Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Optical coherence tomography (OCT) is an innovative catheter-based imaging technology that uses light and fiberoptic technology to obtain unique details of the vessels on a microscopic scale. OCT provides real-time, full tomographic views of the coronary arteries with accurate measurements.
OCT is an efficient method to rapidly map the extension and type of coronary artery disease, guiding precise percutaneous coronary interventions (PCIs) in complex patient and lesion cohorts (i.e., acute coronary syndromes, long lesion, bifurcations to be treated with two stent strategy, calcified vessels) or in presence of angiographic ambiguity. Because of its unique properties and high axial imaging resolution, OCT is able to overcome many of the limitations of coronary angiography and intravascular ultrasound (IVUS), including assessment of extension and thickness of calcium, identification of thrombus and thereby the responsible lesion for acute coronary syndromes, detection of causes of stent failure, and addressing more precise and effective coronary interventions.
OCT can reliably detect and quantify atherosclerotic plaque characteristics (fibrous cap thickness and integrity, thrombus, neovessels, lipid pool, macrophage accumulations), thus differenciating early-stage coronary lesions from advanced ones, with close correlation with pathology. Identification of the underlying etiology in acute coronary syndrome patients (atherothrombotic: ruptured plaque, eroded plaque, calcified nodules; nonatherosclerotic: spontaneous coronary dissections, embolic disease, spasm, external compression) based on OCT features may prompt different treatment regimens. In addition, OCT provides quantitative indices that can be used to assess the effectiveness of novel pharmacologic interventions on plaque stabilization.
Based on accurate vessel mapping, a procedural plan during PCI, including stent length, size, and landing zone, can be generated. Recent OCT (FD-OCT) systems produce a clear visualization of long coronary segments within a few seconds, with a more complete picture of the longitudinal vessel involvement (long view), which is a better guide for PCI compared with standard cross-sectional methods. Automatic lumen detection border allows for immediate identification of the site and severity of coronary stenosis, lesion length, and reference lumen and/or vessel dimensions, for appropriate stent sizing and length.
A major limitation of the current practice of intracoronary imaging is that the information is not immediately correlated with the images obtained by the coronary angiography. OCT coregistration enables real-time, point-to-point, correspondence between OCT imaging and the angiographic lumen contour, leading to full integration of OCT into PCI workflow.
Direct tableside control of OCT acquisition and analysis by the operator enables the use of OCT in daily practice, and automatic visualization of stent struts malapposition and stent underexpansion facilitates stent optimization.
Three-dimensional (3D) reconstruction of OCT images after complex stent positioning (i.e., dual stents in bifurcation, or provisional stent across a large side branch that needs to be recrossed) may facilitate rewiring in the appropriate cell to avoid stent distortion and/or strut malapposition.
The authors thank Keisuke Satogami, MD (Department of Cardiovascular Medicine, Wakayama Medical University, Wakayama, Japan); Paolo Canova, MD (Department of Cardiovascular, ASST Papa Giovanni XXIII, Bergamo, Italy) for their help with the figures; Maoen Xu, MD, Tao Chen, MD and Lijia Ma, MD (Department of Cardiology, the Second Affiliated Hospital of Harbin Medical University, Harbin, China) for providing the case used in Fig. 67-22 ; Kostantinos Koskinas, MD (Department of Cardiology, Bern University Hospital, Bern, Switzerland) for his help with the references; Antonio Sorropago, MD (Univerity of Milano-Bicocca, Milan, Italy), Francesco Moretti, MD (University of Pavia, Pavia, Italy) and Francesca Fenili for assistance with manuscript preparation and revision.
