Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In head and neck reconstruction, a comprehensive assessment of the patient’s disease status, defects of the oral cavity, tongue, and mandible are critical to achieving optimal functional results with minimal complications.
Thorough evaluation of the defect includes its size, shape, geometry, and relationship to the adjacent structures.
A strategic approach to flap selection of soft-tissue flap or osseous flap should accurately reconstruct the oral defects and restore the functional and aesthetic status.
Understanding the different osteocutaneous flaps' anatomical characteristics may increase the likelihood of selecting the appropriate donor flap for mandibular reconstruction.
For the reconstruction of type III mandibular defects, several options exist, including the use of a soft-tissue flap with a reconstruction plate, one osteocutaneous flap with one pedicled flap, double free flaps, chimeric flaps, a composite scapular flap, or a composite osteomusculocutaneous peroneal artery-based combined (OPAC) flap.
The fibula flap's evolution from a bone-only flap to an osteoseptocutaneous flap and an OPAC flap may increase the clinical application of fibula-based flap for type III mandibular defects.
Access video and video lecture content for this chapter online at Elsevier eBooks+
![]()
Reconstructions of the oral cavity, tongue, and mandible are complex and challenging to achieve both ultimate functional and aesthetic outcomes. An ideal reconstruction should mimic the missing tissue with regard to structure, geometry, and tissue character. For an oral cavity reconstruction without bony involvement, the perfect reconstruction involves a soft-tissue flap that is like the missing tissue in size, shape, and quality.
The anatomy of the oral cavity involves complex functions of speech, swallow, and facial expression. Disease status and tumor staging may affect the resection margin and result in variable defects, which requires comprehensive assessment for functional restoration and ultimate cosmesis.
Reconstruction of the tongue is complex due to its essential role in articulation, deglutition, and airway protection. Tongue cancer is the most common oral cavity cancer in Western countries. The incidence of tongue cancer remains as high as buccal cancer due to the popularity of habitual betel nut chewing, smoking, and alcohol consumption in Asian countries. Any reconstructive attempt must restore the bulk and mobility for the neo-tongue to be functional.
The mandible plays a crucial functional role in mastication, swallowing, speech, and smiling, as well as the esthetic shape, contour, and vertical height of the lower third of the face. Mandibular defects can result from resection of tumors, osteomyelitis or osteoradionecrotic lesions, or gunshot trauma. Soft-tissue involvement surrounding the mandible results in more complicated defects that require delicate bone and soft-tissue components of the flaps used for reconstruction. Advanced oral tumors may involve many adjacent structures, such as the buccal mucosa, tongue, floor of the mouth, external skin of the cheek, lower lip, masseter muscle, buccal fat pad, parotid gland, and partial maxilla. These composite defects make the reconstruction more complicated.
The goals of composite mandibular reconstruction include the restoration of the bony scaffold, adequate oral continence and deglutition, obliteration of dead space, and re-establishment of optimal cosmesis. Dead space created by the destruction of masticatory muscles, the buccal fat pad, and the parotid gland can lead to fluid accumulation and infection. If soft-tissue defect and dead space are not adequately restored, it can lead to a sunken appearance from soft-tissue contracture, trismus, plate exposure, fistula, and impaired speech and swallowing function. These conditions may be exacerbated by postoperative radiotherapy. Choosing an optimal flap with suitable flap size, thickness, pliability, and adequate pedicle length and diameter for reconstruction should be based on the clinical situation, patient’s preference, and surgeon’s experience.
The oral cavity is bounded by the lips anteriorly, the oropharynx posteriorly, the hard palate superiorly, the tongue and mouth floor inferiorly, and the cheek laterally. The inner oral cavity is lined with buccal mucosa. Beneath the buccal mucosa is the buccal fat pad and the muscles for facial expression, mouth movement, and oral competence. Between the intraoral mucosa and cheek are bony structures, including the mandible and maxilla, which form and maintain the lower face framework.
The buccal mucosa starts anteriorly from the upper and lower lip's inner surface and then extends to the alveolar ridge. Before reaching the alveolar ridge, the buccal mucosa forms a valley-like structure called the sulcus, which helps with chewing and swallowing and directs the saliva and food toward the oropharynx. Laterally and posteriorly, the buccal mucosa connects at the pterygomandibular junction. On the lateral surface, multiple small intrinsic salivary glands underneath the buccal mucosa secrete saliva and moisten the oral cavity together with the three major salivary glands. The buccal mucosa's mobile character helps with natural facial expression, speech, and deglutition and, therefore, is vital in daily life and social activities.
The tongue is a specific functional unit in speech and swallowing in the oral cavity. The oral tongue's primary component is the striated muscle, which determines the tongue’s shape and function. Multiple extrinsic muscles (genioglossus, hyoglossus, styloglossus, and palatoglossus muscles) originating outside the tongue insert into the tongue and control its movement. The anterior two-thirds of the tongue is located inside the cavity and moves freely above the mouth floor. On the dorsal surface, the tongue has multiple small papillae that contain taste buds. The dorsal surface is lined by rough mucosa for taste and mastication. Free movement of the oral tongue is necessary for most of its function. The posterior one-third of the tongue is in the oropharynx. The tongue's base is fixed to the hyoid bone and is therefore not as mobile as its anterior two-thirds. However, in situations where most of the tongue has been resected, the tongue base preserves some tongue function.
Mastication involves mixing food with saliva and chewing the food to form a food bolus for further swallowing. Mixing the food requires a freely movable tongue that can move the food effectively. The tongue helps to mix the food with saliva with its “intrinsic” muscles; it also pushes the food to the inter-dental space for chewing. A stable mandible and temporomandibular (TM) joint provide steady, voluntary movement that promotes chewing and grinding. Finally, the tongue acts again to push the food to the oropharynx. The tongue tip first moves the food backward, then the tongue base lifts to push the food bolus into the oropharynx. During this process, the hard and soft palate are also necessary to manipulate the space between the oral cavity and the nasal cavity. The entire mastication and swallowing process depends on the mobile mucosa, a freely movable tongue, a stable mandible, and functional TM joints.
Approximately 86% of all oral tumors are squamous cell carcinomas, while other tumors, such as verrucous carcinoma, sarcoma, melanoma, or lymphoma, occur less frequently. The age-adjusted incidence of oral cavity cancer is 11 per 100,000 in the United States; this value could be higher in specific areas where smoking and betel nut chewing are popular. In 2008, it was estimated that head and neck cancers comprised 2%–3% of all cancers and accounted for 1%–2% of all cancer deaths in the US. Most patients with head and neck cancer have metastatic disease at the time of diagnosis, with regional nodal involvement in 43% and distant metastasis in 10%. Moreover, patients with head and neck cancer often develop second primary tumors at an annual rate of 3%–7%. The male–female ratio has been reported as 3 : 1 for the incidence of oral cavity and pharyngeal cancers.
Statistical analysis of a 10-year period revealed a trend toward earlier diagnosis of head and neck cancer. Surgery with or without radiation and chemotherapy remains the standard of care. Since the first introduction of the free intestinal flap 1959 and the first fasciocutaneous free flap for head and neck reconstructions in 1976, free flap transfer has become the gold standard for reconstruction due to its high flap survival rate, improved cosmetic and functional results, and acceptable incidence of donor site morbidity. These techniques also facilitate resection of even more advanced but localized tumors.
Inadequate oral hygiene and contamination may increase the risk of infection and also compromise the survival of any marginally perfused tissue. Irradiation produces detrimental acute and chronic effects in the periosteum, mandible marrow, and the surrounding soft tissue. With chronic post-radiation hypoxia, cellular and vascular damage lead to skin atrophy and increase the susceptibility to wound breakdown and decreased healing potential following minor trauma. Vascular changes initially occur in the microcirculation; however, with progression, larger blood vessels can also be affected. Many of these issues are addressed by the transplantation of well-vascularized tissue with bone and skin components. Vascularized bone resists infection, does not resorb, and is not dependent on the recipient bed’s vascularity for survival.
Currently, most oral cancer patients present at an advanced stage. Most patients suffer from oral ulcers, sore throat, a tongue mass, and swallowing pain. Cuffari et al . reported that the type of pain reported was related to the TNM staging of the tongue and floor of mouth. A thorough physical examination, radiographic study of the tumor, and histologic examination with TNM staging should be performed by both a surgical oncologist and a reconstructive surgeon before surgery. The gene expression of the tissue sample can provide a sub-classification based on genes and mRNA expression, which plays a role in predicting the clinical outcome after radiotherapy. Liao et al . demonstrated that upregulation of centromere protein H is correlated with poor prognosis and progression in tongue cancer patients. Chronic ulcers, leukoplakia, and tumor growth are regularly seen at specialized centers. Visual loss as an initial symptom of squamous cell carcinoma of the tongue was reported by Foroozan et al . It is crucial to keep in mind that rare constellations of symptoms may require differential diagnosis, such as a tumor or abscess of the tongue being a sign of atypical metastasis of lung cancer. A rare Schwannoma of the tongue was reported by Cohen and Wang. and malignant fibrous histiocytoma of the tongue was reported by Rapidis et al .
