Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The human brain is the most complex tissue in the body. It mediates behavior ranging from simple movements and sensory perception to learning, memory, and consciousness. It is the organ of the mind and accounts for the human capacity for invention, discovery, and language. Many of the brain's functions are poorly understood. In fact, the most prominent function of the human brain, its capacity to think, is hardly understood at all. Its capacity to reflect upon itself is a philosophical paradox. Our lack of knowledge about fundamental aspects of brain function stands in marked contrast to the level of comprehension that we have about the primary functions of other organ systems such as the heart, lungs, and kidneys. Nevertheless, tremendous strides have been made in the past few decades.
In this part of the book, we present the physiology of the nervous system in a manner that is intended to be complementary to texts on neurobiology and neuroanatomy. In this chapter, we review the basic cellular, developmental, and gross anatomy of the nervous system. In Chapter 11 , we discuss the fluid environment of the neurons in the brain, how this environment interacts with the rest of the extracellular fluid of the body, and the role of glial cells. Chapters 12 and 13 focus on the broad physiological principles that underlie how the brain's cellular elements operate. Another major goal of this section is to provide more detailed information on those parts of the nervous system that play key roles in the physiology of other systems in the body. Thus, in Chapter 14 , we discuss the autonomic nervous system, which controls viscera such as the heart, lungs, and gastrointestinal tract. Finally, in Chapters 15 and 16 , we discuss the special senses and simple neuronal circuits.
The manner in which the nervous system is subdivided is somewhat arbitrary. All elements of the nervous system work closely together in a way that has no clear boundaries. Nevertheless, the traditional definitions of the subdivisions provide a useful framework for talking about the brain and its connections, and are important if only for that reason.
The central nervous system (CNS) consists of the brain and spinal cord ( Table 10-1 ). It is covered by three “membranes”—the meninges. The outer membrane is the dura mater; the middle is the arachnoid; and the delicate inner membrane is called the pia mater. Within the CNS, some neurons that share similar functions are grouped into aggregations called nuclei. The CNS can also be divided into gray matter, which contains neuron cell bodies, and white matter, which is rich in myelin (see pp. 199–201 ).
| SUBDIVISION | COMPONENTS | SPECIAL FEATURES |
|---|---|---|
| Central | Brain (including CN II and retina) and spinal cord | Oligodendrocytes provide myelin Axons cannot regenerate |
| Peripheral | Peripheral ganglia (including cell bodies); sensory receptors; peripheral portions of spinal and cranial nerves (except CN II), both afferent and efferent | Schwann cells provide myelin Axons can regenerate |
| Autonomic | Selected portions of the CNS and PNS | Functionally distinct system |
The peripheral nervous system (PNS) consists of those parts of the nervous system that lie outside the dura mater (see Table 10-1 ). These elements include sensory receptors for various kinds of stimuli, the peripheral portions of spinal and cranial nerves, and all the peripheral portions of the autonomic nervous system. The sensory nerves that carry messages from the periphery to the CNS are termed afferent nerves (from the Latin ad + ferens [carrying toward]). Conversely, the peripheral motor nerves that carry messages from the CNS to peripheral tissues are called efferent nerves (from the Latin ex + ferens [carrying away]). Peripheral ganglia are groups of nerve cells concentrated into small knots or clumps that are located outside the CNS.
The autonomic nervous system (ANS) is that portion of the nervous system that regulates and controls visceral functions, including heart rate, blood pressure, digestion, temperature regulation, and reproductive function. Although the ANS is a functionally distinct system, it is anatomically composed of parts of the CNS and PNS (see Table 10-1 ). Visceral control is achieved by reflex arcs that consist of visceral afferent (i.e., sensory) neurons that send messages from the periphery to the CNS, control centers in the CNS that receive this input, and visceral motor output. Moreover, visceral afferent fibers typically travel together with visceral efferent fibers.
Nervous tissue is composed of neurons and neuroglial cells. Neurons vary greatly in their structure throughout the nervous system, but they all share certain features that tailor them for the unique purpose of electrical communication (see Chapter 12 ). Neuroglial cells, often simply called glia, are not primary signaling cells and have variable structures that are suited for their diverse functions (see Chapter 11 ).
The human brain contains ~10 11 neurons and slightly more glial cells. Each of these neurons may interact with thousands of other neurons, which helps explain the awesome complexity of the nervous system.