Optical coherence tomography (OCT) is an innovative real-time, tomographic imaging modality able to assess and display vascular structure at very high resolution (axial resolution from 10 to 15 μm), providing close correlation with pathology. OCT delivers near-infrared light to the wall of the coronary artery through small-diameter optical fibers. The light that illuminates the vessel is absorbed and reflected (backscattered) by the structures in the tissue to different degrees. Different tissue types have different optical characteristics and the wavelength was selected so that lipid-rich tissue can be differentiated from fibrous and calcified tissue. This feature makes OCT especially useful for following plaque progression and regression and the vascular response to coronary stent implantation, including neoatherosclerosis deposits. Although OCT resolution is not sufficient to distinguish individual cells (including regenerating endothelium), OCT is able to measure the density of cellular aggregates and to provide information about their composition. For example, the OCT imaging signal characteristics observed in ex vivo coronary arteries in the presence of accumulated foamy macrophages (high signal intensity with high attenuation, sharp shadowing, and rapid changes from frame to frame) have been demonstrated to correlate with foam cell density, whereas in preclinical model, fibrin deposition on stent struts, as detected by light microscopy and scanning electron microscopy (SEM), has specific attenuation properties by OCT. Recent data also indicate that OCT can measures the collagen and smooth muscle cell in atherosclerotic plaques. The current generation of OCT systems has significantly improved the signal-to-noise ratio, with superior near-field image quality and faster acquisition. The lumen profile, displayed at the end of a fast (2 to 3.5 seconds) pullback and based on automatic lumen detection border in every frame, provides immediate identification of the minimal luminal area (MLA) and the maximum degree of stenosis for a more accurate planning of the percutaneous coronary intervention (PCI) procedure. OCT may be used at different times as part of diagnostic and interventional procedures, before and after stent implantation ( Fig. 67.1 ). Quantitative measurements of lumen, stent, and neointimal areas obtained by OCT are highly reproducible among different assessors and corelabs.
The ability of OCT to create tomographic cross-sectional images of a vessel is based on optical interference of near-infrared light. Light is emitted and collected back from the tip of a small-diameter optical fiber that rotates rapidly inside a transparent sheath of the catheter. For longitudinal scanning of the vessel, a drive motor unit on the table pulls back the fiber as it rotates within the sheath. The collected light carries information about the depth-resolved backscattering reflectivity of the tissue structures at the illuminated spot. Unlike ultrasound, in which the depth information can be resolved by measuring the time-of-flight of acoustic reflections, light is too fast to resolve depth information by direct measurement of photon time-of-flight. To overcome this property, the collected light is combined with a reference light beam to generate lower-frequency optical interference signals. These modulated interference signals can be measured with electronic components and digitally decoded to generate an optical backscattering profile of the tissue at the illuminated spot (A-scan). Multiple backscattering profiles are collected as the catheter core rapidly rotates within the vessel. These profiles are then represented in a two-dimensional (2D) image, the OCT B-scan frame displayed to the user.
In current Frequency-Domain OCT (FD-OCT), interference signals generated by an interferometer at various wavelengths are mathematically processed by Fourier transformation to determine the amplitudes of reflections returning from different depths. As in magnetic resonance imaging, the frequency of the recorded signal encodes position in the image.
The current generation of commercial OCT systems have a high image acquisition rate (158 to 180 frames/s), with a ranging depth of 4.6 to 5 mm in tissue. A pullback length of 75 up to 150 mm is obtained in 2 to 3.5 seconds at maximum pullback speed of 40 mm/s.
The current generation of OCT systems improved image quality and significantly increased the speed of image acquisition by a factor of 10 compared with early generation systems. OCT uses a contrast flush (3 to 4.5 mL/s depending on vessel size) to clear the blood column and allow a clear path for the light beams to be emitted, reflected, and detected. Imaging catheters are designed for rapid exchange over a 0.014-inch guidewire delivery, with a crossing profile of 2.4 to 2.8 Fr, compatible with 6-Fr or larger guiding catheters. Because of the very high speed of the pullback, OCT has the ability to scan longer segments of artery without need to occlude the vessel, resulting in minimal ischemic electrocardiographic changes and no major arrhythmias during imaging acquisition.
The safety of use of OCT in current clinical practice (including patients presenting with ST-elevation myocardial infarction [STEMI] and/or stent thrombosis) has been repeatedly demonstrated. Recently, Van der Sijde and colleagues reported the safety of intracoronary imaging (intravascular ultrasound [IVUS] and OCT) in more than 3600 consecutive diagnostic or interventional procedures (1142 performed with OCT and 2476 performed with IVUS). Invasive imaging-related complications, such as transient ST elevation, bradycardia, coronary spasm, thrombus formation, dissection, or stent deformation, were infrequent (OCT: n = 7, 0.6%; IVUS: n = 12, 0.5%; P = .6) and easily treatable. No major adverse events, prolongation of hospital stay, or enduring patient harm were observed.