Surgery for head and neck cancers has been the standard of treatment since the 1940s. The concept of immediate reconstruction following tumor resection was introduced in 1951 and became considered the gold-standard treatment for oral cavity cancers. Surgery at this time was for tumors with early stages, since the reconstruction was limited to small defects due to the inability to reconstruct a larger defect. Options largely followed the reconstructive ladder, from primary closure, to skin grafts, to pedicled flaps such as the infrahyoid myofascial flap, pectoralis major (PM) musculocutaneous flap, or the trapezius island pedicle flap.
Over time, local and regional flaps were introduced to address the reconstruction of extensive head and neck defects after wide tumor resection of advanced-stage cancers. The deltopectoral (DP) musculocutaneous flap, PM musculocutaneous flap, and latissimus dorsi (LD) musculocutaneous flap were the three commonly used flaps. The clinical applications of these flaps were limited by the pedicle's length and the flap's size. The requirement of a two-stage procedure and the less reliable blood flow at the distal part of the flap also made these flaps less desirable. In 1959, a free jejunum flap was first transferred as a microsurgical flap for an esophageal conduit. This surgery introduced the concept of microsurgical free tissue transfer, which was gradually popularized for head and neck reconstruction.
The evolution of head and neck reconstruction gained speed after microsurgical free tissue transfer was accepted. The first free sensate fasciocutaneous flap, a sensate dorsalis pedis flap, was introduced in 1979. Four years later, the free radial forearm flap was applied to oral cavity reconstruction. Microsurgical free tissue transfer eliminated the requirement for a two-stage surgery and allowed more freedom for flap inset to achieve better cosmetic and functional outcomes. In the early 1980s, free flaps were limited to axial fasciocutaneous flaps and myocutaneous flaps. The most used free flaps were the radial forearm flap, transverse rectus abdominis myocutaneous (TRAM) flap, or vertical rectus abdominis myocutaneous (VRAM) flap, and LD musculocutaneous flap. Perforator flap became popular with the advancement of surgical instruments and techniques and better understanding of flaps’ anatomy and vascularity,. The free-style free flap concept based on any sizable perforator made more donor sites available for flap harvest with fewer donor site morbidities. Currently, the radial forearm flap, TRAM flap, LD flap, and anterolateral thigh (ALT) musculocutaneous flap are still the most used flaps in buccal reconstruction. The preferred flaps vary between countries, as flap selection is often influenced by cultural preferences and the patient’s body habitus.
Mandibular reconstruction historically involved use of a prosthesis, then gradually progressed into the use of non-vascularized bone grafts, tubed osteocutaneous skin flaps, pedicled osteomusculocutaneous flaps, and finally microsurgical osteocutaneous flaps. Hansmann was the first surgeon to apply plates and screws for mandibular fractures in 1886, however, this was associated with high failure rates due to infections, plate exposures, plate fractures, wound dehiscence, and other complications. At the end of the nineteenth century, Sykoff performed a non-vascularized bone graft transplantation for mandibular reconstruction when he used a graft from the contralateral mandible to bridge a 4-cm mandibular defect. During World War I, this technique was widely used, with the tibia and rib serving as additional donor sites. In their book Gunshot Wounds of the Mandible , Klapp and Schröder described various ways to bridge mandibular defects. Intermaxillary fixation and wired osteosynthesis were also used during that time. The survival rates of these grafts depend mainly on revascularization from the recipient site and remain an option for defects less than 4 cm in length.
In 1976, Spiessl reported the use of metal plates to bridge a mandibular defect after tumor resection. Originally, plates were made of stainless steel; titanium plates are typically used today due to their biocompatibility. More recently, Kim et al . demonstrated that locking screws/plates, combined with vascularized bone grafts, are associated with significantly fewer complications than non-locking plates due to less compressive forces between the plate and the underlying bone. Despite major disadvantages, there are still indications for using plates for mandibular reconstruction in patients where medical conditions preclude a prolonged operation time or extended surgical reconstruction procedures.
Freeze-dried allogeneic mandibular cribs with autologous iliac cancellous bone grafts were introduced by De Fries et al . for mandible reconstruction in 1971. A combination of autologous cancellous bone with fibrin glue was proposed by Tayapongsak et al . in 1994. Marx et al . also demonstrated the use of platelet-rich plasma as growth factor enhancement for these cancellous bone grafts. Despite these additional developments, bone resorption, wound dehiscence, and infection still occurred, leading to graft loss.
Bardenheuer, in 1891, was the first to transplant a pedicled frontal bone graft with soft tissue to the mandible. Regional osteomusculocutaneous flaps became popularized in the 1980s after the introduction of the pectoralis major (PM) musculocutaneous flap in 1968 by Hueston and McConchie. Cuono and Ariyan reported the first pedicled rib graft with the PM musculocutaneous flap. Robertson and Green et al . demonstrated the feasibility of using the PM musculocutaneous flap with the sternum as a bone graft. A composite pedicled myocutaneous flap transferred with clavicle bone graft has also been reported.
Mowlem et al . demonstrated the osteogenic potential of cancellous bone grafts in 1945. Autologous bone cancellous marrow grafts showed a higher osteogenesis rate and a lower surgical complications rate than cortical grafts. Due to the lack of cohesion, the cancellous bone grafts were placed into different frames and cribs. Boyne began using metal cribs in 1969. Cribs of titanium, Vitallium, tantalum, chrome cobalt, and stainless steel were used with variable results. Modification of a titanium mesh system was addressed by Tideman and Lee et al . in 1996, and 2009. Biodegradable poly(L-lactide) mesh was used successfully along with the cancellous bone graft by Kinoshita et al .
The advent of vascularized bone grafts revolutionized mandibular reconstruction. McKee was the first to describe a microvascular free rib graft for mandible reconstruction in 1971. Various donor sites for vascularized bone have been implemented successfully, including the metatarsal graft, radial forearm, scapula, iliac crest and fibula bone.
Taylor et al . first introduced the free fibula osseous flap for clinical application in 1975. The anatomical study and reliability of the fibula skin paddle nourished by the osteoseptocutaneous perforators were well established by Wei et al . The fibula flap was first applied for mandible reconstruction by Hidalgo and has now become the workhorse flap in vascularized bone replacement of mandibular defects. The reliability and consistent aesthetic and functional results with minimal bony resorption obtained using a free fibula flap were further confirmed by Cordeiro et al . at a 10-year follow-up.
Soft-tissue reconstruction is as vital as bone reconstruction in composite mandibular defects. Wide tumor resection requires effective dead space obliteration to prevent fluid accumulation, resulting in postoperative infection and compromise flap survival. The residual dead space can also result in soft-tissue contraction, which can significantly alter swallowing, chewing, speech, and aesthetic appearance. The fibula osteoseptocutaneous flap is the first choice for mandibular reconstruction. It provides a long bony segment that can be safely osteotomized and transferred with a reliable skin paddle. For extensive defects where multiple tissue components are missing, more than one flap may be required for optimal reconstruction. A common choice is the fibula osteoseptocutaneous flap combined with the ALT musculocutaneous flap, or TRAM or VRAM rectus abdominus muscle flap. In a select group of patients, osseointegrated implants are offered for osseointegrated dental implant placement. This can be performed during the primary operation in patients with mandibular defects resulting from the excision of benign lesions; otherwise, these procedures are performed secondarily due to the subsequent radiation for cancer patients. Although used less frequently, the iliac crest free flap and the scapula free flap are alternative options for reconstructing composite mandibular defects. A new alternative to provide both bone and soft-tissue components as a combined flap for mandibular reconstruction is the osteomyocutaneous peroneal artery-based combined (OPAC) flap, which is the modification of fibula osteoseptocutaneous flap with a cuff of soleus.