Few, if any, of the receptors, ion channels, or cells in the human brain are unique to humans. The unparalleled capabilities of the human brain are presumed to result from its unique patterns of connectivity and its large size.
The brain's diverse functions are the result of tremendous regional specialization. Different brain areas are composed of neurons that have special shapes, physiological properties, and connections. One part of the brain, therefore, cannot substitute functionally for another part that has failed. Any compensation of neural function by a patient with a brain lesion (e.g., a stroke) reflects enhancement of existing circuits or recruitment of latent circuits. A corollary is that damage to a specific part of the brain causes predictable symptoms that enable a clinician to establish the anatomical location of the problem, a key step in diagnosis of neurological diseases.
In 1838, Schleiden and Schwann proposed that the nucleated cell is the fundamental unit of structure and function in both plants and animals. They reached this conclusion by microscopic observation of plant and animal tissues that had been stained to reveal their cellular composition. However, the brain proved to be more difficult to stain than other tissues, and until 1885, when Camillo Golgi introduced his silver-impregnation method, “the black reaction,” there was no clear indication that the brain is composed of individual cells. The histologist Santiago Ramón y Cajal worked relentlessly with the silver-staining method and eventually concluded that not only is nervous tissue composed of individual cells, but the anatomy of these cells also confers a functional polarization to the passage of nervous signals; the tapering branches near the cell body are the receptive end of the cell, and the long-axis cylinder conveys signals away from the cell. In the absence of any reliable physiological evidence, Ramón y Cajal was nevertheless able to correctly anticipate how complex cell aggregates in the brain communicate with each other.
The pathologist Heinrich von Waldeyer referred to the individual cells in the brain as neurons. He wrote a monograph in 1891 that assembled the evidence in favor of the cellular composition of nervous tissue, a theory that became known as the neuron doctrine. It is ironic that Golgi, whose staining technique made these advances possible, never accepted the neuron doctrine, and he argued vehemently against it when he received his Nobel Prize along with Ramón y Cajal in 1906. ![]() N10-1 The ultimate proof of the neuron doctrine was established by electron microscopic observations that definitively demonstrated that neurons are entirely separate from one another, even though their processes come into very close contact.
N10-1 The ultimate proof of the neuron doctrine was established by electron microscopic observations that definitively demonstrated that neurons are entirely separate from one another, even though their processes come into very close contact.
For more information about Santiago Ramón y Cajal and the work that led to his Nobel Prize, visit http://www.nobel.se/medicine/laureates/1906/index.html (accessed October 2014).
Neurons are specialized for sending and receiving signals, a purpose reflected in their unique shapes and physiological adaptations. The structure of a typical neuron can generally be divided into four distinct domains: (1) the cell body, also called the soma or perikaryon; (2) the dendrites; (3) the axon; and (4) the presynaptic terminals ( Fig. 10-1 ). The shape and organelle composition of these domains depends strongly on their cytoskeleton, which consists of three fibrillary structures: neurofilaments (i.e., intermediate filaments; see p. 23 ), microtubules (see pp. 23–25 ), and thin filaments (see pp. 25–28 ). The cytoskeleton—especially the microtubules and thin filaments, is dynamic and imbues axons and dendrites with the capacity to change shape, a plasticity believed to participate in the synaptic alterations associated with learning and memory.
