Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Proctocolectomy with ileal pouch–anal anastomosis (IPAA) has become the standard restorative procedure for patients requiring surgery for ulcerative colitis. Since the operation's introduction in 1978 by Sir Alan Parks, several refinements have occurred as new medications and technologies have come into practice. New biologic therapies have become a standard in medical management, and they have forced the surgeon to better understand the physiologic state of the patient and reconsider the optimal timing of surgery. Advances in stapling technology and emergence of the double-stapled anastomosis have allowed for more efficient pouch construction and improved functional outcomes, respectively. Minimally invasive procedures have gained support because of more rapid recovery, better cosmesis, and potential long-term benefits. The procedure continues to carry the risk of complications, such as pelvic sepsis and delayed onset of Crohn disease, which influence long-term pouch function and durability. Careful preoperative evaluation and timely postoperative intervention are critical to improving surgical outcomes. This chapter will focus on two- and three-stage restorative proctocolectomy via straight and hand-assisted laparoscopic approaches. Alternative procedures to the IPAA will also be discussed.
Patients with ulcerative colitis may require surgery either because they have failed to respond to medical therapy or because the risks of medical therapy outweigh its benefits. Furthermore, the disease can cause debilitating symptoms and lead to a poor quality of life despite appropriate medical treatment. Acute colitis that cannot be controlled is the most common presentation of failed medical management, but chronic issues such as persistent anemia, malnutrition, and protein-losing enteropathy may also prompt consideration of surgical intervention. Close consultation between the patient, gastroenterologist, and surgeon is important to ensure the most appropriate timing for surgery. Delayed operative intervention for patients with acute severe ulcerative colitis is associated with an increased risk for postoperative complications. Patients must be counseled about the short- and long-term risks and benefits of the medical and surgical options so that an informed decision can be derived. It is also important to consider the value of each treatment modality. Patients who undergo definitive surgery for ulcerative colitis have reduced direct medical costs in the 2 years after surgical recovery, and surgical intervention is associated with long-term economic benefit.
Patients with ulcerative pancolitis for more than 8 years are at increased risk of colorectal cancer, approximating 0.5% to 1% per year. Those persons with primary sclerosing cholangitis as a complication of their ulcerative colitis have been shown to have an even higher incidence of dysplasia and cancer. Colonoscopic surveillance with random and chromoscopic-directed biopsies has been recommended in patients with long-standing ulcerative colitis. An obstructing lesion and unresectable dysplasia generally warrant surgery. Historically, high-grade dysplasia and multifocal low-grade dysplasia were widely accepted as clear indications for colectomy because of the high rate of an occult malignancy within the colon. However, there are circumstances in which dysplastic lesions within a field of normal colonic mucosa can be adequately managed endoscopically. This is an evolving area of management that requires a clear understanding of the lesion itself, the patient's disease course, and the comfort of the treating physicians.
Patients with a severe attack of ulcerative colitis should be resuscitated and medically treated if signs of life-threatening hemorrhage or perforation are absent. Deterioration of the patient's condition and failure to improve within a predetermined time period are indications for surgical intervention. Patients initially managed with corticosteroids should improve after 3 days. Rescue therapy includes treatment with cyclosporin or infliximab, and improvement with these agents should occur within 5 to 7 days. Intervention for failed therapy should take the form of colectomy with preservation of the rectum and creation of an end ileostomy. The rectosigmoid stump can be closed and left intraperitoneally, brought up as a closed stump in the subcutaneous tissue, or matured as a mucous fistula above skin level.
Toxic megacolon, which is dilation (5 to 6 cm) of the colon, due to inflammation-induced paralysis of the colon or obstruction proximal to severe disease, is a potentially life-threatening condition. Patients are very ill and often manifest high fever, abdominal pain and tenderness, tachycardia, and leukocytosis. Prompt resuscitation and medical therapy are essential. Early decision to operate is life saving.
Massive hemorrhage is uncommon and accounts for less than 10% of emergency colectomies in patients with ulcerative colitis. Perforation of the colon is a clear indication for surgery. If it occurs in the absence of megacolon, the possibility of Crohn disease should be considered. High doses of corticosteroids often mask these symptoms and signs. Strictures causing an obstruction in patients with chronic ulcerative colitis are uncommon and represent malignant lesions until proven otherwise.
A significant number (30%) of patients who undergo surgery have had some exposure to biologic agents. Anti–tumor necrosis factor (TNF)-α therapies may increase infectious complications, compromised pouch function, and even pouch loss. Controversy persists regarding their use, timing of surgery, and risk for complications of surgery. In a meta-analysis by Selvaggi et al. patients receiving biologic agent therapy prior to restorative proctocolectomy were at higher risk of developing short-term, local pelvic complications (odds ratio [OR] 4.12; 95% confidence interval [CI] 2.3 to 7.1). However, studies included in this analysis demonstrated heterogeneity in reporting of procedures and complications that may contribute to variable outcomes between biologic agent and nonbiologic agent comparison groups.
The Crohn's and Colitis Foundation of America published a position statement regarding anti-TNF-α therapy management for inflammatory bowel disease (IBD). This position statement supports the safety of total colectomy with end ileostomy in the setting of anti-TNF-α therapy as part of a three-stage approach to restorative proctocolectomy. Use of a two-stage technique is associated with increased risk of postoperative complications, and it is recommended that a two-stage approach may be used only in selected patients. Anti-TNF-α therapy is considered an absolute contraindication to single-stage restorative proctocolectomy, based on limited data.
Fortunately, as operative experience in the setting of current and novel biologic agent therapies matures, greater understanding of the impact of these therapies on operative outcomes will guide the surgeon's ability to make decisions.
Restorative procotocolectomy with IPAA is usually performed in two or three stages based on the patient's physiology and the surgeon's judgment. In a two-stage operation, the abdominal colon is mobilized, a nerve-sparing rectal dissection is used, and the proctocolectomy specimen is removed in its entirety. The ileum is preserved and dissected free of its retroperitoneal and duodenal attachments to allow for a tension-free IPAA. Typically, an ileal J pouch is created using 30 to 45 cm of distal ileum, with each limb of the pouch measuring 15 to 20 cm in length. The IPAA is performed by either mucosectomy with a handsewn technique or preservation of the anal transitional zone (ATZ) with a stapled technique, depending upon indication for surgery or surgeon's preference. A temporary ileostomy is created to protect the ileal pouch and the anastomosis. The ileostomy is closed approximately 12 weeks later, following confirmation of proper pouch healing with contrast enema and digital examination.
In a three-stage operation, the abdominal colon is removed to the distal sigmoid colon or upper rectum, and an end ileostomy is matured. The superior hemorrhoidal artery may be left intact to maintain positioning of the rectal stump and preserve anatomic planes of dissection at the time of future proctectomy. Following medical and nutritional optimization of the patient, the second stage of restoration consists of a nerve-sparing completion proctectomy followed by takedown of the end ileostomy and mobilization of the small bowel mesentery from its retroperitoneal and duodenal attachments. An ileal pouch is created as previously described, and a diverting loop ileostomy is brought up through the existing ileostomy aperture.