Comprehensive assessment of the patient’s general condition, the defects of the buccal mucosa, tongue, and mandible, and the available donor sites are required for a reconstructive plan. A thorough understanding of the missing tissue and its geometrical relation to each structure will elucidate the functional and aesthetic requirements of reconstruction and facilitate optimal reconstructive methods ( Tables 12.1–12.3 ).
| Flap character/flap type | Skin or mucosa | Flap dimension | Flap thickness | Pedicle size | Pedicle length | Dissection difficulty |
|---|---|---|---|---|---|---|
| Local/regional flap | ||||||
| Nasolabial flap | ++ | + | ++ | − | − | − |
| Buccal fat pad flap | − | + | + | − | − | − |
| Facial artery musculomucosal flap | ++ | + | ++ | − | − | − |
| Submental flap | +++ | +++ | ++ | − | − | − |
| Deltopectoral flap | ++ | +++ | +++ | − | − | − |
| Pectoralis major flap | ++++ | ++++ | ++++ | − | − | − |
| Free flap | ||||||
| Radial forearm flap | ++++ | ++++ | ++ | +++ | ++++ | ++++ |
| Ulnar forearm flap | ++++ | ++++ | ++ | +++ | ++++ | ++++ |
| Lateral arm flap | +++ | ++ | ++ | ++ | + | +++ |
| Rectus abdominis musculocutaneous flap | ++++ | +++ | ++++ | ++++ | +++ | ++++ |
|
++++ | ++++ | +++ | ++++ | ++++ | + |
| ++++ | ++++ | ++++ | ++++ | +++ | + | |
| Thoracodorsal artery perforator flap | ++++ | +++ | +++ | +++ | +++ | ++ |
| Medial sural artery perforator flap | ++++ | +++ | ++ | +++ | ++++ | ++ |
| Femoral profunda artery perforator flap | ++++ | +++ | ++ | +++ | +++ | ++ |
| Superficial circumflex iliac artery flap | ++++ | +++ | +++ | + | ++ | +++ |
| Flap character/flap type | Small mucosa defect | Large mucosa defect | Mucosa trigon | Through-and-through | Mucosa and partial maxilla | Mucosa and marginal mandibulectomy |
|---|---|---|---|---|---|---|
| Local/regional flap | ||||||
| Nasolabial flap | + | − | − | − | − | − |
| Buccal fat pad flap | ++ | ++ | − | − | − | − |
| Submental flap | ++ | ++ | − | − | − | − |
| Facial artery musculomucosal flap | ++ | − | − | − | − | − |
| Deltopectoral flap | − | +++ | ++ | +++ | ++ | +++ |
| Pectoralis major flap | − | ++++ | ++ | +++ | ++ | ++++ |
| Free flap | ||||||
| Radial forearm flap | − | ++++ | ++ | + | + | + |
| Ulnar forearm flap | − | ++++ | ++ | + | + | + |
| Lateral arm flap | − | ++ | ++ | + | + | + |
| Rectus abdominis musculocutaneous flap | − | ++ | ++ | +++ | ++++ | ++++ |
|
− | +++ | +++ | +++ | ++ | +++ |
| − | ++ | +++ | ++++ | ++++ | ++++ | |
| Thoracodorsal artery perforator flap | − | ++++ | ++++ | ++ | ++ | +++ |
| Medial sural artery perforator flap | − | +++ | ++ | + | + | + |
| Femoral profunda artery perforator flap | − | +++ | +++ | ++++ | ++++ | ++++ |
| Superficial circumflex iliac artery flap | ++ | +++ | ++ | ++ | ++ | ++ |
| Cheng’s classification | Tongue defect | Considerations | Preferred options | Alternatives |
|---|---|---|---|---|
| I | Hemi or less | Thin and pliable skin flap, motility | Radial forearm flap/ulnar forearm flap |
|
| IIA | Two-thirds | Bulky skin flap | Anterolateral thigh perforator flap |
|
| IIB | Three-quarters | Bulky musculocutaneous flap | Anterolateral thigh musculocutaneous flap |
|
| III | Total | Large musculocutaneous flap with adequate volume for swallowing | Pentagonal anterolateral thigh musculocutaneous flap |
|
Many oral cavity cancer patients have a history of smoking and alcohol consumption, which increases the risk of perioperative pulmonary and overall complications. These factors can also affect the microvascular anastomosis in a free flap transfer. Smoking should be stopped at least 2 weeks before a lengthy operation to reduce pulmonary complications. Diabetes mellitus is a risk factor for peripheral vasculopathy and is associated with a higher incidence of postoperative infection. A patient with end-stage renal disease undergoing a prolonged operation is at greater risk of developing postoperative fluid overload and other associated complications. Patients with Child’s class B or C cirrhosis have more complications, including pulmonary issues, acute renal failure, and sepsis than those with class A cirrhosis (80% vs. 19.1%). Old age is not a contraindication for a microsurgical head and neck reconstruction. However, medical problems associated with chronological age, such as cardiopulmonary diseases, atherosclerosis, and previous stroke, indicate a higher incidence of postoperative medical complications. These advanced oromandibular cancer patients are often malnourished, impacting routine wound healing, pulmonary function, and postoperative recovery. For a malnourished patient, a short period of tube feeding before surgery can correct malnutrition status and improve wound healing and general recovery after surgery.
It is ideal to replace missing tissue with like tissue. The assessment of excision defects should include the size, volume, and components of the involved soft tissue, the length and location of the mandible defect, the available recipient vessels, and the quality of the external skin. When a severe medical comorbidity precludes complex microsurgical reconstruction, the reconstruction ladder may be downgraded to achieve wound healing for further chemoradiotherapy.
The commonly applied reconstructive options are as follows.
A skin graft is a relatively straightforward procedure, however its application in oral cavity reconstruction has largely been replaced by pedicled or free flaps since the environment of the oral cavity is not conducive to the survival of a skin graft. Scar contracture progression from a skin graft often limits the oral mucosa and tongue’s mobility, compromising the postoperative oral cavity function.
| Considerations | Details | |
|---|---|---|
| 1 | Patient’s risk factors |
|
| 2 | Defects |
|
| 3 | Recipient vessels | Ipsilateral or contralateral |
| 4 | Selection of donor flaps | See Tables 12.1, 12.2, 12.3 & 12.6 |
| 5 | Technique considerations | |
| Plating | Reconstruction plate or miniplates, preoperative 3D CT simulation, pre-bending plate, occlusion with intermaxillary wiring | |
| Osteotomy |
|
|
| Flap inset |
|
|
| Microsurgical anastomosis | Artery first or vein first | |
| Osseointegration dental implants |
|
|
Before the development of free flap transfer, local and regional flaps were the treatment of choice. Today, pedicled flaps’ applications are limited to reconstructing minor defects or treating patients where free flap transfer is not indicated.
The advent of microsurgical free tissue transfer has significantly increased reconstructive alternatives through the ability to use larger flaps and the increased versatility to ensure a better fit of the defect. Free tissue transfer for composite reconstruction has allowed the restoration of increasingly complex defects in a single stage with better functional and aesthetic outcomes.
Different decision-making processes for the most common buccal, tongue, and mandibular reconstructions are discussed below.
When the defect involves only the buccal mucosa, the reconstruction is relatively straightforward. The size of the defect should be measured with the mouth maximally open. A tongue retractor should be applied between the upper and lower teeth to facilitate adequate exposure. A thin and pliable flap is generally the first choice for reconstruction ( Figs. 12.1–12.3 ). Trismus will develop if the resurfacing is inadequate. The pliability of the flap allows for a better inset to fit the contour of the defect. It provides better functional restoration of postoperative mouth movement, eating, speech, and facial expression.
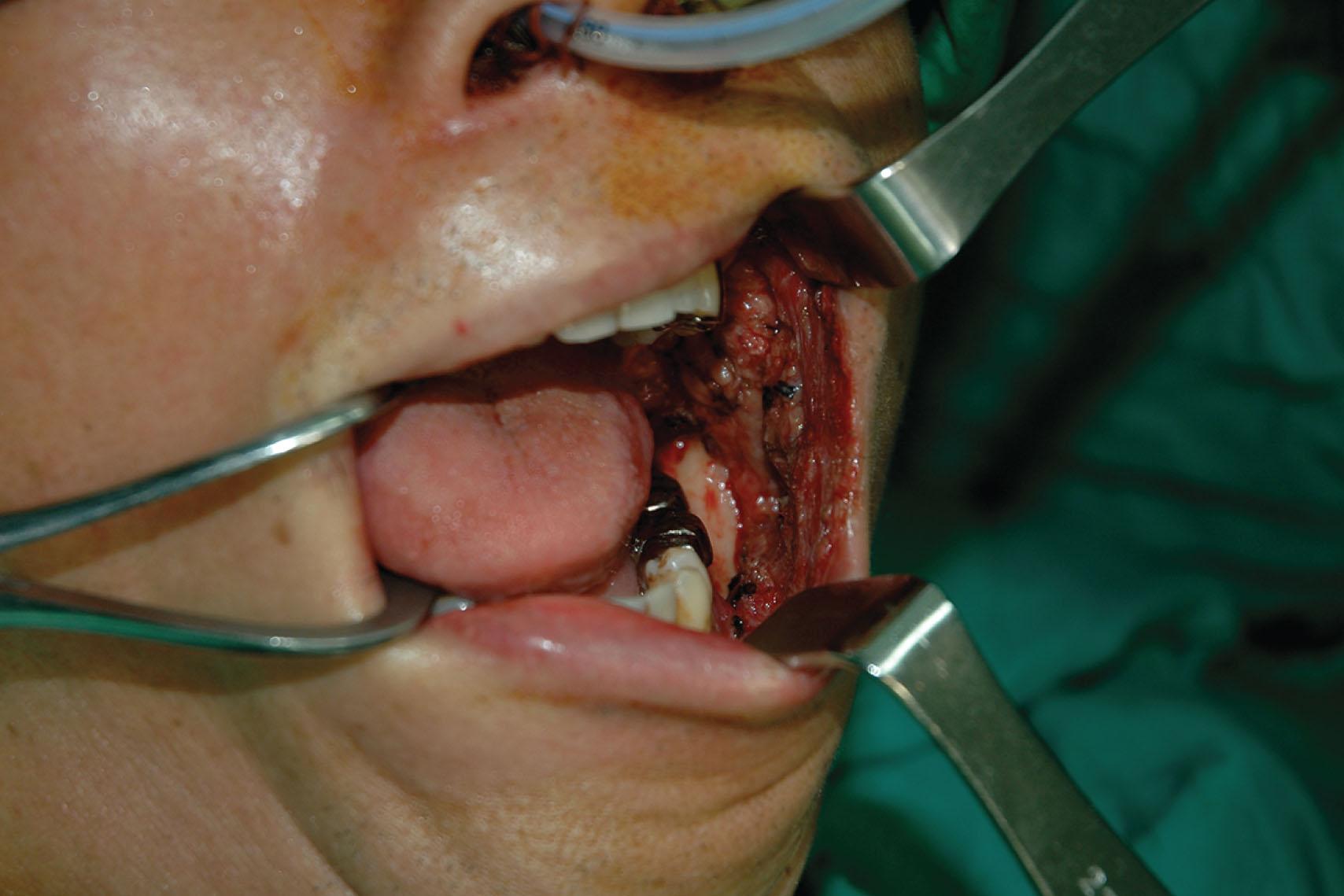


It is best to recreate the sulcus when it is involved in a buccal defect. A slight folding of a thin flap is required to form the sulcal shape. It is also expected that the lower or upper lip's inner surface will be involved. Although the wound between the edge of the lip and the lower gum can be closed primarily, it is not recommended since it results in an unnatural appearance and function.
