leads to tracheoesophageal defects. BMP signaling also controls the expression of SOX2 within the foregut. , Retinoic acid signaling is also critical for the specification of both the trachea and the esophagus. Retinoic acid activity is seen in the anterior portion of the foregut both in mesenchyme and in epithelium and its alteration leads to foregut developmental anomalies. Moreover, a dorsoventral expression pattern exists for retinoic acid receptors. For example, retinoic acid receptor β 2 in mice is localized to the dorsal epithelium and becomes more restricted during the tracheoesophageal separation. Genetic deletion of retinoic acid receptors abrogates the tracheoesophageal separation.
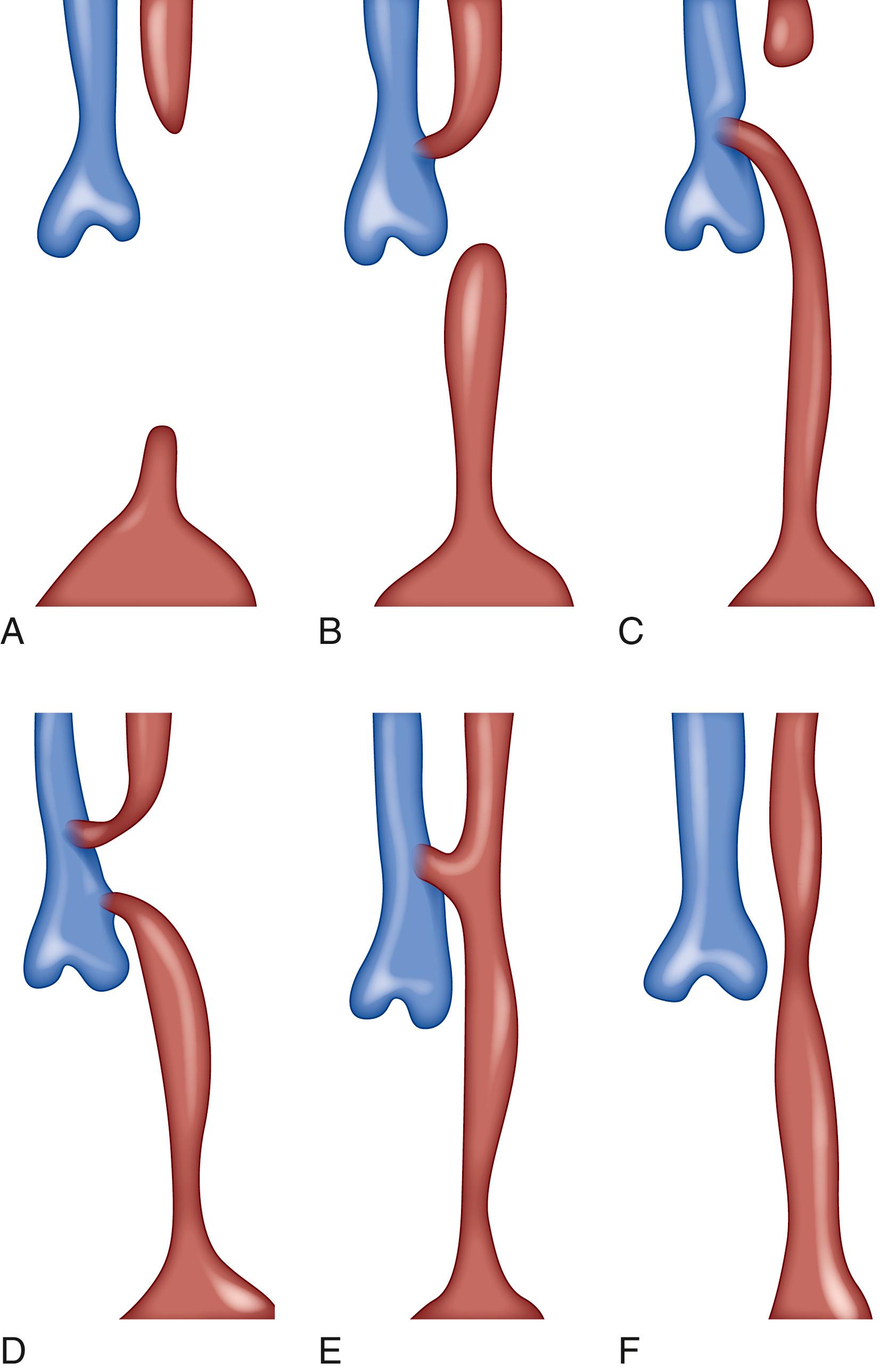
Esophageal atresia and tracheoesophageal fistula are commonly found in association and represent the most frequent congenital esophageal abnormalities. This defect results from the abnormal separation of the tracheal diverticulum from the foregut. The esophageal atresia is an interruption of the esophageal continuity resulting from a recanalization defect of the primitive gut during the eighth week. Esophageal atresia with tracheoesophageal fistula occurs in 1 in 3000 to 1 in 5000 live births. Five types of esophageal atresia with or without fistula have been classified. The most common is type C—esophageal atresia with distal tracheoesophageal fistula—with an incidence of 86.5% ( Fig. 82.2 ). ,
Esophageal stenosis is a narrowing of the esophagus that can be more frequently found in the middle to distal third of the esophagus and presents as a web (membranous diaphragm) or a long segment of narrowed esophagus (fibromuscular stenosis). This anomaly results from incomplete recanalization of the esophagus during the eighth week. It may also result from the absence of vascular ingrowth within the area or an incomplete tracheoesophageal separation as suggested by the presence of respiratory tissue. The incidence of this congenital defect is 1 per 25,000 live births. This type of esophageal stenosis has been classified into three groups. Another form of esophageal stenosis called esophageal ring is found within the lower third of the esophagus and is created by the presence of concentric extension of the normal esophageal tissue. These rings may result from an incomplete vacuolization of the columnar epithelium during the eighth week. Three types have been classified, with type B or Schatzki’s ring the most common.