Artifacts can be detected in all types of imaging, including OCT. Some artifacts are common to OCT and IVUS, whereas others are unique to the OCT imaging systems. Nonuniform rotational distortion (NURD) is caused by increased friction on the rotating components of the catheter during image acquisition and results in circumferential imaging loss or shape distortion ( Fig. 67.2B ). Data on bench tests have shown fewer NURD artifacts with OCT compared with IVUS. The sew-up artifact results from rapid artery or imaging wire movement during single-frame imaging formation, leading to single-point misalignment of the luminal border (see Fig. 67.2A ). Increased friction and deformation on the rotating components of the catheter, mainly caused by tortuous segments of coronary arteries or narrow calcified lesions, may result in coexisting multiple artifacts. The fold-over artifact is more specifically related to the new generation of FD-OCT devices. It is the consequence of “phase wrapping” or “aliasing” along the Fourier transformation when structure signals are reflected from outside the system’s field of view (see Fig. 67.2F ). Eccentric catheter position may affect OCT images differently before and after stent implantation. Tangential signal dropout due to eccentric catheter position adjacent to the luminal wall can lead to misinterpretation of lesions, generating a false impression of thin-cap fibroatheroma (TCFA), even giving the impression of a ruptured plaque under certain circumstances (see Fig. 67.2E ). In stented coronary arteries, eccentric catheter position may distort the image of the struts at distance from the scanning fiber as a result of the longer distance between each A-line and reduced lateral resolution.
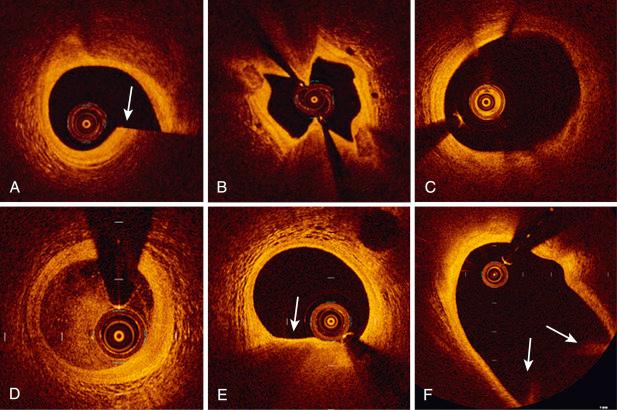
All cross-sectional images (frames) obtained with a pullback need to be initially screened for quality assessment and should be excluded from analysis if any portion of the image is out of the screen or if the image had poor quality caused by residual blood, artifacts, or reverberation (see Fig. 67.2 ).
OCT tissue characterization is based on signal intensity (backscattering) and signal attenuation. The high resolution of OCT allows superb definition in the near field with precise bordering of the intima and media within the coronary arterial wall. In normal coronary artery, the intima appears as a signal-rich layer nearest to the lumen, whereas the media is visualized as a signal-poor middle layer that encircles the vessel (three-layer structure) ( Fig. 67.3A ). The ability to evaluate subtle intimal thickening in vivo allows detection of the early phase of coronary atherosclerosis and monitoring of the plaque progression over time (see Fig. 67.3B ). The OCT measurements of the intimal thickness are well correlated with findings on histologic examination. Yabushita and coworkers developed objective OCT imaging signal criteria for differentiating distinct components of atherosclerotic tissues. In their histology-controlled OCT study of 357 autopsy segments from 90 cadavers, calcified plaques appeared as signal-poor region (i.e., low backscattering) with low attenuation (signals behind can be appreciated) and with very sharp border zones ( Fig. 67.4F ); lipid-rich plaques, covered by a fibrous cap were characterized by a hyperintense internal layer (high backscattering), an underlying signal-poor layer with indefinite borders (high attenuation) and no other structures visible deeper in the arterial wall (see Fig. 67.4D ); fibrotic plaques were seen as homogenous layers with high signal intensity (high backscattering) and low attenuation so that signals behind (e.g. media, adventitia) can still be detected. Validation tests revealed good intraobserver and interobserver reliability ( κ = 0.83 to 0.84), as well as excellent sensitivity and specificity: 71% to 79% and 97% to 98%, respectively, for fibrous plaques, 95% to 96% and 97% for fibrocalcific plaques, and 90% to 94% and 90% to 92% for lipid-rich plaques. These definitions formed the basis for the in vivo assessment of plaque components and were subsequently endorsed by a Consensus Document on standardization and validation. Using these definitions, Kawasaki and colleagues reported that OCT provided better separation of coronary plaque constituents than integrated backscatter or conventional IVUS. For fibrotic plaques, the sensitivities were 98%, 94%, and 93%, respectively, and the specificities were 94%, 84%, and 61%. For calcified plaques, the sensitivities were 100%, 100%, and 100%, and the specificities were 100%, 99%, and 99%, respectively. For lipidic plaques, the sensitivities were 95%, 84%, and 67%, and the specificities were 98%, 97%, and 95%, respectively.