The mandible is often exposed when the buccal defect extends to the trigone region, and such defects commonly involve the posterior tongue. Although the tissues in this area are relatively loose, making primary wound closure possible in some cases, it can distort the natural anatomy of the tonsillar pillar and tether the tongue. An anatomical change in the tonsillar pillar that separates the oro- and nasopharynx can result in food regurgitation into the nasal cavity. Tongue tethering also limits eating and speaking.
Flap selection for pure buccal reconstruction depends on the thickness of the flap required. According to the author’s experience, a free radial forearm or ulnar forearm flap is usually adequate. A relatively thin anterolateral thigh perforator flap is needed for a thicker defect. A medial sural artery perforator flap is a feasible alternative.
In severe trauma or post-resection of buccal cancer, the defects can extend from the mucosa to the external skin (through-and-through defect), often requiring a bulky myocutaneous flap or a thick fasciocutaneous flap with partial de-epithelialization. Alternatively, a chimeric flap design with two separate skin paddles for intraoral and cheek reconstructions can achieve good aesthetic results. Each skin paddle can be customized according to the defect, preventing distortion of the mouth angle.
If the buccal mucosa resection is accompanied by marginal mandibulectomy, sufficient coverage of the exposed mandible is essential. The reconstruction is necessary to resurface the defect and provide adequate bulk replacement of the resected teeth and alveolar bone for a more symmetrical facial contour. An ALT flap with or without vastus lateralis is the preferred choice for reconstruction for the through-and-through cheek defect ( Figs. 12.4–12.7 ). The femoral profunda artery perforator (PAP) flap is another good alternative.



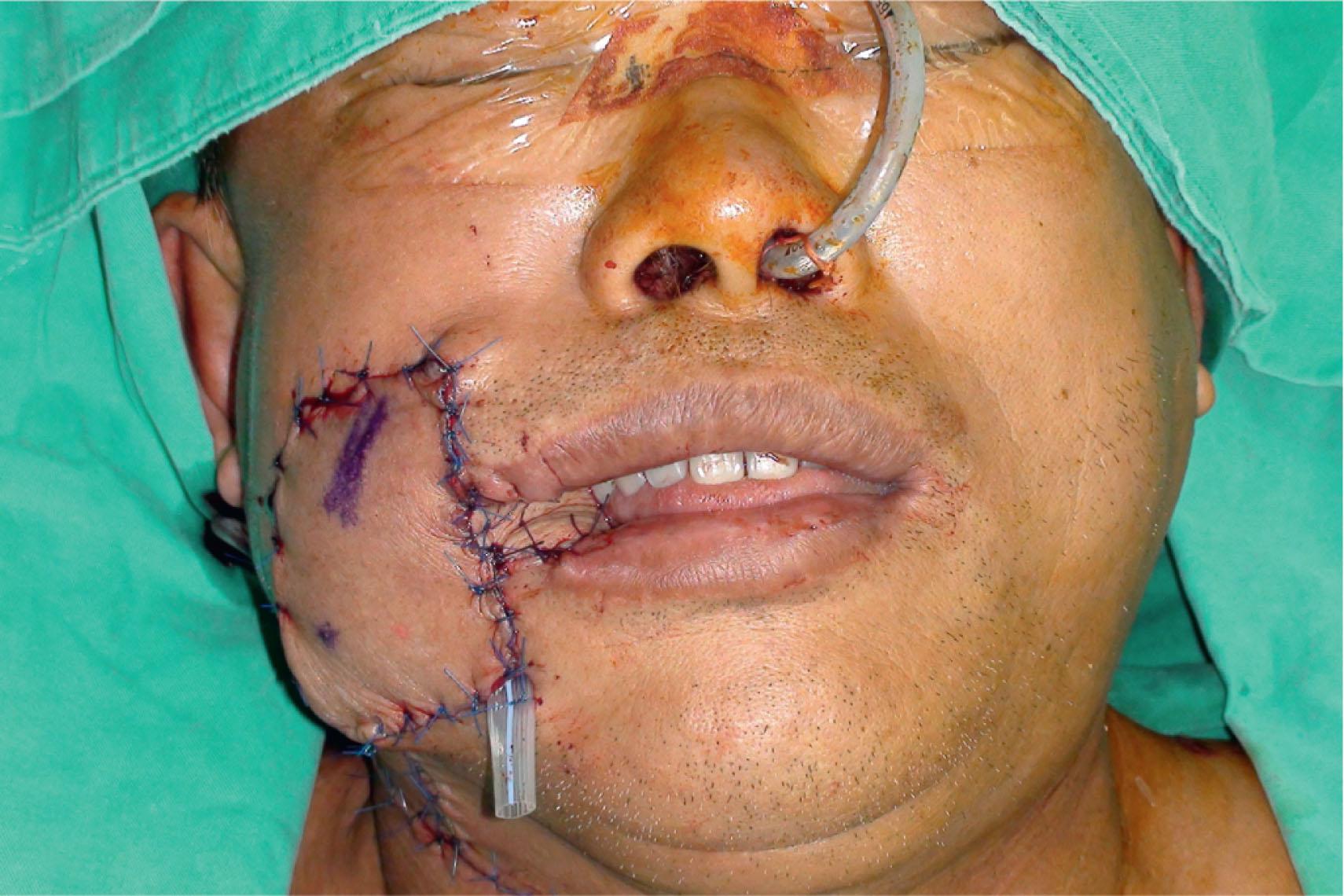
An inferior maxillectomy after excision of an advanced buccal mucosal tumor may result in a dead space in the inferior maxillary area that requires soft tissue obliteration to avoid fluid accumulation and postoperative infection ( ) ( Figs. 12.8–12.11 ). The anterior maxillectomy can be reconstructed with a rib bone graft ( ). It is not uncommon that part of the soft or hard palate is involved in the resection. Few palatal wounds can be closed primarily and inadequate tissue resurfacing will result in oronasal fistula, food regurgitation, and hypernasality. An ALT flap with vastus lateralis muscle is used most frequently in the author’s experience. Alternatively, a free TRAM or VRAM flap or a PAP flap can be used.