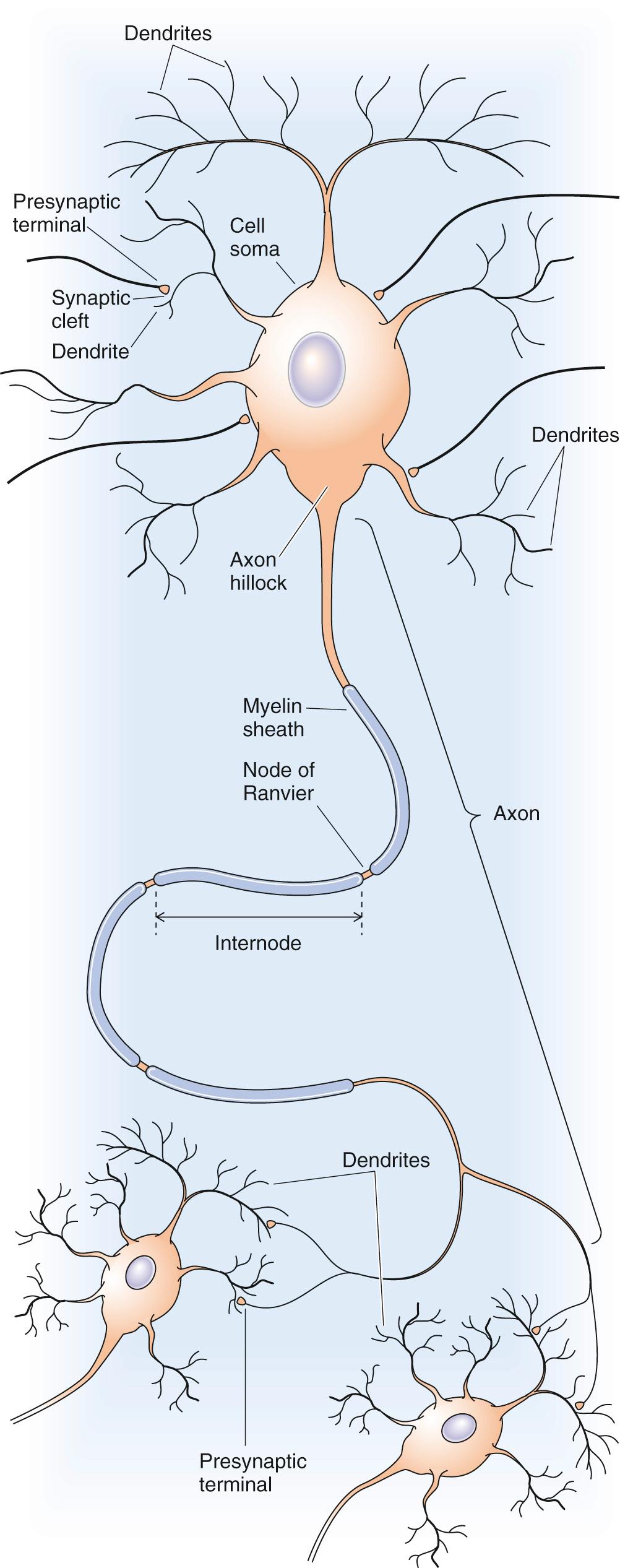
As the name perikaryon implies, the cell body is the portion of the cell surrounding the nucleus. It contains much of the cell's complement of endoplasmic reticular membranes as well as the Golgi complex. The cell body appears to be responsible for many of the neuronal housekeeping functions, including the synthesis and processing of proteins.
These structures are tapering processes of variable complexity that arise from the cell body. Dendrites, and to a lesser extent the cell body, are the main areas for receiving information. Thus, their membranes are endowed with receptors that bind and respond to the neurotransmitters released by neighboring cells. The chemical message is translated by membrane receptors into an electrical or a biochemical event that influences the state of excitability or function of the receiving neuron. The cytoplasm of the dendrites contains dense networks of microtubules as well as extensions of the endoplasmic reticulum.
Perhaps the most remarkable feature of the neuron, the axon is a projection that arises from the cell body, like the dendrites. Its point of origin is a tapered region known as the axon hillock. Just distal to the cone-shaped hillock is an untapered, unmyelinated region known as the initial segment. This area is also called the spike initiation zone because it is where an action potential (see p. 176 ) normally arises as the result of the electrical events that have occurred in the cell body and dendrites. In contrast to the dendrites, the axon is thin, does not taper, and can extend for more than a meter. Because of its length, the typical axon contains much more cytoplasm than does the cell body, up to 1000 times as much. The neuron uses special metabolic mechanisms to sustain this unique structural component. The cytoplasm of the axon, the axoplasm, is packed with parallel arrays of microtubules and neurofilaments that provide structural stability and a means to rapidly convey materials back and forth between the cell body and the axon terminus. Axons are self-reliant in energy metabolism, taking up glucose and oxygen from their immediate environment to produce ATP. Specialized glial cells called oligodendrocytes contribute in complex ways to axon integrity (see pp. 292–293 ).