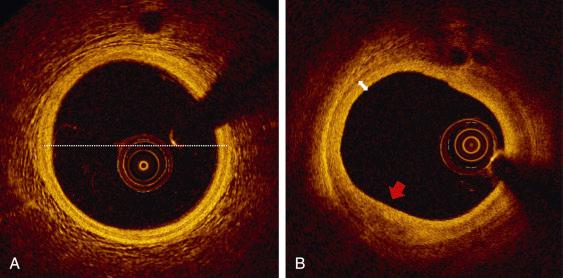
Distinguishing the three main tissue types from each other has a primary role in planning the PCI strategy in terms of lesion preparation and optimal stent results, as the presence of large calcifications (e.g. >180 degrees, thickness >0.5 mm, length >5 mm), is a relevant predictor of poor stent expansion, whereas lipid-rich tissue may increase the risk of plaque embolization and of no-reflow phenomenon. Furthermore, careful evaluation of the landing zone to avoid stent edge positioning in a segment with significant residual disease (i.e., >50% plaque burden) and especially in a lipid-rich plaque with a large lipid arc (>185 degrees) may reduce the stent edge recurrences.
OCT detection of the external elastic lamina (EEL) can be used as a practical tool for assessing plaque composition and eventually appropriate stent size selection. If EEL (rope) and adventitia (mesh) are visualized in a cross-sectional image, overlying normal tissue (three layered) or fibrous plaque (high intensity) can be anticipated. Conversely, if EEL cannot be seen underneath a plaque and OCT signal changes across the vessel wall (without evidence of thrombus into the lumen), high attenuation identifies a lipid plaque, whereas low attenuation and clear border recognize a calcified plaque (see Fig. 67.4 ).
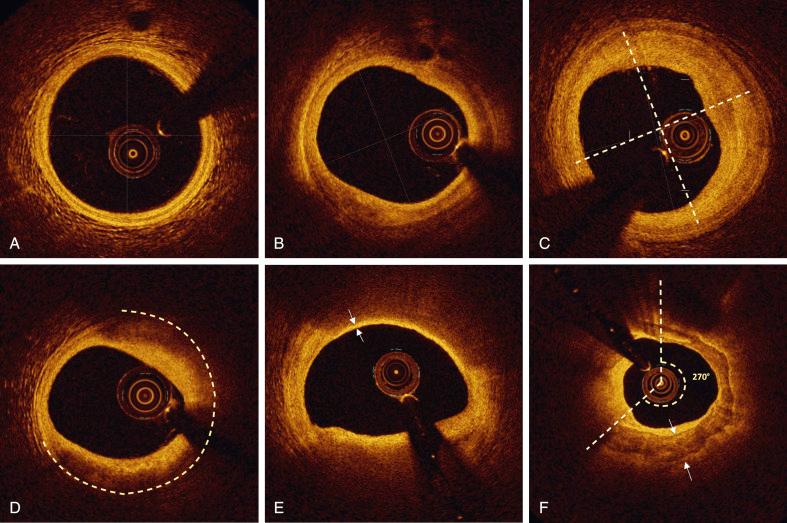
During PCI, the knowledge of the composition of the vessel wall at the landing zones may be relevant to avoid geographical miss and select appropriate stent sizing. The threshold required to make EEL-based decisions is the visualization of at least 180 degrees of EEL at cross-sectional level. In the ILUMIEN III randomized controlled trial, the EEL identification at the reference segments was possible in 84% of the PCI cases. Local sites EEL identification was similar with OCT and IVUS, whereas core lab assessment found slightly better EEL visualization with IVUS. OCT-guided PCI using a specific EEL-based stent optimization strategy was noninferior to IVUS-guided PCI for achieving minimum stent area (MSA) and resulted in superior stent expansion and procedural success compared with angiography-guided PCI.