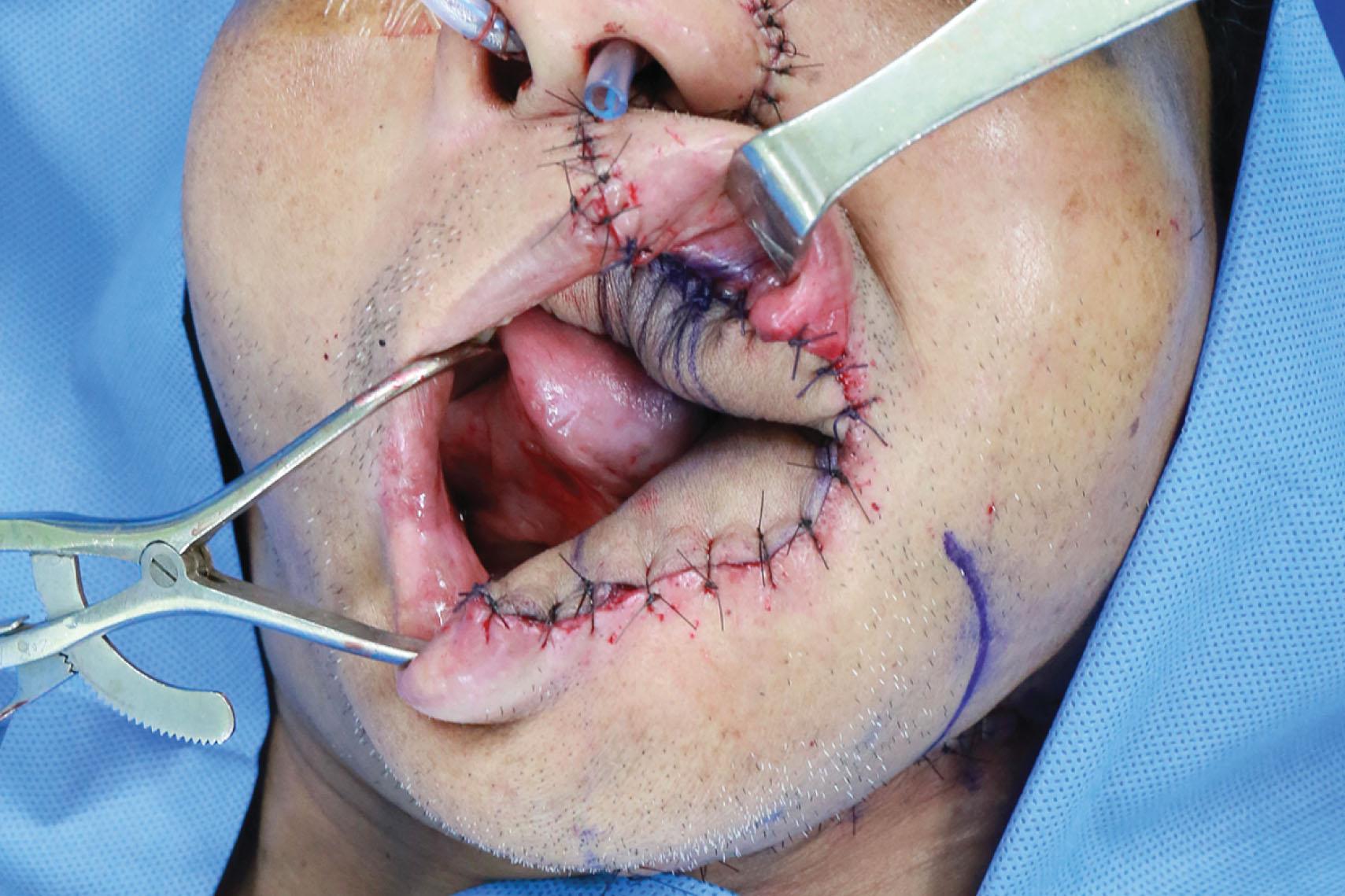
Table 12.1 summarizes the characteristics of each available flap and its clinical applications in buccal reconstruction. Table 12.2 provides guidelines for flap selection for variable buccal defects ( Algorithm 12.1 ).

An algorithm for the strategic selection of the most appropriate donor flap for buccal and mandibular defects for maximal functional and cosmetic outcomes. ALT, Anterolateral thigh; DIEP, deep inferior epigastric perforator; MC, musculocutaneous; PAP, femoral profunda artery perforator; RA, rectus abdominis; SCIP, superficial circumflex iliac perforator.
Reconstruction of the tongue requires a delicate procedure due to the tongue’s central role in articulation, deglutition, and airway protection. Several studies have used flap inset techniques to reconstruct a tongue with a three-dimensional shape closer to that of the native tongue. Hsiao et al . described a radial forearm flap design with a narrow waist to recreate the omega-shaped profile of the tongue. Chepeha et al . used a rectangular template with a more straightforward design and more dynamic reconstruction. Davison et al . used a combination of native tongue tip rotation and wedge de-epithelialization in the flap for optimizing tongue-tip sensation and to reduce pooling on the mouth floor. Chiu et al . further expanded on this technique by describing their semicircular design with wedge de-epithelialization or resection to increase tongue elevation and deepening of the tongue floor for a neo-sulcus. Very little has been reported regarding the refinement of total tongue reconstruction, probably due to the mistaken notion that such reconstructions serve no purpose other than volumetric restoration.
Current tongue reconstructive options either maintain mobility or provide bulk. Flaps that maintain mobility include the infrahyoid myofascial flap, medial sural artery perforator flap, radial forearm flap, and ulnar forearm flap. Flaps that provide bulk include the TRAM or VRAM flap, latissimus dorsi (LD) myocutaneous flap, PM myocutaneous flap, and trapezius island flap. The ALT flap has emerged in recent decades as a popular option for head and neck reconstruction due to its reliability, long pedicle, and acceptable donor morbidity. Due to its versatility, this flap has been used both to provide bulk and mobility.