Laparoscopic surgery appeals to patients due to the potential benefit of reduced disability, enhanced recovery, and better cosmesis. The majority of reports tout the benefits of laparoscopy over open surgery, including reduced major and minor complications, decreased narcotic use, and lower rate of intraoperative transfusion, at the expense of longer operative times. Length of stay is similar between the laparoscopic and open groups owing to the need for ileostomy education before discharge, but the laparoscopic approach has fewer postoperative complications and better cosmesis. Studies have failed to show a significant difference in the quality of life associated with the two operative approaches. Several single institutional reports demonstrate the safety of laparoscopic colectomy in the setting of acute ulcerative colitis. The minimally invasive approach preserved fertility and decreased the incidence of incisional hernia. Laparoscopic, hand-assisted, single-port, or robotic-assisted approaches are not different. A randomized trial comparing hand-assisted laparoscopic with open restorative proctocolectomy found no difference in postoperative narcotic use, morbidity, length of stay, or quality of life at 3 months after surgery. Hand-assisted laparoscopy approach is associated with a 55-minute reduction in operative time compared with straight laparoscopy, with no difference in short-term outcomes. Robotic IPAA is possible with minimal complications.
Further consideration should be given to surgical approach based on the indication for the procedure in light of a randomized controlled trial comparing laparoscopic versus open proctectomy for rectal cancer that failed to demonstrate noninferiority of pathologic outcomes following laparoscopic proctectomy. Oncologic principles of resection are required, and careful consideration of operative approach should be given to the colitis patient with rectal cancer.
The patient is placed in dorsal lithotomy positioning with adequate space left between the legs to allow for access to the anus and operator standing room. A bean bag with chest wrap can be used to secure the patient during steep Trendelenburg positioning. The patient's arms are padded and tucked at the sides. The hips are positioned with 0 degrees of flexion to help keep the thighs out of the way during dissection in the upper quadrants.
The peritoneum is entered via a periumbilical incision using either an open or closed insertion technique. A 12-mm port should be ideally placed in the midline, halfway between the pubis symphysis and the xiphoid process, which allows for the camera to be at the apex of the 12-mm Hg pneumoperitoneum. A 10-mm, 0- or 30-degree laparoscope can be used. In one of the more commonly used approaches, at least three 5-mm working ports are placed (see figures).
Extraction of the specimen is possible through a planned ileostomy site, periumbilical site, Pfannenstiel incision, or left lower quadrant muscle-splitting incision.
Our sequence of procedures follows right colon mobilization, opening the lesser sac and transverse colon mobilization, ligation of the right and transverse colon mesentery, mobilization of the left colon, and ligation of its mesentery and the rectal dissection. Right colon mobilization starts with accessing the retroperitoneum, followed by mobilization of the right colon off the retroperitoneum, division of the lateral attachments, entering the lesser sac, and ligation of the mesentery. Regardless of a posterior, medial to lateral, or lateral approach, the steps are the same, but the order in which they are performed are different. The posterior approach will be presented in this chapter.
The abdomen is explored (including small bowel evaluation to look for Crohn disease), the patient is placed in a steep Trendelenburg position so the small bowel can be placed in the right upper quadrant. The posterior aspect of the small bowel mesentery is exposed from the fourth portion of the duodenum to the cecum. The right hand grasper elevates the small bowel mesentery to expose the fourth portion of duodenum. The energy source is in the left hand (for a left-handed surgeon), and the peritoneum is incised. The duodenum is swept away from the right colon mesentery, which is elevated off the retroperitoneum in a medial to lateral direction. The patient is then placed right side up to allow the small bowel to fall into the left side of abdomen. The lateral attachments are finally divided from the cecum toward the hepatic flexure.
The patient is placed in reverse Trendelenburg position, and the lesser sac is entered through the gastrocolic ligament, separating the lesser omentum from its attachments to the transverse colon mesentery. The major challenge with this maneuver is to avoid dissecting into the transverse colon mesentery. Therefore the cut edge of the lesser omentum is elevated to expose its avascular attachments to the transverse colon and its mesentery. Near the pylorus, the lesser omentum and transverse colon mesentery appear fused, but with careful dissection under tension, the omentum and mesocolon can be separated. The lesser omentum is then divided toward the splenic flexure, which fully exposes the lesser sac and facilitates exposure of the transverse colon mesentery.
The mesenteric vessels can now be ligated. The ileocecal junction is grasped and pulled to the right lower quadrant to facilitate exposure of the ileocolic pedicle. The ileocolic pedicle is ligated at the superior mesenteric artery (SMA) away from the second portion of the duodenum. The transverse colon mesentery is elevated to expose the bare area to the left of the middle colic vessels. The peritoneum is incised toward the right branch of the middle colic vessels and the previously cut edge of the right colic mesentery. The middle colic vessels are free to be isolated and ligated.
Attention is now turned to the mobilization of the left colon. The patient is placed in a steep Trendelenburg and right side down position to facilitate placement of the small bowel to the right upper quadrant. The left colon is mobilized in a medial to lateral approach. The retroperitoneum can be accessed either at the level of the sacral promontory or medial to the inferior mesenteric vein. At the sacral promontory, the superior rectal vessels are elevated and the peritoneum is incised while avoiding damage to the sympathetic nerves. The wider the incision and bigger the window created, the greater the mobility of the vessel and exposure of the retroperitoneum. After the left ureter is identified and swept free into the retroperitoneum, the inferior mesenteric artery is isolated and ligated at its origin. The inferior mesenteric artery can also be divided distal to the vessel's branching. The inferior mesenteric vein is elevated off the retroperitoneum, isolated, and transected near the inferior border of the pancreas. The first assistant elevates the cut edge of the transverse colon mesentery, and the surgeon elevates the ligated inferior mesenteric vein pedicle, exposing the remaining colonic mesentery. Depending upon the extent of transverse colon mesentery divided during the right-sided mobilization, only a few vascular pedicles on the left transverse mesocolon remain for division. The left colon mesentery is released from the retroperitoneum beyond the lateral aspect of the colon, from the level of the sigmoid colon to the splenic flexure. The lateral attachments are incised starting at the pelvic brim during medial traction on the sigmoid colon. The prior plane of dissection is entered and the lateral attachments are divided to the splenic flexure. Any intact omental adhesions are divided, and the mesenteric attachments along the inferior border of the pancreas can be cut to finish mobilization of the abdominal colon.
The rectal dissection can be the most technically challenging aspect of the procedure because of the confined space of the pelvis, limited angling of the instruments, stapling difficulties at the pelvic floor, and mesenteric inflammation from the proctitis. It is easiest to perform the dissection immediately outside the fascia propria of the mesorectum, but care must be taken to avoid injury to the sympathetic and parasympathetic nerves. Given the limited space of the pelvis and the angles of visualization, achieving adequate exposure and tension can be difficult. Conceptually, the rectum and mesorectum form a cylinder within the cylinder of the pelvis. The dissection is achieved with curvilinear sweeping incisions in the areolar tissue plane following the curves of the pelvis. Ideal tension is achieved when traction is perpendicular to the point of dissection (i.e., anterior to posterior, medial to lateral) rather than pulling the rectum out of the pelvis. Small changes in the force vectors of retraction can profoundly impact tension and exposure. The dissection begins with entering the presacral plane by retracting the rectum directly anterior. As the dissection moves laterally, the angle of retraction is rotated to the contralateral side to maintain tension and exposure.