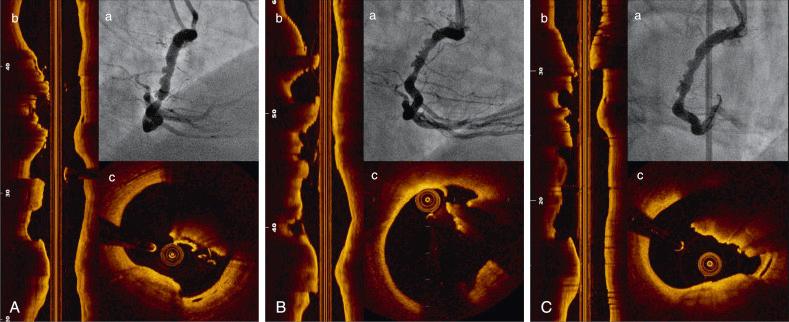
Unlike IVUS, coronary thrombus can be easily detected by OCT in various clinical settings, including acute coronary syndromes (ACSs) and stent thrombosis. A close correlation with pathology was demonstrated in 108 coronary arterial segments at postmortem examination. White thrombus is imaged as a homogeneous, signal-rich, irregular mass with low-backscattering attenuation, whereas red thrombi are irregularly shaped, mural or luminal masses protruding into the lumen, characterized by high-backscattering attenuation and signal-free shadowing. Using a measurement of OCT signal attenuation within the thrombus, Kume and colleagues demonstrated that a cutoff value of 250 μm in the half-width of signal attenuation can differentiate white from red thrombi with high sensitivity (90%) and specificity (88%). Thrombus detection may have important clinical implications, including identification and characterization of culprit plaque in patients presenting with ACS and unclear angiography, identification of thrombus forming acutely during PCI ( Fig. 67.5 ), and monitoring of mechanical and/or pharmacologic interventions for thrombus removal. Various type of thrombus may require different approaches and interventions ( Fig. 67.6 ).
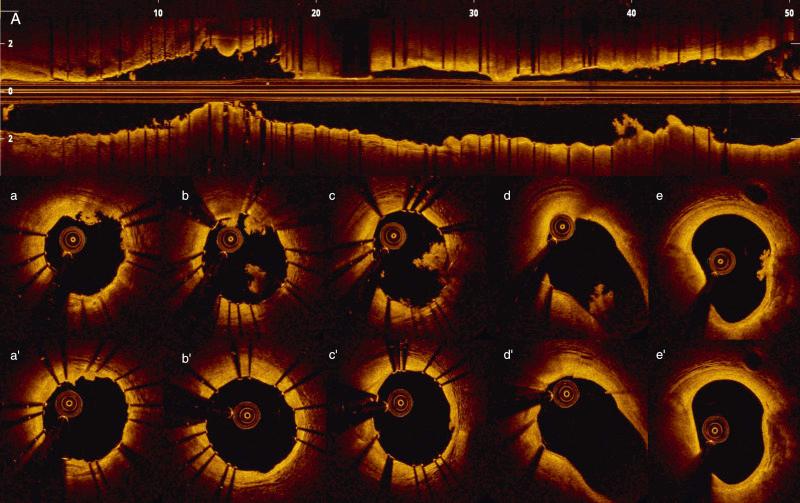
Among various plaque components with clinical impact, macrophage accumulation plays a major role to trigger progression and instability of atherosclerotic coronary lesions. Autopsy series have shown that the thin caps of ruptured plaques are heavily infiltrated by macrophages. Given the close proximity to the lumen and its unique axial resolution, OCT enables detection of the pool of foamy macrophages confined to the luminal surface of the plaque. Macrophages are identified in OCT images by the presence of high-intensity, signal-rich, linear or confluent punctuate regions (bright spots) accompanied by high attenuation and confirmed in adjacent consecutive frames. Tearney and associates tested ex vivo the ability of OCT to assess macrophage distribution within the fibrous cap. A high degree of positive correlation was reported between OCT signals and histologic measurements of fibrous cap macrophage density ( r < 0.84, P < .0001). A range of OCT signal standard deviation thresholds (6.15% to 6.35%) yielded 100% sensitivity and specificity for identification of caps containing greater than 10% CD68 staining (see Fig. 67.6 ). Concerns have been raised about the specificity of bright-spot signals with intense attenuation in identifying macrophages. OCT may fail to discriminate macrophages from cellular fibrous tissue, calcium-fibrous tissue interfaces, microcalcifications, or cholesterol crystals.