Axons are the message-sending portion of the neuron. The axon carries the neuron's signal, the action potential, to a specific target, such as another neuron or a muscle. Some axons have a special electrical insulation, called myelin, that consists of the coiled cell membranes of glial cells that wrap themselves around the nerve axon (see pp. 292–293 ). If the axon is not covered with myelin, the action potential travels down the axon by continuous propagation. On the other hand, if the axon is myelinated, the action potential jumps from one node of Ranvier (the space between adjacent myelin segments) to another in a process called saltatory conduction (see pp. 200–201 ). This adaptation greatly speeds impulse conduction.
At its target, the axon terminates in multiple endings—the presynaptic terminals—usually designed for rapid conversion of the neuron's electrical signal into a chemical signal. When the action potential reaches the presynaptic terminal, it causes the release of chemical signaling molecules in a complex process called synaptic transmission (see Chapters 8 and 13 ).
The junction formed between the presynaptic terminal and its target is called a chemical synapse. Synapse is derived from the Greek for “joining together” or “junction”; this word and concept were introduced in 1897 by the neurophysiologist Charles Sherrington, whose contributions led to a share of the 1932 Nobel Prize in Medicine or Physiology. ![]() N10-2 A synapse comprises the presynaptic terminal, the membrane of the target cell (postsynaptic membrane), and the space between the two (synaptic cleft). In synapses between two neurons, the presynaptic terminals primarily contact dendrites and the cell body. The area of the postsynaptic membrane is frequently amplified to increase the surface that is available for receptors. This amplification can occur either through infolding of the plasma membrane or through the formation of small projections called dendritic spines.
N10-2 A synapse comprises the presynaptic terminal, the membrane of the target cell (postsynaptic membrane), and the space between the two (synaptic cleft). In synapses between two neurons, the presynaptic terminals primarily contact dendrites and the cell body. The area of the postsynaptic membrane is frequently amplified to increase the surface that is available for receptors. This amplification can occur either through infolding of the plasma membrane or through the formation of small projections called dendritic spines.
For more information about Sir Charles Scott Sherrington and the work that led to his Nobel Prize, visit http://www.nobel.se/medicine/laureates/1932/index.html (accessed October 2014).
The molecules released by the presynaptic terminals diffuse across the synaptic cleft and bind to receptors on the postsynaptic membrane. The receptors then convert the chemical signal of the transmitter molecules—either directly or indirectly—back into an electrical signal.
In many ways, neurons can be thought of as highly specialized endocrine cells. They package and store hormones and hormone-like molecules, which they release rapidly into the extracellular space by exocytosis (see pp. 34–35 ) in response to an external stimulus, in this case a nerve action potential. However, instead of entering the bloodstream to exert systemic effects, the substances secreted by neurons act over the very short distance of a synapse to communicate locally with a single neighboring cell (see p. 47 ).
In a different sense, neurons can be thought of as polarized cells with some of the properties of epithelial cells. Like epithelial cells, neurons have different populations of membrane proteins at each of the distinct domains of the neuronal plasma membrane, an arrangement that reflects the individual physiological responsibilities of these domains. Thus, the design of the nervous system permits information transfer across synapses in a selective and coordinated way that serves the needs of the organism and summates to produce complex behavior.
Synapses can undergo long-term changes based on certain patterns of prior activity. This plasticity (see pp. 328–333 ) is believed to underlie learning and memory.
Neurons are compartmentalized in both structure and function. Dendrites are tapered, have limited length, and contain neurotransmitter receptor proteins in their membranes. Axons can be very long and have a high density of Na + channels. Dendrites and the cell body contain messenger RNA, ribosomes, and a Golgi apparatus.
How does this compartmentalization come about? The molecular orientation of microtubules (see pp. 23–25 ) together with microtubule-associated proteins (MAPs) play important roles in dictating the vectorial transport of organelles and proteins. (Note that these MAPs are totally unrelated to the mitogen-activated protein [MAP] kinase; see p. 69. ) Two major classes of MAPs are found in the brain: high-molecular-weight proteins such as MAP-1 and MAP-2 and lower-molecular-weight tau proteins. Both classes of MAPs associate with microtubules and help link them to other cell components. MAP-2 is most abundant in dendrites, where it may assist in dendrite formation. Tau proteins are confined to axons; their suppression in cultured neurons prevents formation of the axon without altering formation of the dendrites. Hyperphosphorylated tau proteins can assemble into pathological aggregates called neurofibrillary tangles, which are a hallmark of Alzheimer Disease.