The posterior dissection is carried down to the pelvic floor, and at the tip of the coccyx, the plane of dissection sweeps anterior to the pelvic outlet. The peritoneum on the lateral aspects of the mesorectum is divided all the way to the anterior peritoneal reflection, which exposes the anterior and deep lateral planes. The lateral dissection on both sides of the pelvis progresses by retracting the rectum medially and superiorly so the plane can be dissected in a posterior to anterior fashion. The anterior dissection begins, after completion of the lateral dissection, by pulling the peritoneum overlying the cervix or posterior bladder/prostate in an anterior direction and the rectum in a posterior fashion. The rectum is rotated to the contralateral side to maintain the tension in a plane that is perpendicular to the point of dissection. A laparoscopic stapler is directed into the pelvis to divide the rectum in a single firing, if possible. It is best to divide the rectum with three or fewer staplings oriented in the same direction. A right lower quadrant port or suprapubic port or a Pfannenstiel incision can be accessed to divide the rectum.
Prior to extraction of the specimen through an abdominal wall incision, it is important to ensure that the colon is anterior to the small bowel to avoid avulsion of the small bowel mesentery, or a volvulus of the small bowel mesentery. Proper orientation of the ileum during extraction of the specimen facilitates construction of the ileal pouch. After construction of the pouch (see later), a circular stapling anvil is secured in the apex of the ileal pouch and the pouch is returned to the abdomen. The extraction site is closed, and the IPAA is completed under direct laparoscopic visualization. A proximal loop of ileum is matured into a diverting loop ileostomy in the right lower quadrant. The stoma should be brought through the stoma aperture under direct laparoscopic vision to ensure proper orientation. A closed suction drain may be placed into the pelvis through the left lower quadrant port site to facilitate evacuation of blood and fluid that can lead to pelvic fibrosis and impact pouch compliance.
Alternatively, a hand-assisted technique via a 6- to 8-cm Pfannenstiel or lower midline incision can be considered. Use of the hand-assisted approach is a matter of surgeon's preference, and based on the previous data, it offers the surgeon another tool for their toolbox.
The sequence of the proctocolectomy is the same as described previously, but this section will describe how the hand is used for the procedure. The camera port should be placed in the supraumbilical position so that the skirt of the hand port does not interfere with the camera. A 5-mm working port is placed on the right and left side, lateral to the rectus muscle and midway between the camera and hand port. Additional 5-mm working ports can be placed in the right and left upper quadrants as needed. The posterior approach is used for mobilization of the right colon. The surgeon stands on the patient's left side with her/his left hand in the abdomen, and the right hand holds the energy device. With the patient in steep Trendelenburg and the small bowel reflected to the right upper quadrant, the posterior aspect of the small bowel mesentery is exposed. The mesentery is grasped and elevated with the middle finger and thumb. The index finger is used to further expose the fourth portion of the duodenum ( Fig. 162.1 ).
The base of the terminal ileal mesentery is incised from the third portion of the duodenum to the cecum. After the retroperitoneum is entered, the hand is placed palm down into the retroperitoneum to elevate the right colon mesentery so the duodenum and retroperitoneal structures can be pushed posteriorly with the instrument in the right hand ( Fig. 162.2 ). The dissection is extended beneath the hepatic flexure and ascending colon. To divide the lateral attachments, the patient is tilted right side up so the small bowel is displaced to the left side of the abdomen, exposing the lateral attachments and hepatic flexure. The hand is placed into the retroperitoneum, under the right colon mesentery, and the colon is retracted medially to expose the lateral attachments at the level of the cecum. The first assistant then divides the lateral attachments toward the hepatic flexure. The lesser sac is entered, and the lesser omentum is divided toward the splenic flexure as far as possible ( Fig. 162.3 ).
The ileocecal angle is grasped and retracted to the right lower quadrant to expose the ileocolic pedicle for isolation and ligation at the level of the SMA ( Fig. 162.4 ).
The middle colic vessels are exposed by passing the hand through the mesenteric defect, created by ligation of the ileocolic pedicle, and into the lesser sac. The fingers encircle the base of the middle colic vessels. The peritoneum is scored over the bare area adjacent to the ligament of Treitz and the vessels are isolated, sealed, and divided. As long as the vessels are ligated anterior to the pancreas, there is no risk of injuring the superior mesenteric vessels.
For resection of the left colon and rectum, the left-handed surgeon stands on the patient's right side with her/his right hand in the abdomen and left hand on the energy source. The right-handed surgeon also stands on the right side, with the left hand in the abdomen and right hand on the energy source in the right lower quadrant port. The patient is in steep Trendelenburg and left side up to keep the small bowel in the right upper quadrant. The retroperitoneum can be accessed either at the level of the sacral promontory or medial to the inferior mesenteric vein. Starting at the sacral promontory, the superior rectal vessels are grasped and elevated, and the retroperitoneum is entered. With the left ureter identified and safely swept into the retroperitoneum, the index finger with the pad toward the anterior abdominal wall elevates and applies tension on the superior rectal vessels. The middle finger then sweeps free all the tissue adjacent and lateral to the vessels. This maneuver allows exposure of the bare area cephalad to the inferior mesenteric artery and medial to the inferior mesenteric vein. The peritoneum medial to and along the inferior mesenteric vein is incised allowing entry into the retroperitoneal plane of dissection. Once it has been confirmed that the left ureter is away from the course of the inferior mesenteric artery, the artery can be safely divided ( Fig. 162.5 ). The inferior mesenteric vein is then isolated near the inferior border of the pancreas in the same manner described for the artery ( Fig. 162.6 ). When ligating the inferior mesenteric vein, the index and middle fingers are placed into the retroperitoneal space to isolate the vessel, while the fourth and fifth fingers remain in the peritoneal space covering the small bowel to prevent the energy source injury. The remaining transverse colon mesentery is divided in the same fashion as described previously ( Fig. 162.7 ).