Duplications can be found in direct communication with the esophagus or they may lie within the mediastinum completely separate from the esophagus. The origin of those intramural duplications within the esophagus could be caused by a failure of the early foregut to become completely vacuolated. The duplication originates from the foregut and thus could be attached to the esophagus or the tracheobronchial system. These duplications develop independently and are rarely in continuity with the esophagus but are contiguous with some gastrointestinal segments.
Esophageal webs are thin transverse membrane consisting of a mucosal and submucosal layer covered by normal squamous epithelium. These webs are more frequently found in the cervical esophagus associated with heterotopic gastric mucosa. The webs may result from the incomplete vacuolization of the columnar epithelium.
The stomach is cranially connected to the esophagus and caudally connected to the duodenum by the pylorus. The average volume of the stomach is about 30 mL in the newborn, increasing to 1.5 to 2 L in the adult. The stomach is divided into four regions: the cardia, the fundus, the body, and the antrum.
The stomach arises during the fourth week from the dilation of the posterior portion of the foregut. At 26 days of gestation, the foregut elongates to form a primitive stomach that expands in a spindle-shaped structure. During the fifth week the dorsal part of the stomach expands further than the ventral part, thus resulting in the formation of the greater and the lesser curvatures. By the end of the seventh week the expansion of the superior part of the greater curvature results in the formation of the fundus and the cardiac incisure. During the seventh and eighth weeks the stomach undergoes a 90-degree clockwise rotation (toward subject’s left) around an anterior-posterior axis positioning the greater curvature to the left side and the lesser curvature to right side. The stomach also rotates slightly around a dorsoventral axis so the greater curvature is slightly upward and the lesser curvature downward. By approximately eight weeks of gestation, formation of the stomach is complete.
During the formation of the stomach, anterior-posterior boundaries are maintained by specific transcriptional factors. SOX2 is expressed in the foregut epithelium and BarH-like homeobox 1 (BARX1) is expressed in the foregut mesenchyme. Both transcription factors block the intestinal program relying on Wnt signaling and the activation of the transcription factor CDX2. , Thus they establish the distinction between the stomach and the intestines. Also, other transcription factors are expressed along the anterior-posterior axis to delineate the forestomach and the posterior portion of the stomach. For example, the nuclear receptor COUP transcription factor 2 (NR2F2) is highly expressed in the proximal portion of the gastric mesenchyme and its deletion in the mouse induces a posteriorization of the forestomach. In contrast, Bagpipe homeobox protein homologue 1 (BAPX1, also known as NK3 Homeobox 2 [NKX3-2] ) is expressed in the distal mesenchyme and maintains a normal antrum to the duodenum by the pyloric boundaries. BMP signaling is also established gradually along an anterior-posterior axis where it is posteriorly localized. , PDX1 is expressed in the distal portion of the foregut, demarcating the pancreatic, gastric antral, and proximal duodenal regions. FGF10, produced by the mesenchyme, and its epithelial receptor fibroblast growth factor receptor 2 (FGFR2) act synergistically to control stomach morphogenesis and gland formation. , Crosstalk between the epithelium and the mesenchyme and these regional transcription factors creates and maintains the boundaries within the stomach. Studies in mice have also suggested that BARX1 enhances gastric smooth muscle development along with the expression of left-right transcription factors (ISL LIM homeobox 1 [ISL1], paired-like homeodomain 1 and 2 [PITX1 and PITX2] and Sine oculis homeobox homolog 2 [SIX2]), thus placing the stomach in its final position during the intestinal rotation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here