Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cancer during childhood remains one of the leading causes of disease-related mortality among pediatric patients. With advancements in the treatment of pediatric oncologic disorders and improved survival rates, the pediatric anesthesiologist will encounter these patients in the operating room, radiology suites, radiation therapy, inpatient floors for pain management, and oncology clinic settings. This chapter will review the three most common pediatric oncologic disorders that involve the blood (acute lymphoblastic leukemia [ALL]), brain (medulloblastoma), and abdomen (neuroblastoma). This will include treatment-related morbidity and anesthetic-management concerns.
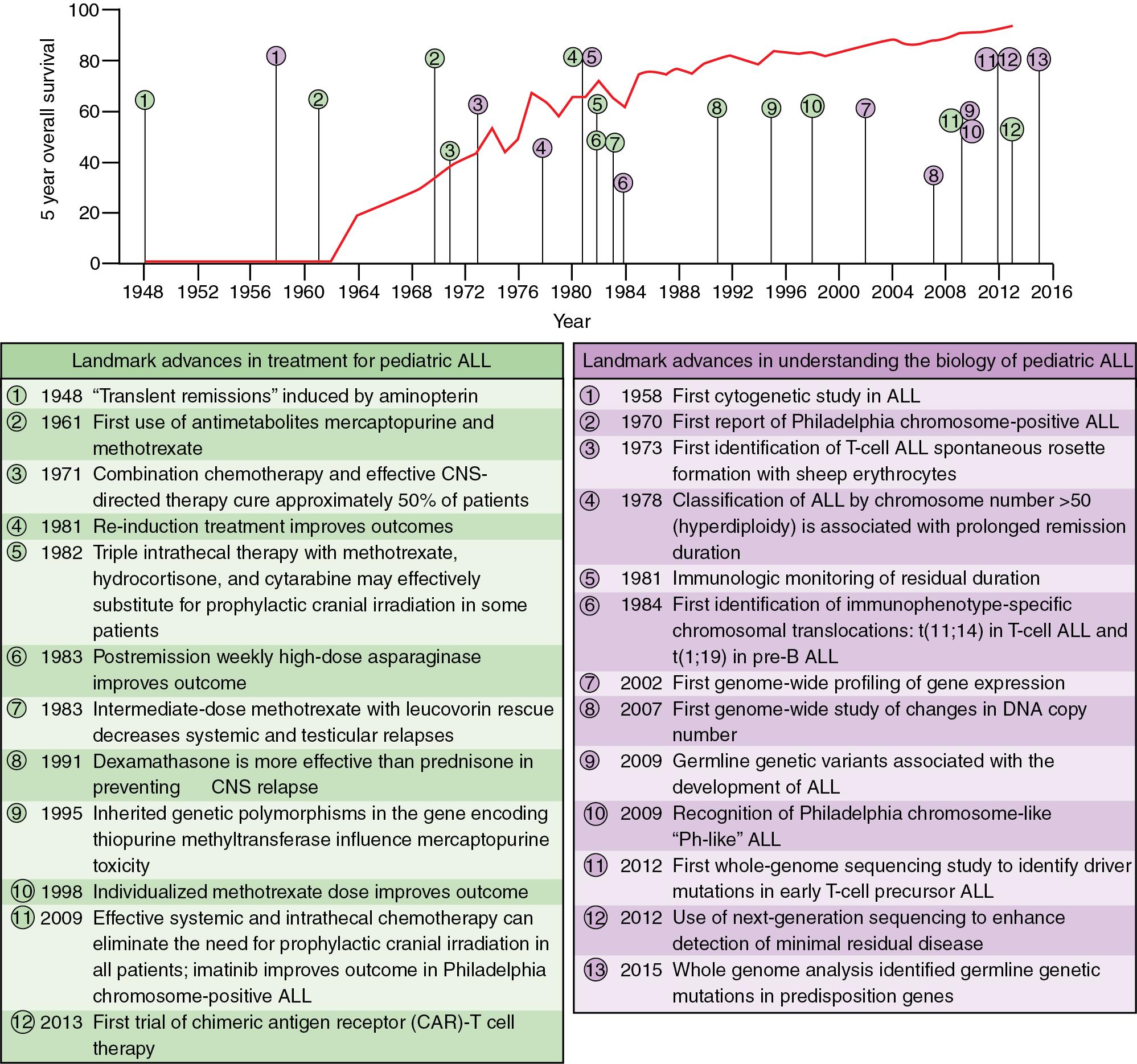
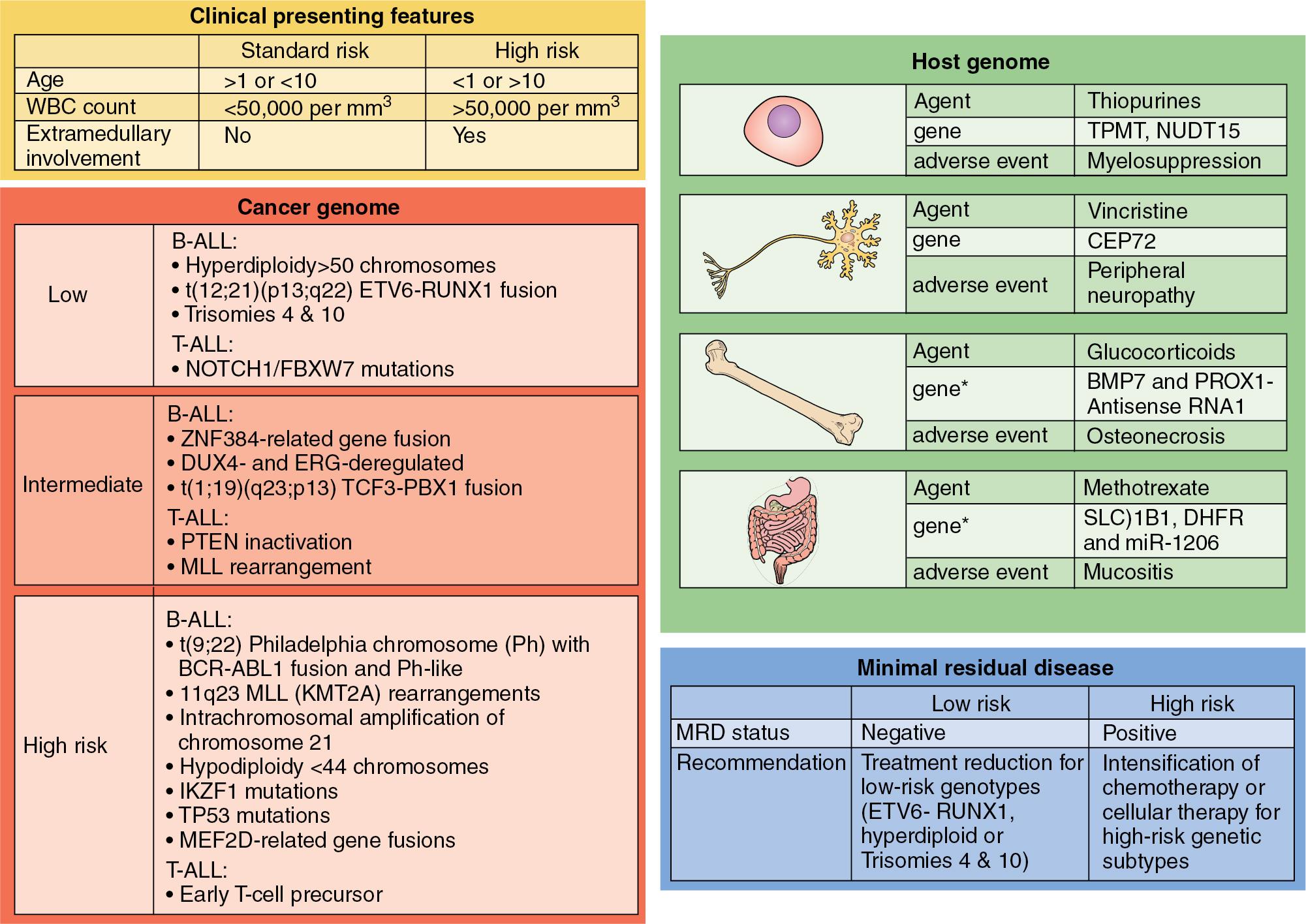
Pediatric ALL is the most common pediatric oncologic diagnosis; however, advancements in treatment and understanding the biology of pediatric ALL over the past 50 years has led to a rapid increase in overall survival (OS). Currently, pediatric ALL has a 5-year OS of greater than 80% ( Fig. 50.1 ) ( ). The treatment for childhood ALL is centered on risk-based stratification. ( Fig. 50.2 ) The main factors affecting patient outcome include age at presentation, white blood cell (WBC) count at time of diagnosis, the presence of sanctuary sites, central nervous system (CNS) involvement, tumor cytogenetics, and therapeutic response to induction agents. Factors that portend a poorer prognosis include age younger than 1 year and older than 10 years, WBC count >50,000 on presentation, presence of extramedullary sites or sanctuary sites that involve disease (CNS and testicles; these sites are difficult to penetrate with systemic chemotherapy), prolonged pretreatment with corticosteroids, immunophenotype of the cell line (T-cell lymphocytes have a poorer prognosis than B cells), and the cytogenetic factor of hypoploidy (loss of one or more chromosomes). In addition to all of the previously mentioned prognostic indicators, the response to initial therapy has emerged as an independent predictor. Evaluation of bone marrow minimum residual disease (MRD) at the end of the induction phase of treatment has proven to be an independent factor predicting outcome ( ; ).