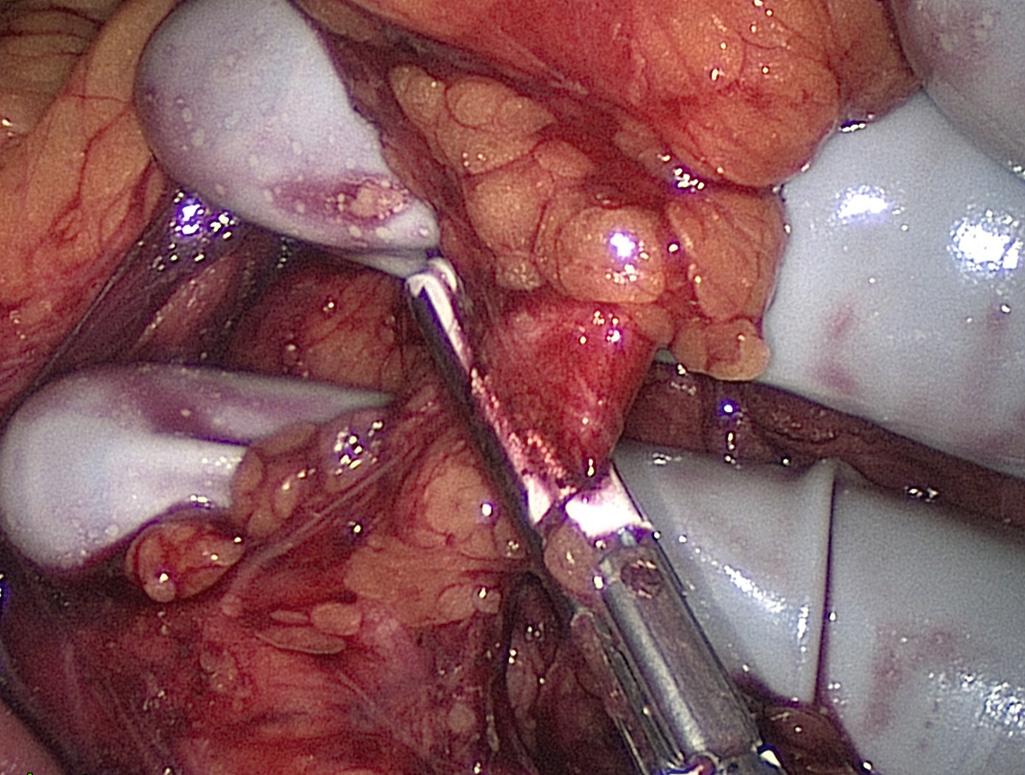
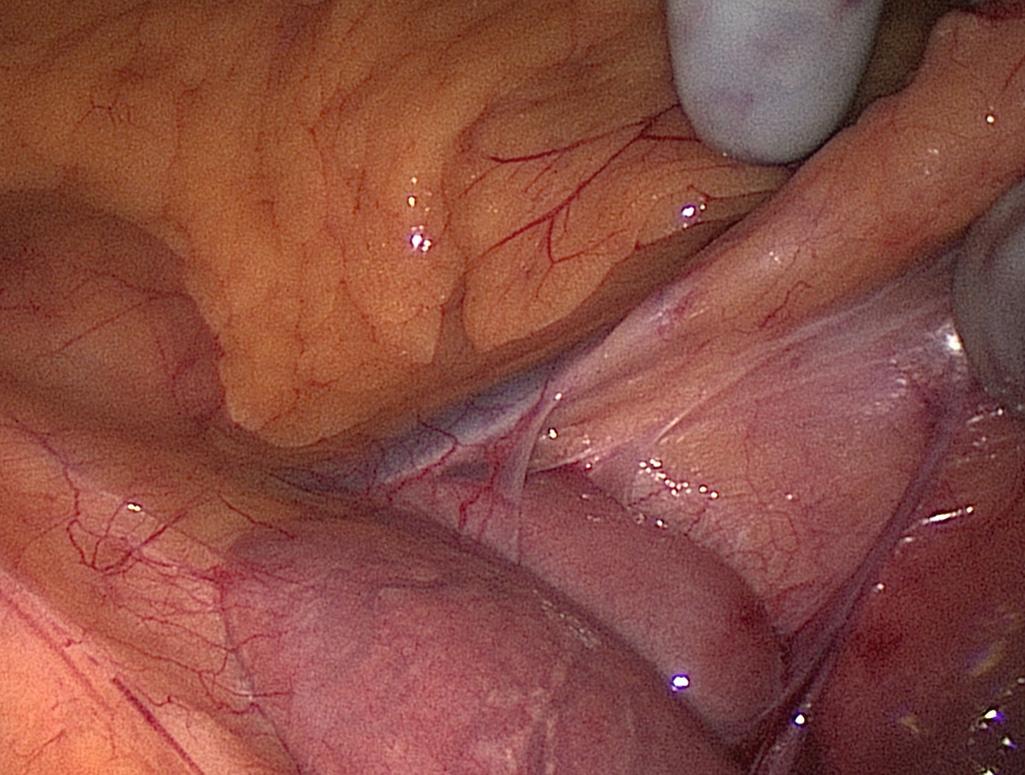
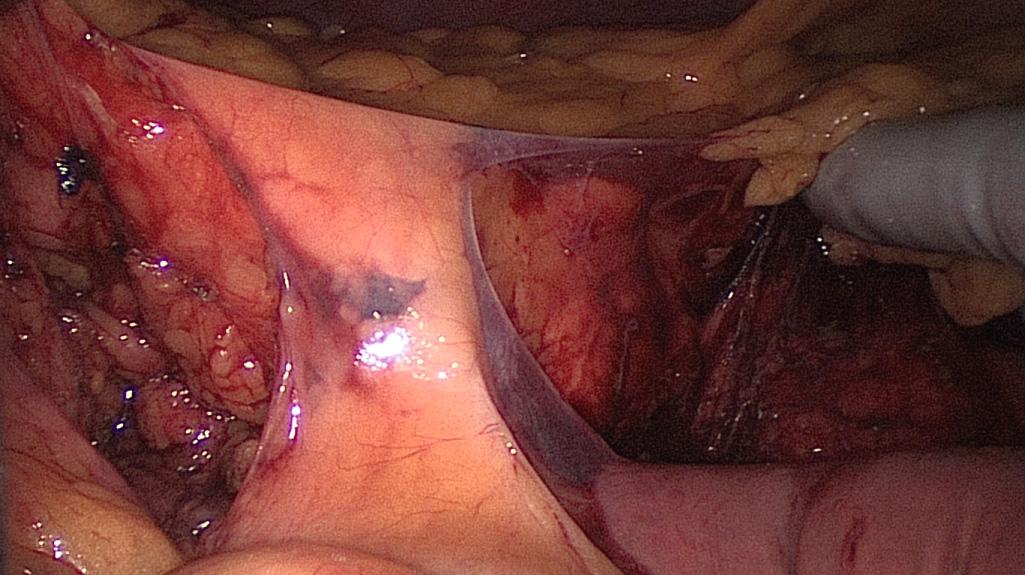
Mobilization of the descending and sigmoid colon mesenteries is facilitated by using the hand placed palm down to elevate the mesentery and an instrument to downward sweep the retroperitoneum. By creating the greatest tension safely possible, this dissection is carried beyond the lateral colon. The lateral attachments of the sigmoid colon are incised and the prior plane of dissection is entered. The hand is placed through this defect, and the remaining attachments are divided toward the splenic flexure ( Fig. 162.8 ).