The polarization of microtubules (see pp. 23–25 ) helps to create remarkable morphological and functional divisions in neurons. In axons, microtubules assemble with their plus ends pointed away from the cell body; this orientation polarizes the flow of material into and out of the axon. The cytoskeletal “order” provided by the microtubules and the MAPs helps define what should or should not be in the axonal cytoplasm. In dendrites, microtubules do not have a consistent orientation, which gives dendrites a greater functional similarity to the soma and perhaps their tapered shape.
The neuron cell body is the main manufacturing site for the membrane proteins and membranous organelles that are necessary for the structural integrity and function of its processes. Axons have little or no intrinsic protein synthetic ability, whereas dendrites have some free ribosomes and are able to engage in limited protein production. The transport of proteins from the cell body all the way to the end of long axons is a challenging task. The neuron also has a second task: moving various material in the opposite direction, from presynaptic terminals at the end of the axon to the cell body. The neuron solves these problems by using two distinct mechanisms for moving material to the presynaptic terminals in an “anterograde” direction and a third mechanism for transport in the opposite or “retrograde” direction ( Table 10-2 ).
| TRANSPORT TYPE | SPEED (mm/day) | MECHANISM | MATERIAL TRANSPORTED |
|---|---|---|---|
| Fast anterograde | ~400 | Saltatory movement along microtubules via the motor molecule kinesin (ATP dependent) | Mitochondria Vesicles containing peptides and other neurotransmitters, some degradative enzymes |
| Fast retrograde | ~200–300 | Saltatory movement along microtubules via the motor molecule MAP-1C (brain dynein, ATP dependent) | Degraded vesicular membrane Absorbed exogenous material (toxins, viruses, growth factors) |
| Slow (anterograde only) | 0.2–8 | Not clear; requires intact microtubules (ATP dependent) | Cytoskeletal elements (e.g., neurofilament and microtubule subunits) Soluble proteins of intermediary metabolism Actin |
If the flow of materials from the soma to the distant axon terminus were left to the whims of simple diffusion, their delivery would be far too slow to be of practical use. It could take months for needed proteins to diffuse to the end of an axon, and the presynaptic terminals are high-volume consumers of these molecules. To overcome this difficulty, neurons exploit a rapid, pony express–style system of conveyance known as fast axoplasmic transport (see Table 10-2 ). Membranous organelles, including vesicles and mitochondria, are the principal freight of fast axoplasmic transport. The proteins (some associated with RNA), lipids, and polysaccharides that move at fast rates in axons do so because they have caught a ride with a membranous organelle (i.e., they are sequestered inside the organelle or bound to or inserted into the organellar membrane). The peptide and protein contents of dense-core secretory granules, which are found in the presynaptic axonal terminals, are synthesized as standard secretory proteins (see pp. 34–35 ). Thus, they are cotranslationally inserted across the membranes of the rough endoplasmic reticulum and subsequently processed in the cisternae of the Golgi complex. They are shipped to the axon in the lumens of Golgi-derived carrier vesicles.
Organelles and vesicles, and their macromolecule payloads, move along microtubules (see p. 23 ) with the help of a microtubule-dependent motor protein called kinesin ( Fig. 10-2 A ). The kinesin motor (see p. 25 ) is itself an ATPase that produces vectorial movement of its payload along the microtubule. This system can move vesicles down the axon at rates of up to 400 mm/day; variations in cargo speed simply reflect more frequent pauses during the journey. Kinesins always move toward the plus end of microtubules (i.e., away from the cell body), and transport function is lost if the microtubules are disrupted. The nervous system has many forms of kinesin that recognize and transport different cargo. It is not known how the motor proteins recognize and attach to their intended payloads.