Treatment of ALL is accomplished in four phases: remission induction, consolidation, maintenance, and CNS-directed therapy. The goal at the end of the induction period is to induce complete remission (defined as <5% detectable blasts on microscopic morphology). This phase generally lasts 4 to 6 weeks, and complete remission generally is achieved in 95% of patients. Chemotherapeutic agents used during induction typically include vincristine, corticosteroids, and asparaginase, with most protocols also administering an anthracycline (doxorubicin or daunorubicin) ( Table 50.1 ). The consolidation phase follows remission induction and is aimed at eradicating submicroscopic residual disease. Patients with higher-risk disease receive longer and more intensive consolidation regimens than patients with standard-risk disease. Therapy during the consolidation phase is usually administered on an outpatient basis, and the chemotherapeutic agents typically include high-dose methotrexate, mercaptopurine, asparaginase, dexamethasone, and vincristine. For high-risk patients, cytarabine and cyclophosphamide may be added to postinduction consolidative chemotherapy (see Table 50.1 ) ( ; ). Maintenance chemotherapy is the final and longest stage of treatment in childhood ALL and uses a markedly less intensive regimen. The prolonged maintenance phase lowers the risk for relapse once remission has been established, and this phase is also administered on an outpatient basis. The cornerstone of maintenance therapy is antimetabolite therapy (weekly methotrexate and daily mercaptopurine, with or without pulses of a corticosteroid plus vincristine ( ). The final component of ALL treatment involves therapy directed against the CNS. CNS treatment is essential because relapse occurs commonly in sanctuary sites, even with bone marrow remission with systemic chemotherapy. The CNS-directed therapy includes both treatment of patients with clinical CNS disease at initial diagnosis and prophylaxis for patients with subclinical disease. Three CNS approaches are used: (1) direct intrathecal administration of chemotherapy, (2) systemic administration of chemotherapy able to penetrate the blood-brain barrier, and (3) cranial radiation. Intrathecal chemotherapeutic agents typically used include intrathecal methotrexate or a combination of intrathecal methotrexate, cytarabine, and hydrocortisone (known as triple intrathecal ). Systemically administered chemotherapeutic agents capable of CNS penetration include dexamethasone, high-dose methotrexate, cytarabine, and asparaginase (see Table 50.1 ). Because of its damaging CNS effects, cranial radiation is generally administered only for patients at highest risk for CNS relapse. However, some institutions defer its use altogether. Hematopoietic stem cell transplantation (HSCT) is generally considered for patients with the highest risk for relapse and/or treatment failure (i.e., patients with hypodiploidy or induction failure) ( ).