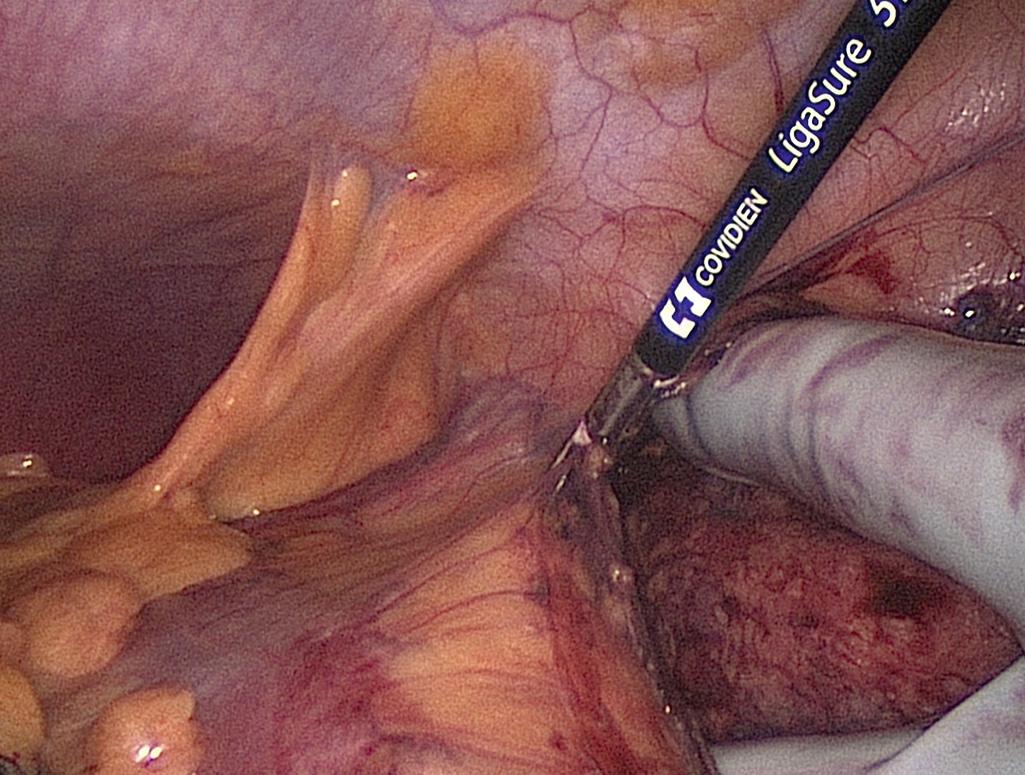
At the splenic flexure, the lesser sac is entered and the medial cut edge of the lesser sac from the transverse colon dissection is identified. Division of the remaining omentum completely opens the lesser sac, leaving only the peritoneal attachments along the inferior border of pancreas. The right-handed surgeon stands between the legs to mobilize the splenic flexure. The left hand lifts the colon toward the midline, and the colon is released from the kidney and spleen using the energy source through the left lower quadrant port. The final attachments to the pancreas are exposed by bringing the hand into the retroperitoneal space from a lateral direction and pulling the transverse colon caudally, which allows the first assistant to divide this remaining tissue.
For the pelvic dissection, the left-handed surgeon stays in the same position with her/his right hand through the port. The dissection begins by entering the avascular plane between the presacral fascia and fascia propria of the mesorectum. The hand is pronated so the palm is up and the right thumb is pointing to the patient's right side. In this position, the hand anteriorly retracts the upper rectum to expose the plane of dissection. The fingers are creating tension as close to the plane of dissection as possible ( Fig. 162.9 ). The right-handed surgeon places the left hand through the suprapubic port, which lifts the rectum. The right hand uses the energy source through the right lower quadrant port to dissect the rectum from the pelvis, as described earlier for straight laparoscopy.
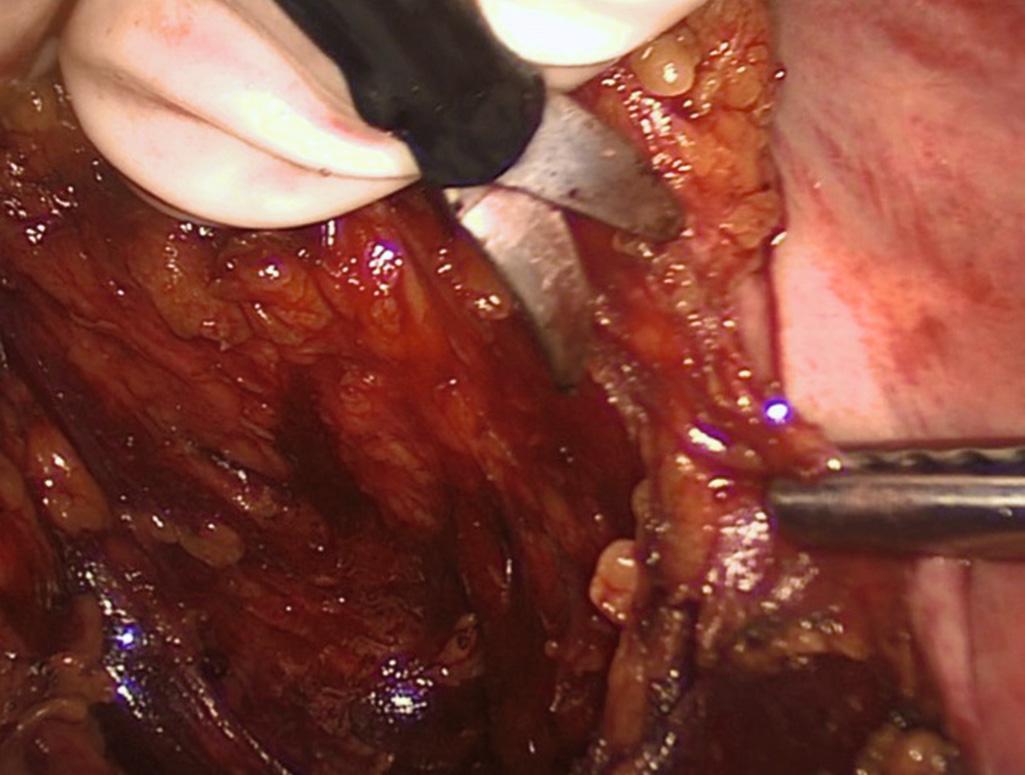
This maneuver allows for tactile feedback as to the appropriate plane. The dissection is carried from sidewall to sidewall, and the hand is rotated right and left to maintain tension as the dissection is carried out toward the opposite sidewall. As the dissection continues deeper into the pelvis, better exposure is gained and tension is created by flexing the fingers rather than pulling the hand and mesorectum out of the pelvis. The lateral dissection is completed by incising the lateral peritoneal attachments down to the anterior peritoneal reflection. The anterior dissection begins with incising the anterior peritoneal reflection as the hand is pronated as described previously to anteriorly retract the bladder/prostate or the uterus/cervix while the first assistant posteriorly displaces the rectum. As the dissection moves deeper into the pelvis, the first assistant rotates the rectum in the direction contralateral to the dissection. For example, as the dissection is carried to the left, the rectum is retracted in a posterior and right direction to keep the tension perpendicular to the plane of dissection. If there is difficulty completing the pelvic dissection in this manner, the procedure can be completed using open instruments introduced through the hand port.
With the pelvic dissection completed to the level of the pelvic floor, the colon needs to be extracted. This is accomplished by passing the small bowel underneath the colon so the cecum can be delivered, which allows the small bowel to be oriented so it is on the patient's left side and the cut edge of the mesentery is facing the patient's right side. Starting in the left upper quadrant, the colon is elevated, and the small bowel is fed underneath the colon. As this maneuver progresses, the patient is tilted left side down to allow gravity to facilitate passing the small bowel to the left upper quadrant. At the conclusion, the hand should have worked its way to the cecum, which is extracted through the hand port, allowing the terminal ileum and its mesentery to be divided. The remaining colon is then easily extracted. The rectum can be divided through the hand access port sleeve with a single transverse staple line at the pelvic floor. Before firing the stapler to divide the rectum, it is best to ensure the anterior tissue in a male and the posterior wall of the vagina in a female are free from the stapler. The construction of the ileal pouch is described later.
The three-stage restorative proctocolectomy begins with removal of the abdominal colon and creation of an end ileostomy. After the patient has mentally and physiologically recovered from the first operation, they undergo completion proctectomy with creation of ileal pouch with anastomosis and diverting loop ileostomy. The third stage is completed with closure of the loop ileostomy.