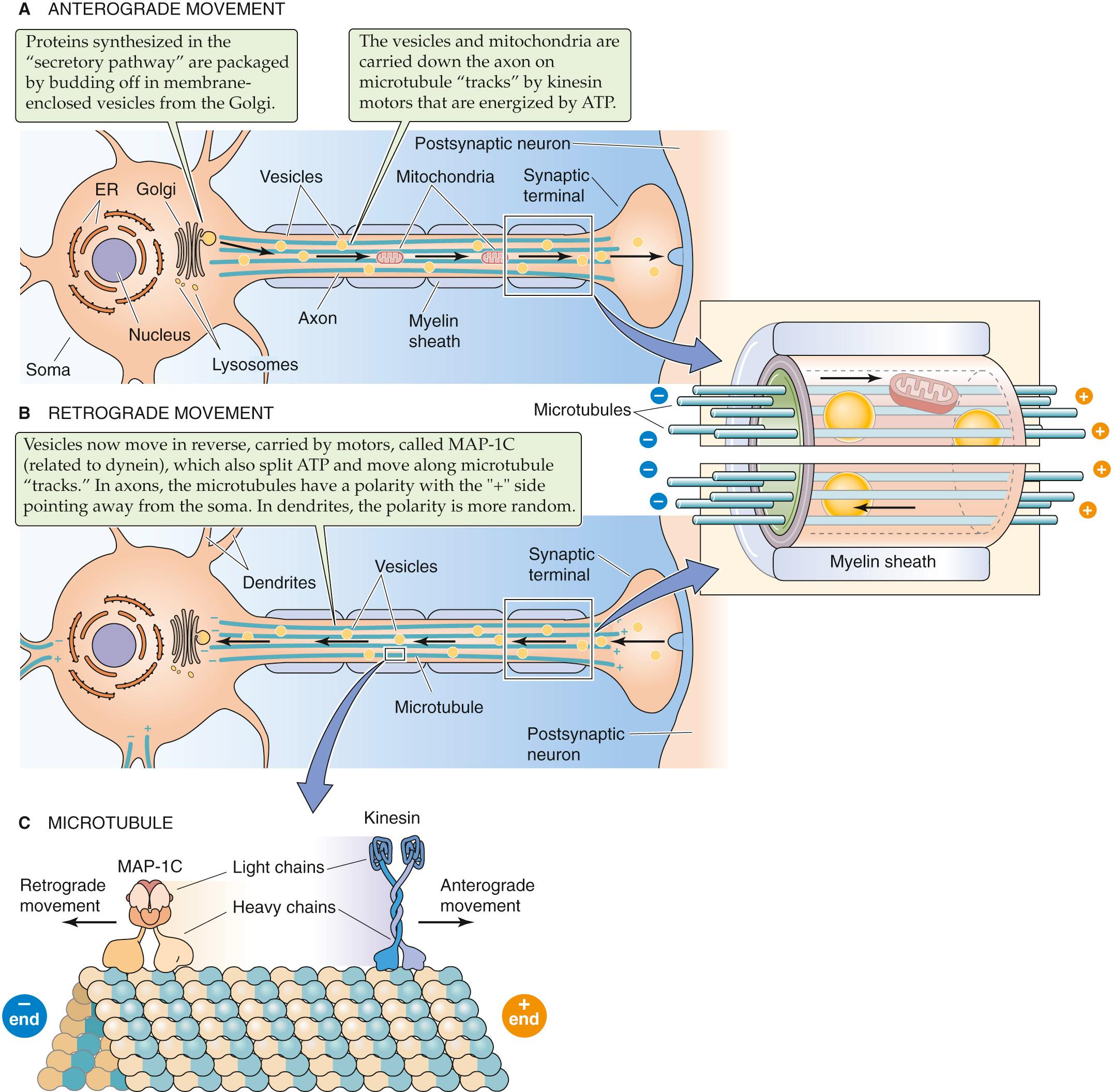
Axons move material back toward the cell body via a different motor protein called brain dynein (see p. 25 ) or MAP-1C (see Fig. 10-2 B ). Like kinesin, MAP-1C moves along microtubule tracks and is an ATPase (see Table 10-2 ). However, MAP-1C moves along microtubules in the opposite direction to kinesin (see Fig. 10-2 C ), though at a slightly slower pace. Retrograde transport provides a mechanism for target-derived growth factors, like nerve growth factor, to reach the nucleus of a neuron where they can influence survival. How this signal is transmitted up the axon has been a persistent question. It may be endocytosed at the axon's terminal and transported to the cell body in a “signaling endosome.” The loss of ATP production in axons, as occurs with blockade of oxidative metabolism, causes fast axonal transport in both the anterograde and retrograde directions to fail.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here