Most publications have reported on the use of a single flap to reconstruct a limited range of tongue defects, while others compared two flaps but generally gave little information for why one flap was selected over another.
Some authors classified tongue defects after tongue resection as hemiglossectomy, sub-total, and total glossectomy defects. A goal-directed classification for tongue defects should provide descriptions and facilitate precise judgment with therapeutic consequences. Cheng’s modified tongue classification (I, IIa, IIb, III) separates tongue defects into three major groups, which indicates the types of donor flap selection ( Algorithm 12.2 ; see Table 12.3 ).
For Cheng’s tongue defect type I patients (defects ≤50%), a radial or ulnar forearm flap is recommended because of its thinness, flexibility, and long pedicle ( Figs. 12.12–12.14 ); but the ALT perforator flap can be used instead in thin patients. Group II defects were further classified into 50%–66% (IIa) and 66%–75% (IIb) for further refinements in flap selection. For type IIa defects, a thin ALT flap is preferred over the radial forearm flap due to the larger available flap size, especially when encountering accompanying floor of mouth or buccal defects. The medial sural artery perforator flap is also a good option ( Figs. 12.15–12.18 ) for tongue type IIa defects. For type IIb defect, the small residual amount of tongue (about 25%) has likely maintained the basic anatomical integrity of the tongue's base, the retromolar trigone, or one side of the pterygoid fossa, depending on its location (see Table 12.3 ). The bulkiness of the subcutaneous tissue in the ALT musculocutaneous flap provides a better neo-tongue profile for defects crossing the midline in Cheng’s types IIb.