The technical steps of the total abdominal colectomy are exactly the same as described previously. The only significant variation is the management of the rectal stump. Given the presence of acute colitis, possible immunosuppression, and state of the patient, a real concern exists related to breakdown of the rectal stump staple line. Therefore various alternatives to managing the rectal stump and its vasculature should be considered. The first option is to ligate the inferior mesenteric artery at its origin as described previously, and divide the rectum and mesorectum just below the rectosigmoid junction. Dissection below the sacral promontory should be minimized to preserve this plane as much as possible. A large (34 French) rectal drain is left in the rectum for a few days to decompress the rectum and reduce the risk of “stump blowout.” The second option involves preserving the superior rectal artery and leaving a long enough rectosigmoid stump to reach the anterior abdominal wall. The rectal stump is then secured in a subcutaneous position through the hand port or extraction site. This option preserves all of the pelvic planes and minimizes injury to the sympathetic nerves, and disruption of the stump becomes an easily controlled mucous fistula. In a retrospective review of 204 patients undergoing laparoscopic colectomy comparing these two approaches, no difference was found in the number of stump leaks in each group (5 intraperitoneal vs. 10 subcutaneous; P = .23). All stump leaks in the subcutaneous group were locally managed without the need for reoperation. However, this group had higher rates of wound infection. Five patients with intraperitoneal stump leak required antibiotic therapy or computed tomography (CT)-guided drainage and one patient required reexploration. No predisposing risk factors for stump leak among these patients were noted.