A milestone document on standardization, validation, and reporting for OCT, published by the International Working Group for Intravascular Optical Coherence Tomography, provides basic notions for collecting and interpreting imaging data with a high standard of quality and reliability.
Postfixation and frozen measurements in excision tissues are not easily comparable to in vivo assessment because of possible changes in arterial optical properties caused by technical preparation and tissue shrinkage. The processes of dehydration, paraffin embedding, sectioning, and staining result in a reduction of the circumference by approximately 19% ± 5% and a reduction in wall thickness of 18% ± 2%. Furthermore, backscattering of OCT signals due to fixation of cross-linked collagen may enhance the delineation between lumen and vessel wall, compared with in vivo lumen measures, resulting in an artificial increase of reflectivity. The gap in lumen dimensions observed between OCT and histology findings in nonstented coronary segments was less clear when the arteries were treated with metallic stent implantation, with approximately 6% greater stent area and 10% greater luminal area by OCT across all stent types.
In measurements of lumen diameter and luminal area, there is a good in vivo correlation between OCT and IVUS.
To clarify differences in measures among current imaging techniques, a prospective, multicenter study comparing quantitative coronary angiography (QCA), IVUS, and OCT (Optical Coherence Tomography Compared with IVUS in a Coronary Lesion Assessment Study [OPUS-CLASS]) was performed by Kubo and colleagues in 100 patients and in a phantom model. OCT accurately measured the MLA compared with the actual phantom, whereas IVUS significantly overestimated the MLA and was less reproducible than OCT ( P < .001 vs. OCT). In the clinical study the minimal lumen diameter (MLD) measured by QCA was significantly smaller than that measured by OCT or IVUS ( P < .001), and the MLA measured by IVUS was significantly greater than that measured by OCT, demonstrating that more accurate measurements of the coronary lumen may be achieved by using OCT compared with IVUS or QCA. In summary, compared with IVUS, OCT systematically underestimates the lumen dimensions by approximately 10%. This has clinically relevant consequences when MLA cutoffs for treatment decisions are considered, because previous validation versus fractional flow reserve (FFR) was mainly derived from IVUS studies (e.g., assessment of lesion significance in left main [LM] disease).