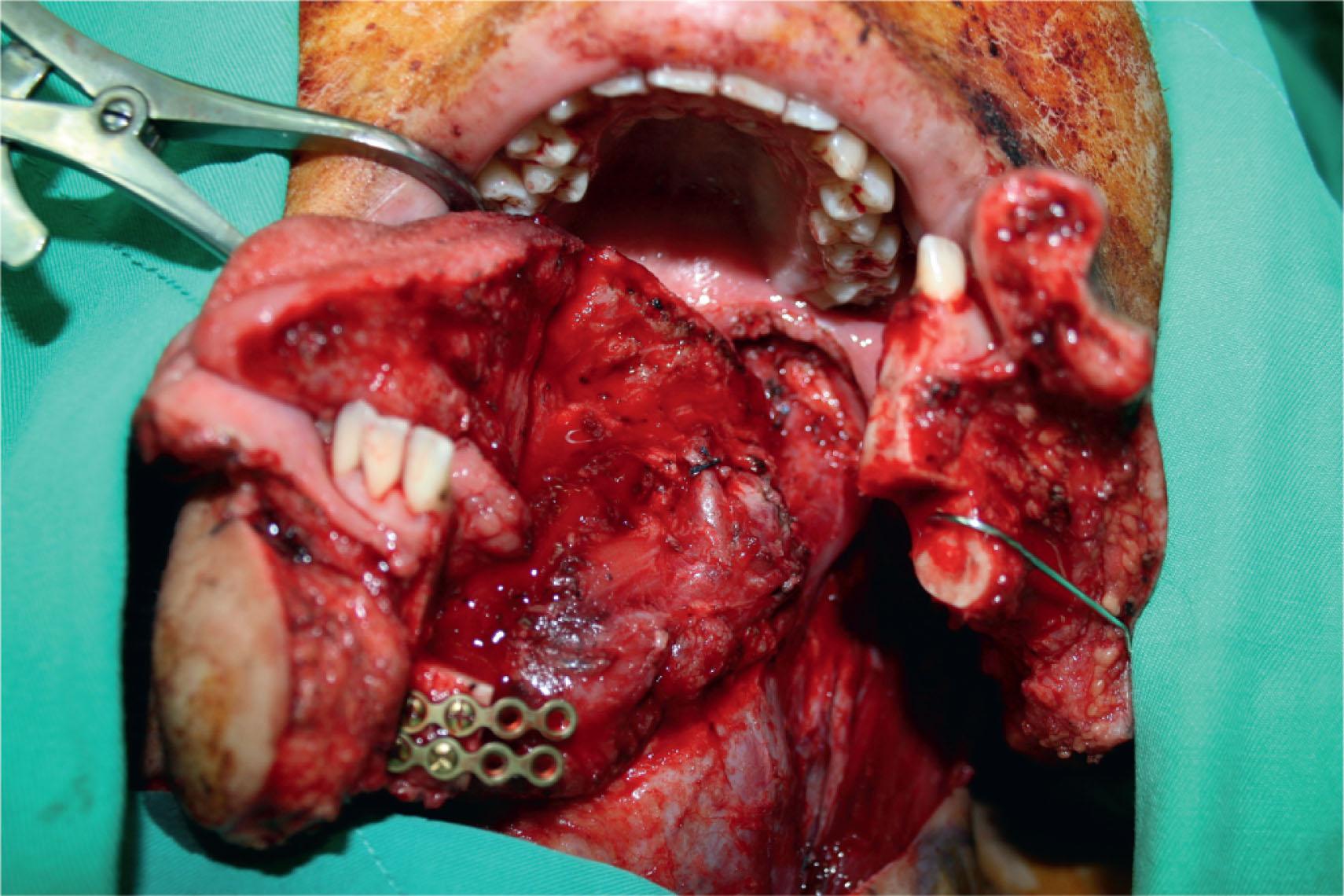




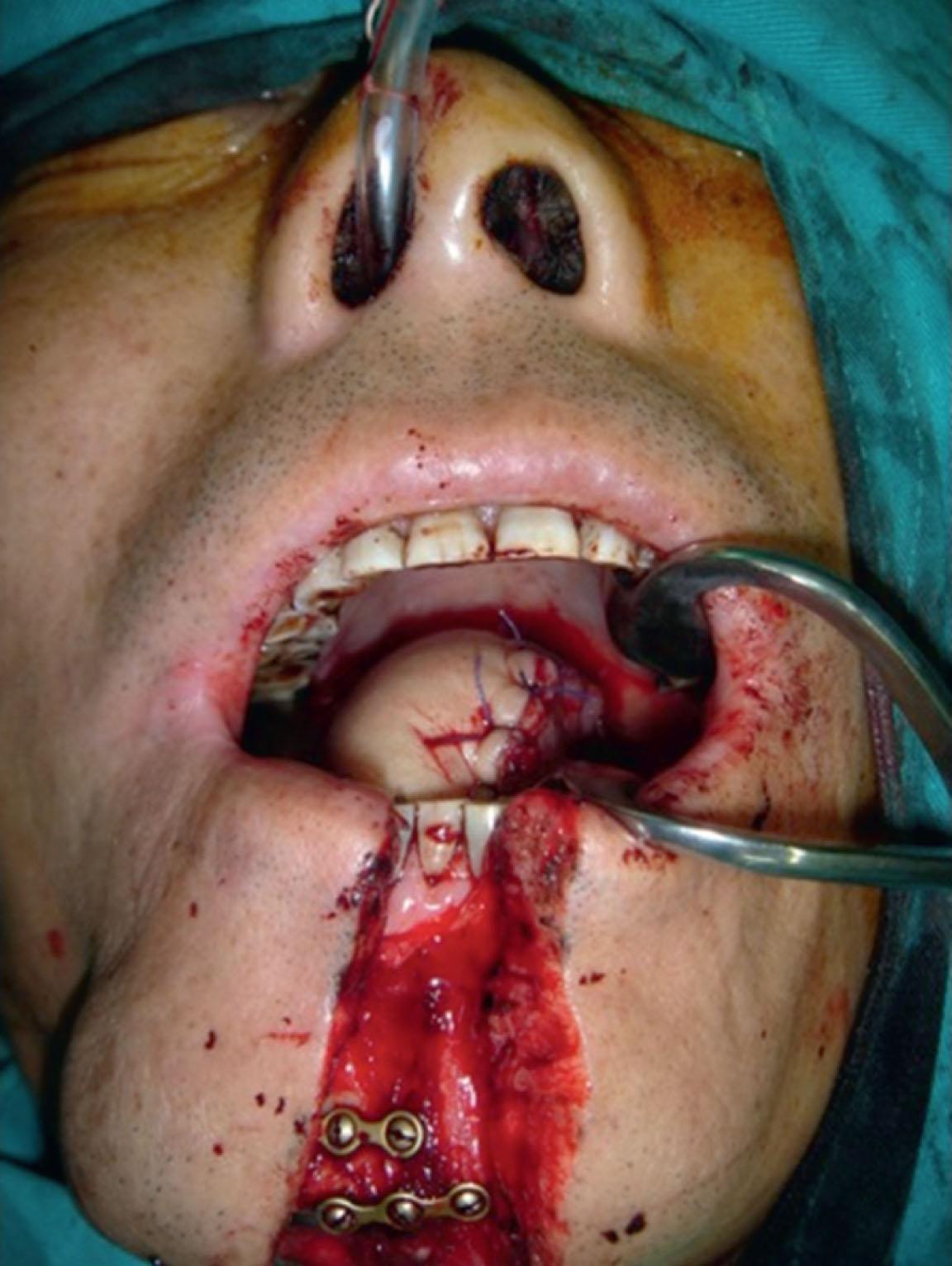

In total glossectomy defect (Cheng’s type III), a specially designed pentagonal-shaped ALT myocutaneous flap provides better flap inset, provides adequate volume, and gives an aesthetically pleasing neo-tongue tip ( Fig. 12.19 ). The “V” shape of the pentagon posteriorly allows a more excellent sloping profile when viewed in cross-section as well as an increasing posterior-to-anterior tongue projection (see Fig. 12.19 ). Such a design yields a well-shaped tissue bulk that resembles a native tongue more closely in its lateral and frontal views. The anterior “I” shape allows increased elevation and mobility of the neo-tongue tip and creates a gingival sulcus that prevents saliva pooling and subsequent drooling. Most of these patients provide ratings of “good” for diet or cosmetic appearance following reconstruction using this method ( Figs. 12.20–12.23 ).
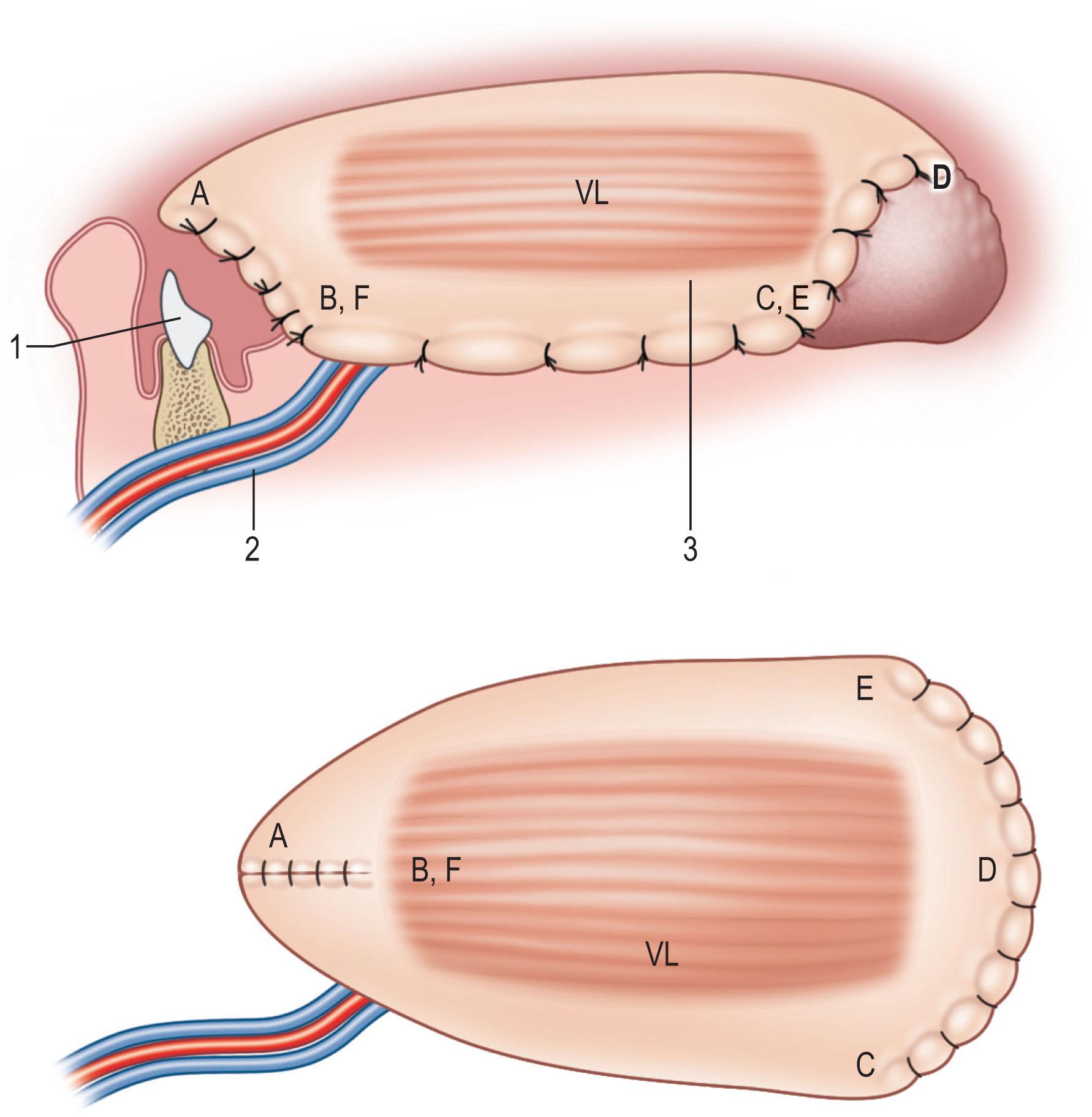

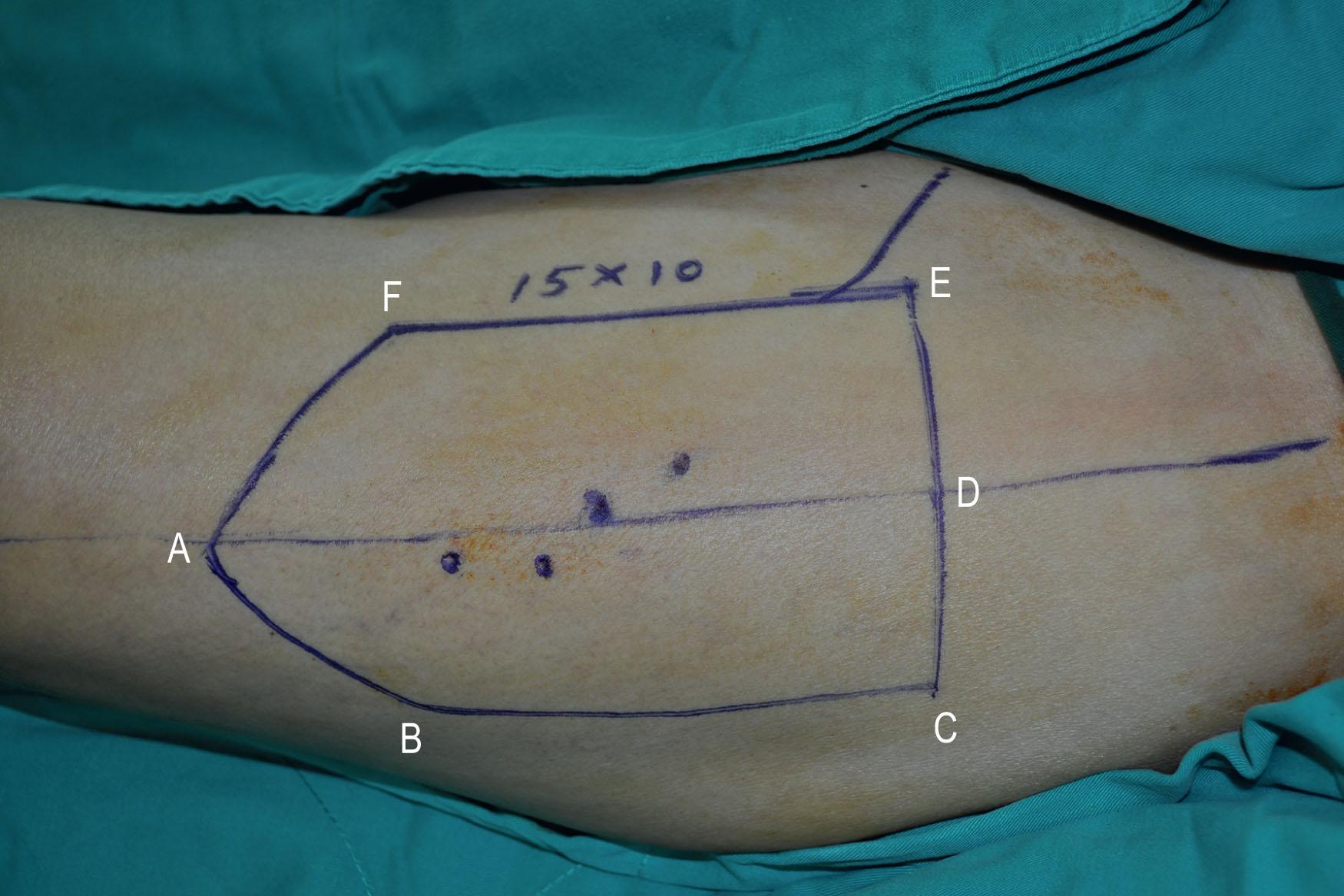
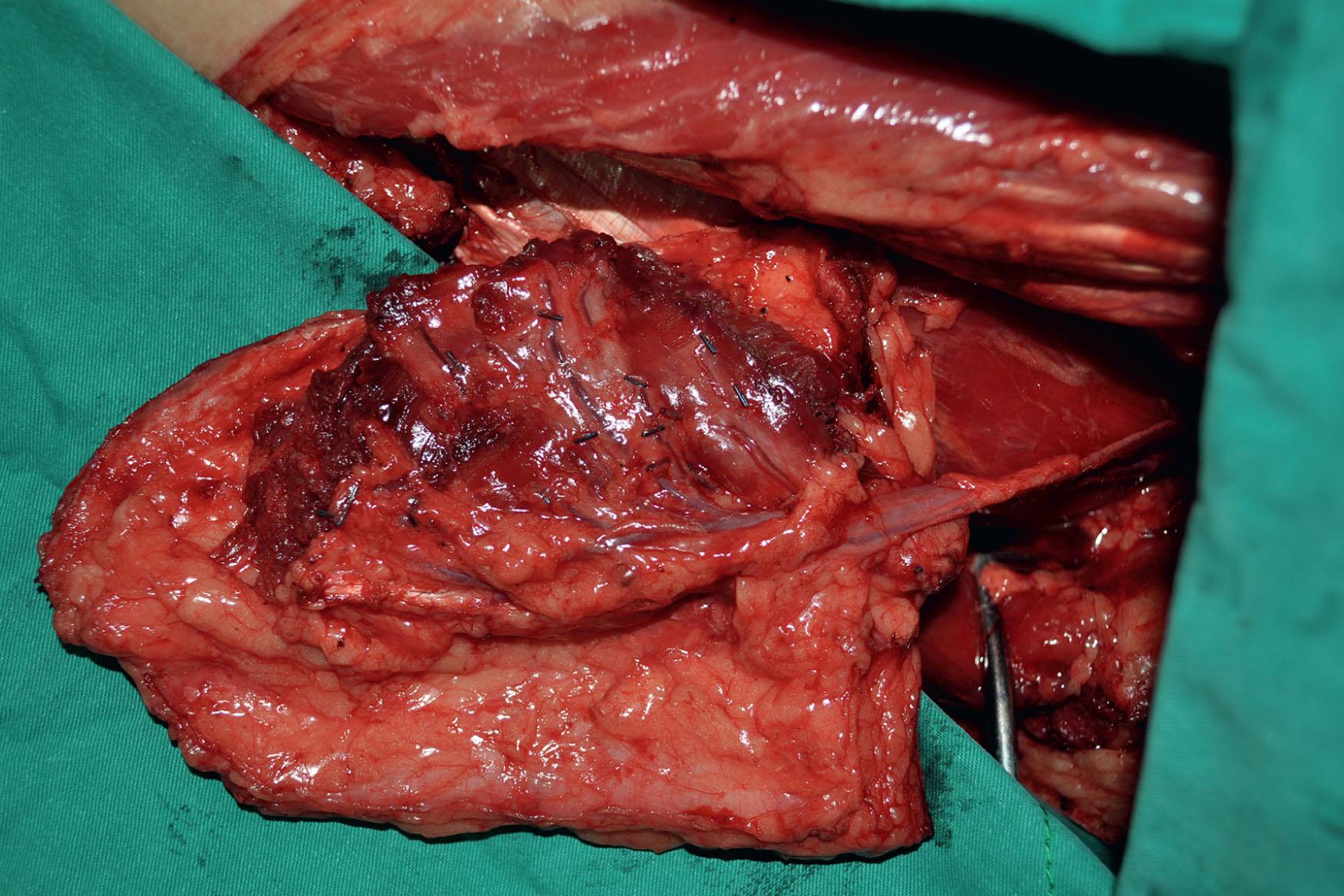

In both Cheng’s IIb and III, the ALT musculocutaneous flap rather than the fasciocutaneous flaps is recommended for reconstruction to provide more bulk to augment the tongue's base. The fullness at the base of the tongue is essential to close off the oropharynx during swallowing with the assistance of the movement of the hyoid bone. The TRAM or VRAM flap, the LD myocutaneous flap, and the PAP flap with adductor magnus are alternatives for Cheng’s IIb and III tongue reconstruction.
Daniel categorized lower jaw defects as isolated, compound, composite, and extensive composite, or en bloc . Isolated defects include any single bone tissue resection; compound defects refer to those involving two tissue layers, such as bone and oral lining or bone and external skin. Composite defects indicate a three-layer defect involving the mucosal lining, bone, and external skin; finally, extended composite or en bloc defects also include extensive soft-tissue loss.