The second stage or completion proctectomy can be approached via the same approach as described previously ( Figs. 162.10–162.14 ).
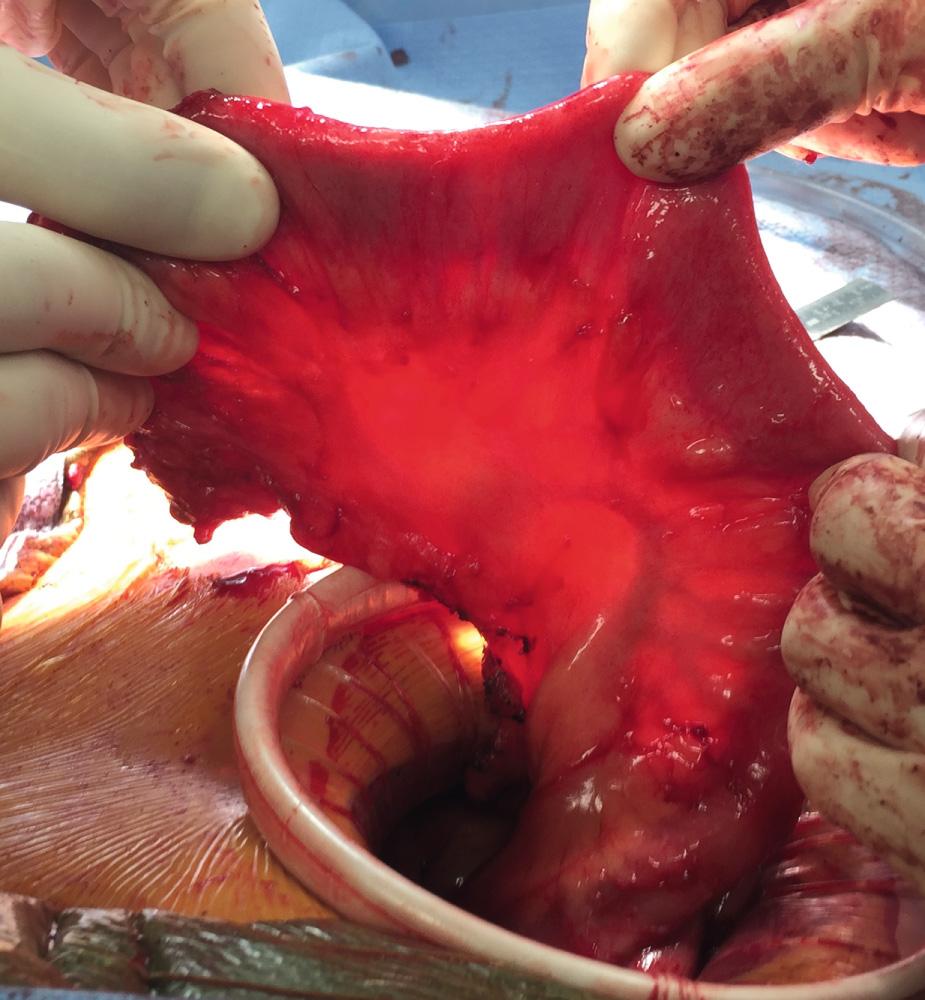
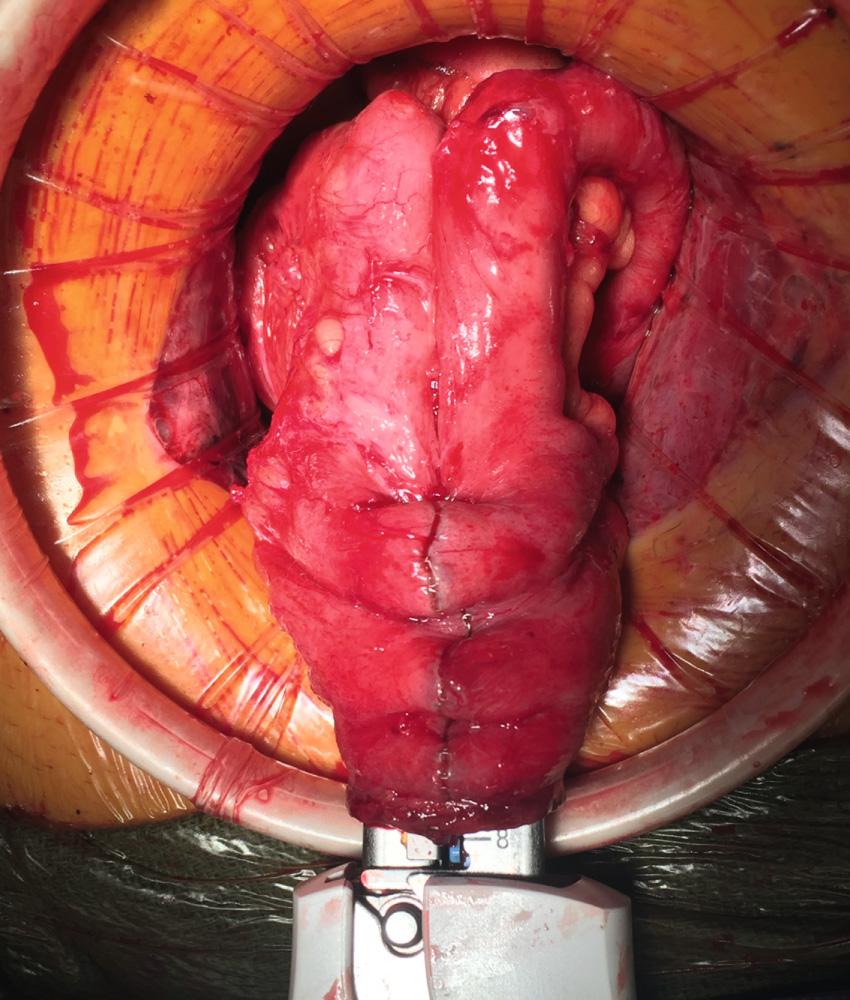
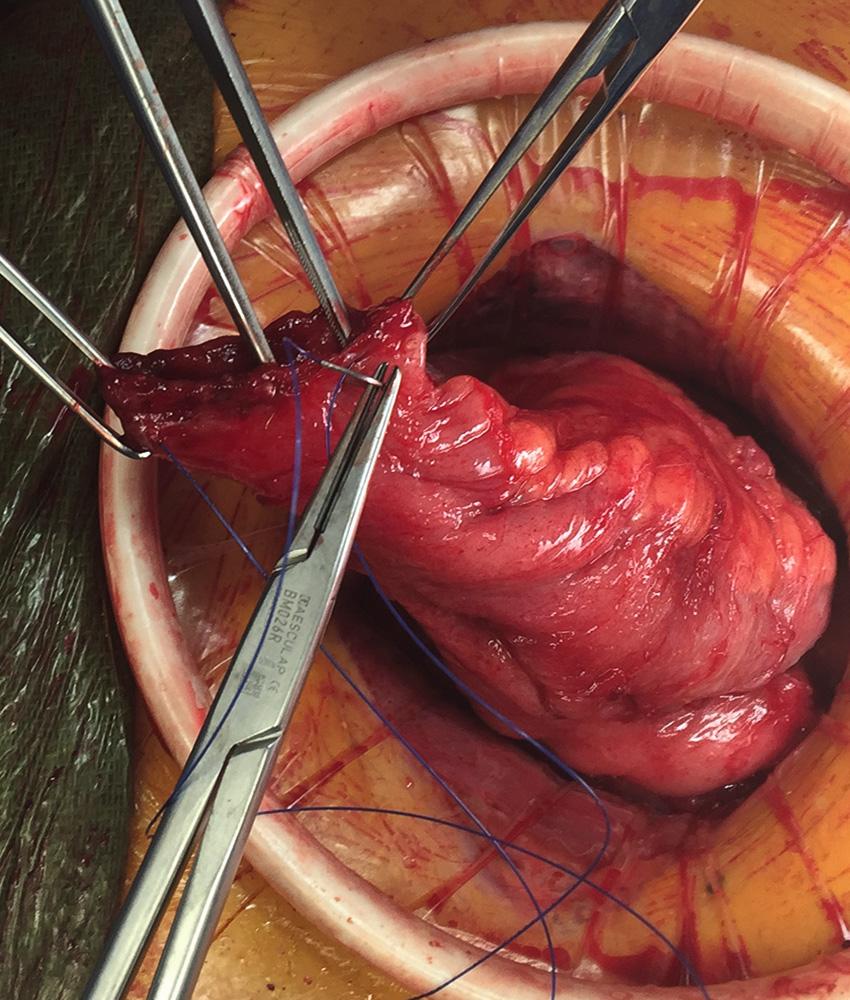
There are occasions when the ileal J pouch will not reach the pelvic floor even after lysis of all adhesions and complete mobilization of the small bowel mesentery over the duodenum. Five maneuvers can be used to create extra length to improve reach of the pouch into the pelvis. First, if the ileocolic pedicle was not divided during colectomy, it can be ligated and a window of peritoneum excised to increase length. Second, the anterior and posterior peritoneum overlying the superior mesenteric vessels can be incised transversely at multiple levels. This maneuver risks injury to the blood supply of the pouch and can result in hematoma. The denuded mesentery can be easily avulsed, which can result in a devastating mesenteric hematoma and ileal pouch loss. Third, the mesentery can be transilluminated and selective division of secondary arcade mesenteric vessels to the apex of pouch can be performed. The arcade to the distal ileum must be preserved. The peritoneum over the arcade is incised, and the individual branches are divided. Division of the secondary arcade vessels typically results in one-for-one increase in length (i.e., every centimeter of transverse incision results in a centimeter of longitudinal lengthening). Fourth, an S pouch can be created. The near-normal orientation of the mesentery and a 2-cm efferent limb allow for extra reach. The details for creating an S pouch are described later. Finally, when despite all of these maneuvers, a tension-free ileoanal anastomosis is not possible, the created pouch can be left suspended in the pelvis as the distal end of a loop ileostomy. The pouch outlet must be closed prior to placing the pouch in the pelvis, and the pouch can be anchored to the pelvic floor with a suture. This maneuver will require another operation to create the anastomosis and can be complicated by reperitonealization of the pelvic floor and rectal stump.