Reproducibility of OCT lumen and length measurements was evaluated by Fedele and associates. In their study, OCT measurements were taken twice at intervals of 5 minutes in 25 patients undergoing coronary angiography. The per-segment and per-frame analyses proved to have excellent reproducibility with high correlation for intraobserver and interobserver measurements and intrapullback assessments of both lumen area and segment length ( R ≥ 0.95 and P < .001 for all).
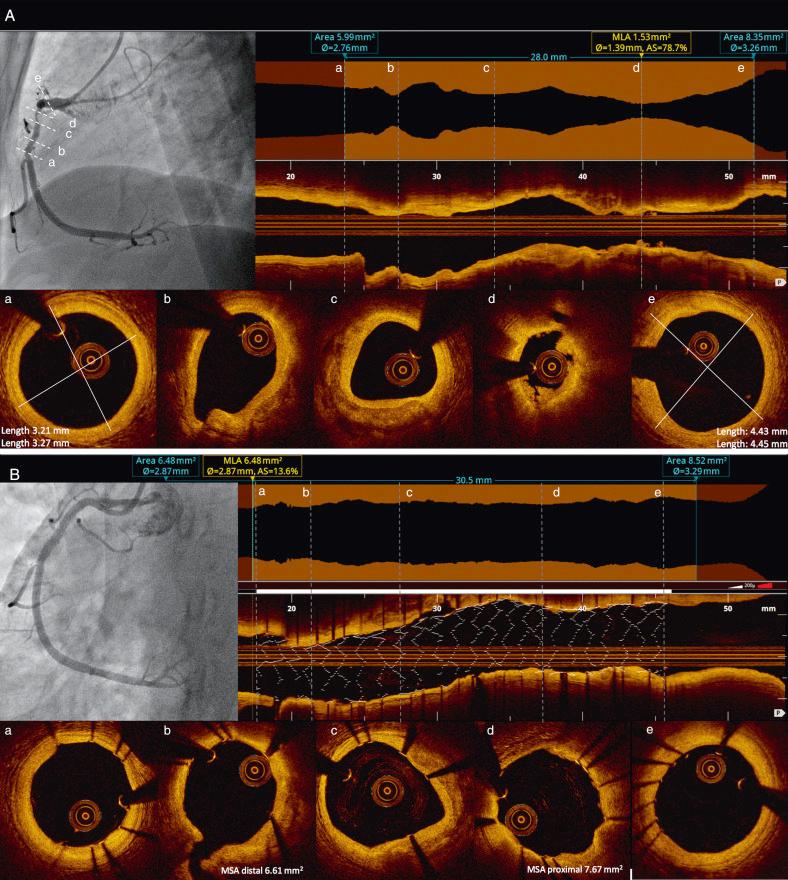
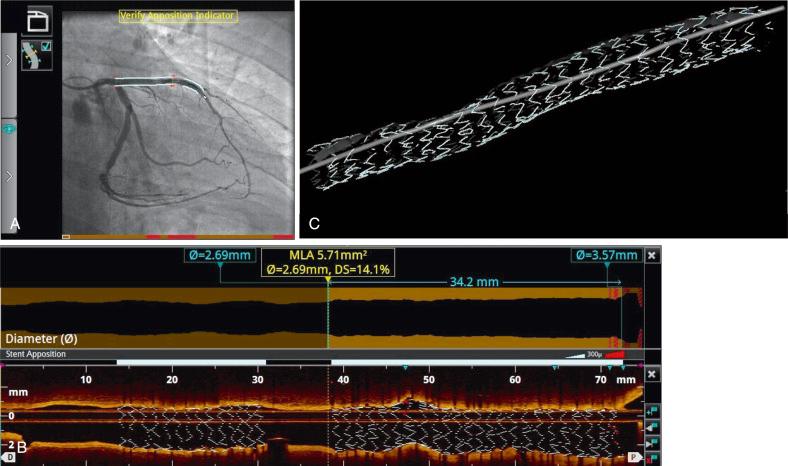
A semiautomated contour-detection software that traces lumen boundaries of the longitudinal (L-mode) view is used for measurements in current generation OCT systems. The software automatically shows the cross-sectional areas calculated at all frames of the pullback as a graph superimposed on the longitudinal view. This lumen contour function enables quick and precise comparison of the automatically detected MLA with the reference areas over the entire length of the vessel imaged by the pullback ( Fig. 67.7 ). A good correlation ( r = 0.99) between manual and quantitative, fully automated three-dimensional (3D) lumen contour detection methods has been demonstrated.
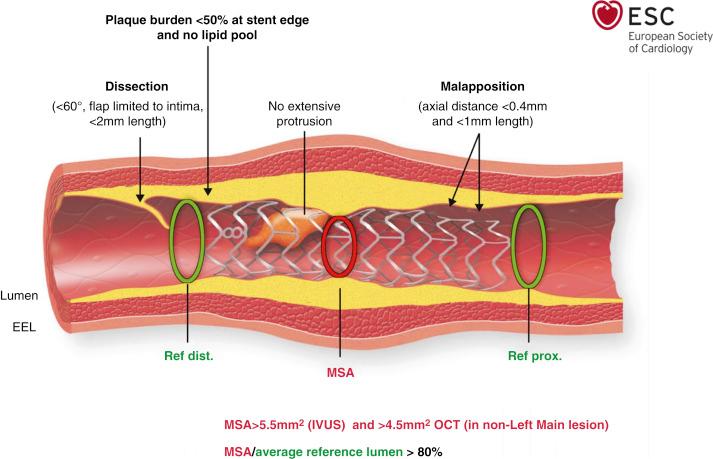
In spite of the technical ameliorations in optics, rapid imaging acquisition, automatic lumen detection and measurements (enabling use in daily practice), the adoption of OCT by cardiovascular centers remains extremely heterogeneous, ranging from routine use in Japan to more selective or occasional use in most other countries. An expert consensus document on clinical use of intracoronary imaging organized by the European Association of Percutaneous Cardiovascular Interventions (EAPCI) appraises current evidence on clinical indications and provides consensus opinion regarding patients or lesions most likely to derive clinical benefit from an imaging-guided intervention, key parameters that characterize optimal stent results, and areas that warrant further research (i.e., algorithm standardization for PCI guidance) ( Fig. 67.8 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here