Jewer classified mandibular defects as central, lateral, or hemimandibulectomy. The classification system was further modified by Urken to consider associated soft-tissue defects and by Boyd to recognize subcategories such as mucosa, skin, or a combination of both. These classifications were comprehensive and based on the availability of reconstructive options. With the development of microsurgical techniques and a better understanding of the perforator flaps concept, more reconstructive alternatives became available. The author recommends a modified (Cheng’s) classification of the mandibular defects to define the component tissues involved in the mandibular defects ( Table 12.5 ) with examples and possible reconstructive options compared and summarized in Tables 12.6 and 12.7 ( Algorithm 12.3 ).
| Cheng | Daniel | Jewer and Boyd | Defects | Available managements | Examples |
|---|---|---|---|---|---|
| IA | Isolated | Central (C) | Bone only | Plating, bone graft, bone flap | Benign tumor, trauma |
| IB | Isolated | Lateral (L) | Bone only | Plating, bone graft, bone flap | Benign tumor, trauma |
| IC | Isolated | Hemimandibulectomy (H) | Bone only | Plating, bone graft, bone flap | Benign tumor, trauma |
| IIA | Compound | HCL + mucosal (m) | Bone and intraoral mucosa | Osteocutaneous flap | Stage 3–4 oromandibular cancer |
| IIB | Compound | HCL + skin (s) | Bone and external skin | Osteocutaneous flap | Osteoradionecrosis of mandible |
| IIC | Compound | — | Bone, external skin and soft tissue | Osteocutaneous flap, OPAC flap | Osteoradionecrosis of mandible |
| IIIA | Composite | HCL + mucosa and skin (ms) | Composite 3 layers | Options in Table 12.7 | Stage 4 oromandibular cancer, gunshot wound |
| IIIB | Extensive composite | —- | Composite 3 layers and partial tongue | Options in Table 12.7 | Stage 4 oromandibular cancer, gunshot wound |
| IIIC | Extensive composite | — | Composite 3 layers and partial maxilla | Options in Table 12.7 | Stage 4 oromandibular cancer, gunshot wound |
| Bone | Skin | Combined muscle | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Height | Firmness | Length | Reliability | Pliability | Availability | Volume | Pedicle length | Donor site morbidity | Disadvantages | |
| Fibula | ++ | ++++ |
|
++++ | ++++ | Soleus | ++++ | +++ | ++++ | Flap inset |
| Iliac | ++++ | ++++ | +++ | + | + | Vastus lateralis | ++++ | + | ++ | Donor site morbidity, partial skin paddle loss |
| Scapula | + | + |
|
++++ | +++ | Latissimus dorsi | ++++ | ++ | +++ | Intraoperative change of position |
| Radius | + | ++ |
|
++++ | ++++ | — | ++++ | + | Radius fracture | |
| Rib | ++ | ++ |
|
++ | ++ | Pectoralis major, serratus anterior | ++ | ++ | +++ | Tenuous periosteal perfusion |
| Second metatarsal | + | ++++ |
|
++++ | ++++ | — | — | +++ | ++ | Donor-site morbidity |
| Cheng’s classification | Defects | Option 1 Soft-tissue flap with reconstruction plate | Option 2 One free flap and one pedicled flap | Option 3 Double free flaps | Option 4 Chimeric flap – LCFA | Option 5 Composite flap – scapula | Option 6 Composite flap – OPAC |
|---|---|---|---|---|---|---|---|
| IIIA | Bone | Reconstruction plate | Fibula | Fibula | Iliac | Scapula | Fibula |
| Mucosa | ALT flap or RA flap | Fibula skin | Fibula skin | ALT flap | Scapula/parascapular skin | Fibula skin | |
| Soft tissue | Vastus lateralis, RA | Pectoralis major, deltopectoral | Vastus lateralis, RA | Vastus lateralis | LD | Soleus | |
| External skin | ALT flap or RA flap | Pectoralis major, deltopectoral | Radial forearm or ALT, RA | Groin skin | Scapula/-Parascapular skin | Fibula skin | |
| IIIB | Tongue | ALT flap or RA flap | Fibula skin | Fibula skin | ALT flap | Scapula/parascapular skin | Fibula skin |
| IIIC | Maxilla | Vastus lateralis, RA | Pectoralis major, deltopectoral | Vastus lateralis, RA | Vastus lateralis | LD | Soleus |

Modified (Cheng’s) classification of the mandibular defects is used to define the component tissues involved in the mandibular defects with the most appropriate reconstructive options for maximal functional and cosmetic outcomes suggested. There are eight configurations related to the mandibular defect sites, donor fibula laterality, peroneal skin paddle transfer, and recipient vessel selection. FA, Facial artery; STA, superficial temporal artery.
The nasolabial flap was first described as a fasciocutaneous flap and transferred as an inferior-based or superior-based flap. A well-studied vasculature makes it possible to design a conventional pedicle flap, a subcutaneous pedicled flap, or an island pedicle flap based on the facial artery. For a small buccal mucosa reconstruction, an inferior-based flap design is recommended. The flap width must be between 2 and 3.5 cm to allow donor site primary closure. The flap can reach the ipsilateral buccal mucosa with careful flap design, representing an excellent reconstructive option in minor defect resurfacing. The nasolabial flap has been applied in the buccal mucosa, lip, mouth floor, and nasal reconstruction.
Egyedi first described the pedicled buccal fat pad flap for oncological reconstruction. The buccal fat pad is an encapsulated fat tissue located in the masticatory space between the buccinator and masseter muscles. It contains a central body with extensions to the buccal, temporal, and pterygoid regions. The central body and the buccal extension comprise up to 50% of the fat pad and are the most familiar portion for reconstructing the buccal mucosa. A rich blood supply network comprising the superficial temporal artery, transverse facial artery, and internal maxillary artery nourishes this flap. It is a reliable flap to reconstruct small to moderate-sized mucosal defects. The major drawback of the pedicled buccal fat pad flap is the lack of a skin paddle for resurfacing. Traditionally, the flap was covered with a split-thickness skin graft for rapid wound healing but tends to lead to postoperative scar contracture. If left uncovered, a spontaneous epithelialization can instead be achieved within 3 weeks.
A buccal fat pad flap of 10 mL in volume and 6 mm in thickness and a length of 4–5 cm can reliably be harvested to reconstruct intraoral defects less than 5 cm in diameter. Approaching the buccal fat pad is relatively easy, as the flap can usually be accessed through the resection wound. By dissecting upward and posteriorly away from the Stensen duct through the buccinator muscle, the capsule fascia of the buccal fat pad can be opened, and the buccal fat pad can be pulled into the oral cavity by gentle distraction and a push force from the external cheek inferior to the zygomatic arch.
The FAMM flap comprises mucosa, submucosa, buccinator muscle, and the underlying facial artery as the flap's pedicle. The facial artery's intraoral course can be traced with a pencil Doppler, and the flap can be designed centered over the pedicle. The flap can be harvested as an inferior-based flap (antegrade flow) or a superior-based flap (retrograde flow). An average flap width of 1.5–2 cm and flap length of 8–9 cm can be obtained. An incision initiates flap dissection through the mucosa, submucosa, and buccinator muscle. The facial artery is found underneath the buccinator muscle. Because the buccinator muscle is part of the oro-sphincter muscle, it is crucial to reapproximate the muscle after flap dissection.
The FAMM flap has the advantage of providing tissue of similar type, color, and texture to that of the buccal mucosa, palatal mucosa, and lip. It is, therefore, a popular flap for small intraoral and vermilion reconstruction. The only drawback is that the limited flap width prevents its application from moderate to extensive defects.
The submental flap can be transferred as a pedicled or free flap to the oral cavity. The submental flap's blood supply derives from the submental artery, a continuous branch of the facial artery, located 5–6.5 cm away from the facial artery's origin. This branch penetrates deep to the submandibular gland below the mandible lower margin, extending medially deep to the digastric muscle's anterior belly. As the vessel travels along the mandible margin, it sends off cutaneous perforators through the platysma muscle to the skin. The vascular anatomy is consistent and the perfusion it provides to the submental skin is reliable.
The flap design is initiated by marking the inferior mandible border as the upper margin. The flap length can extend from the ipsilateral mandible angle to the contralateral mandible angle. The flap width depends on the skin's laxity, but usually 5 cm can be obtained, and even more in older patients with redundant skin. The flap can be elevated as an axial flap or a perforator flap, but the less complicated surgical technique is to elevate the flap as an axial flap without perforator dissection. An incision is made in the inferior margin of the flap directly through the platysma muscle. Then, the dissection is carried out with harvesting of the platysma and anterior belly of the digastric muscle, including the perforator and main submental vessels. When the pedicle is identified, its branches to the submandibular gland should be ligated carefully. Finally, when reaching the inferior mandible border, care should be taken to protect the marginal mandibular nerve. If the flap is going to be transferred as a free flap, dissection of the pedicle can be continued to the facial vessels to obtain a better size and length for anastomosis.
The submental flap is suitable for rotating to the lower third of the face and the entire oral cavity, making it a convenient pedicle flap for oral cavity reconstruction. The major drawback is that the submental skin's continuity and its main pedicle are usually disrupted, particularly during wide tumor excision and neck lymph node dissection at the cancer ablation surgery.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here