Various configurations are described for construction of the ileal pouch. The J, S, and W pouch configurations are well described ( Fig. 162.15 ). Functional outcomes are similar across all of these designs. The choice of pouch configuration is dictated by the experience of the surgeon and the body habitus of the patient.
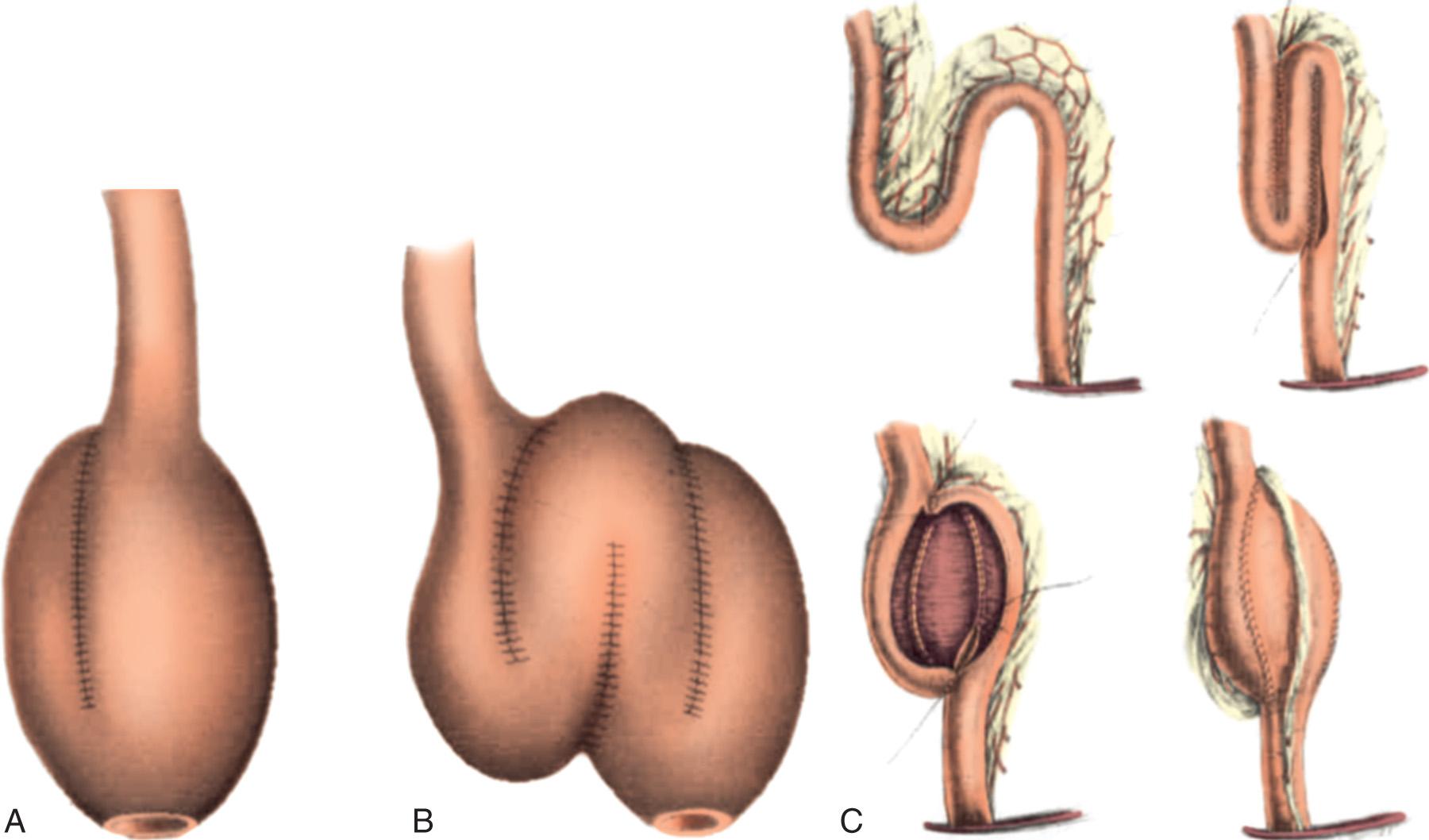
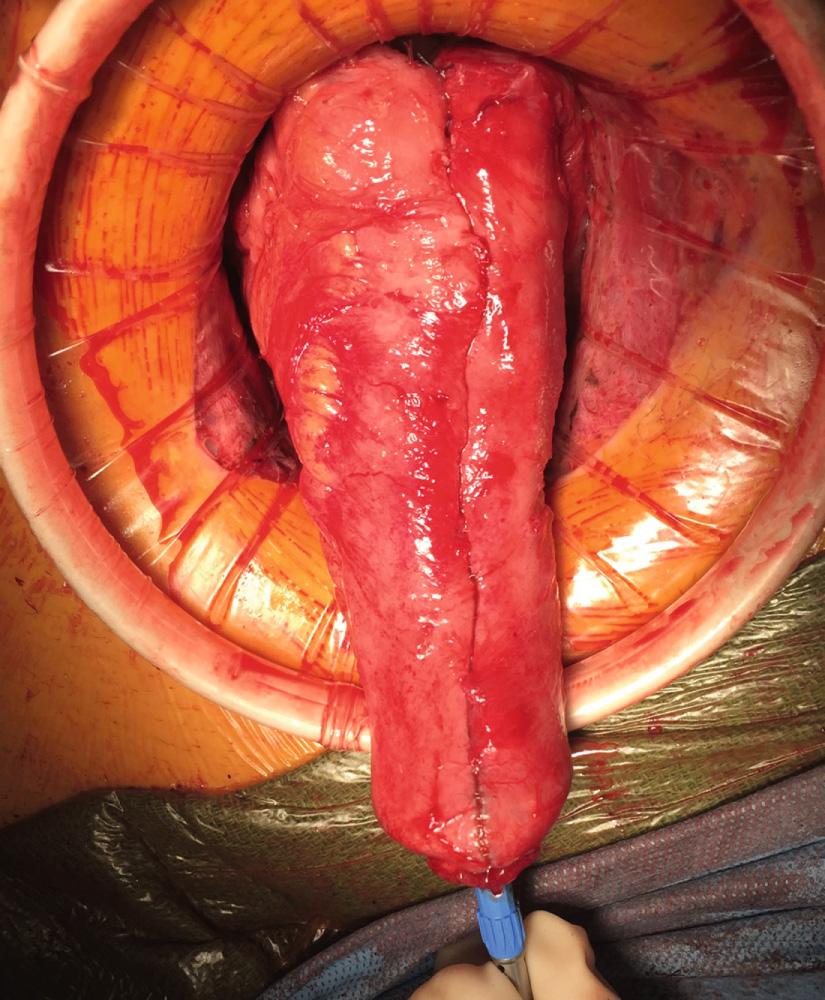
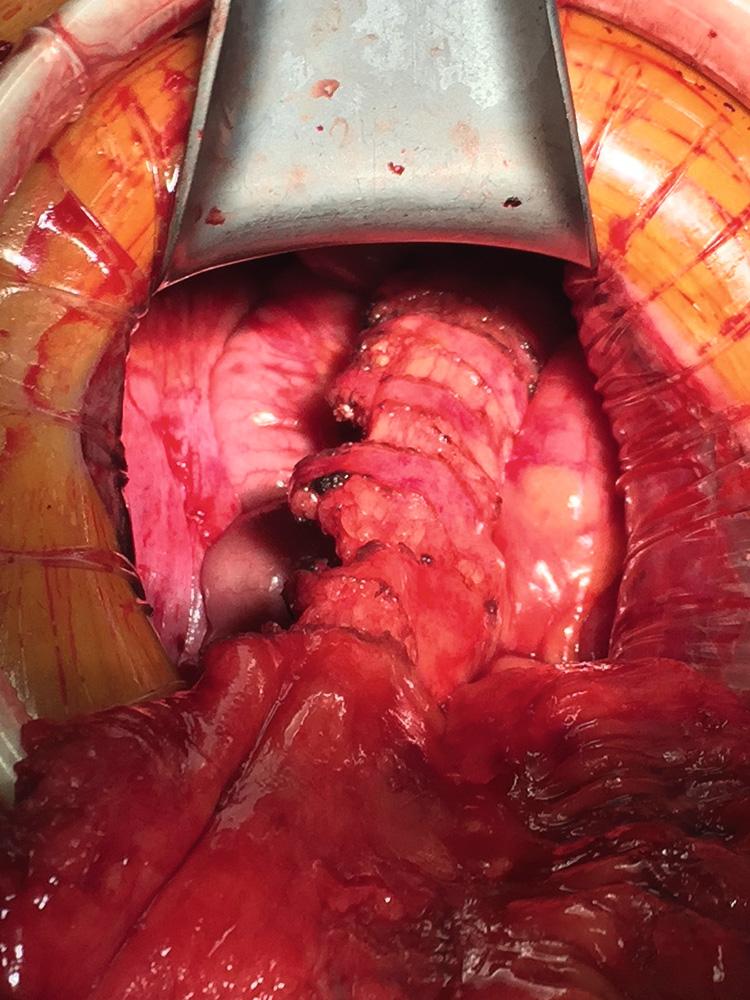
Originally described by Utsunomiya et al. in 1980, the ileal J pouch is the most commonly used configuration. The J pouch is a two-limbed folded ileal pouch. The apex of the pouch must reach the anal canal without tension and is determined as the most dependent part of the reservoir. The length of the pouch is typically 15 to 20 cm, and no functional difference exists between J pouches constructed from two 15-cm limbs or two 20-cm limbs. The reservoir is created by making an enterotomy at the portion of the terminal ileum that most easily reaches below the pubis. A linear stapler is passed up either limb, the antimesenteric borders of the afferent and efferent limbs are aligned, and the stapler fired to create the reservoir. Multiple firings may be required to complete the length of the pouch. Each subsequent stapler is placed into the pouch and the pouch is telescoped onto the stapler so the end of the previous staple line is at the crotch of the stapling device. Seromuscular sutures can be placed between the antimesenteric aspects of each limb to prevent torque on the staple line. The anvil of the circular stapler is fitted into the apex of the ileal pouch and secured with a handsewn purse-string suture in preparation for the IPAA.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here