| Drug | Mechanism of Action or Classification | Indication(s) | Adverse Reactions | Monitory Drug Level | Comments |
|---|---|---|---|---|---|
| Methotrexate | Folic acid antagonist; inhibits dihydrofolate reductase | ALL, non-Hodgkin lymphoma, osteosarcoma, Hodgkin lymphoma, medulloblastoma | Myelosuppression, mucositis, stomatitis, dermatitis, hepatitis With long-term administration, osteopenia and bone fractures With high-dose administration, renal and CNS toxicity With intrathecal administration, arachnoiditis, leukoencephalopathy, leukomyelopathy |
Plasma levels must be monitored with high-dose therapy and when low doses are administered to patients with renal dysfunction and leucovorin rescue applied accordingly | Systemic administration may be PO, IM, or IV; also may be administered intrathecally |
| 6-Mercaptopurine (Purinethol) | Purine analog; inhibits purine synthesis | ALL | Myelosuppression, hepatic necrosis, mucositis; allopurinol increases toxicity | Therapeutic drug monitoring not available or indicated | Allopurinol inhibits metabolism |
| Cytarabine (Ara-C) | Pyrimidine analog; inhibits DNA polymerase | ALL, AML, non-Hodgkin lymphoma, Hodgkin lymphoma | Nausea, vomiting, myelosuppression, conjunctivitis, mucositis, CNS dysfunction With intrathecal administration, arachnoiditis, leukoencephalopathy, leukomyelopathy |
Therapeutic drug monitoring not available or indicated | Systemic administration may be PO, IM, or IV; also may be administered intrathecally |
| Cyclophosphamide (Cytoxan) | Alkylates guanine; inhibits DNA synthesis | ALL, non-Hodgkin lymphoma, Hodgkin lymphoma, soft-tissue sarcoma, Ewing sarcoma | Nausea, vomiting, myelosuppression, hemorrhagic cystitis, pulmonary fibrosis, inappropriate ADH secretion, bladder cancer, anaphylaxis | Therapeutic drug monitoring not available or indicated | Requires hepatic activation and is thus less effective in presence of liver dysfunction |
| Ifosfamide (Ifex) | Alkylates guanine; inhibits DNA synthesis | Non-Hodgkin lymphoma, Wilms’ tumor, sarcoma, germ cell and testicular tumors | Nausea, vomiting, myelosuppression, hemorrhagic cystitis, pulmonary fibrosis, inappropriate ADH secretion, bladder cancer, CNS dysfunction, cardiotoxicity, anaphylaxis | Therapeutic drug monitoring not available or indicated | |
| Doxorubicin (Adriamycin) and daunorubicin (Cerubidine) | Binds to DNA, intercalation | ALL, AML, osteosarcoma, Ewing sarcoma, Hodgkin lymphoma, non-Hodgkin lymphoma, neuroblastoma | Nausea, vomiting, cardiomyopathy, red urine, tissue necrosis on extravasation, myelosuppression, conjunctivitis, radiation dermatitis, arrhythmia | Therapeutic drug monitoring not available or indicated | |
| Dactinomycin | Binds to DNA, inhibits transcription | Wilms’ tumor, rhabdomyosarcoma, Ewing sarcoma | Nausea, vomiting, tissue necrosis on extravasation, myelosuppression, radiosensitizer, mucosal ulceration | Therapeutic drug monitoring not available or indicated | |
| Bleomycin (Blenoxane) | Binds to DNA cleaves DNA strands | Hodgkin disease, non-Hodgkin lymphoma, germ cell tumors | Nausea, vomiting, pneumonitis, stomatitis, Raynaud phenomenon, pulmonary fibrosis, dermatitis | Therapeutic drug monitoring not available or indicated | |
| Vincristine (Oncovin) | Inhibits microtubule formation | ALL, non-Hodgkin lymphoma, Hodgkin disease, Wilms tumor, Ewing sarcoma, neuroblastoma, rhabdomyosarcoma | Local cellulitis, peripheral neuropathy, constipation, ileus, jaw pain, inappropriate ADH secretion, seizures, ptosis, minimal myelosuppression | Therapeutic drug monitoring not available or indicated | IV administration only; must not be allowed to extravasate |
| Vinblastine (Velban) | Inhibits microtubule formation | Hodgkin disease; Langerhans cell histiocytosis | Local cellulitis, leukopenia | Therapeutic drug monitoring not available or indicated | IV administration only; must not be allowed to extravasate |
| L-Asparaginase | Depletion of L-asparagine | ALL; AML, when used in combination with asparaginase | Allergic reaction pancreatitis, hyperglycemia, platelet dysfunction and coagulopathy, encephalopathy | Therapeutic drug monitoring not available or indicated | PEG-asparaginase now preferred to L-asparaginase |
| Pegaspargase (Oncaspar) | Polyethylene glycol conjugate of L-asparagine | ALL | Indicated for prolonged asparagine depletion and for patients with allergy to L-asparaginase | Therapeutic drug monitoring not available or indicated | |
| Prednisone and dexamethasone (Decadron) | Lymphatic cell lysis | ALL; Hodgkin disease, non-Hodgkin lymphoma | Cushing syndrome, cataracts, diabetes, hypertension, myopathy, osteoporosis, infection, peptic ulcer, psychosis | Therapeutic drug monitoring not available or indicated | |
| Carmustine (nitrosourea) | Carbamylation of DNA; inhibits DNA synthesis | CNS tumors, non-Hodgkin lymphoma, Hodgkin disease | Nausea, vomiting, delayed myelosuppression (4–6 wk); pulmonary fibrosis, carcinogenic stomatitis | Therapeutic drug monitoring not available or indicated | Phenobarbital increases metabolism, decreases activity |
| Carboplatin and cisplatin (Platinol) | Inhibits DNA synthesis | Gonadal tumors; osteosarcoma, neuroblastoma, CNS tumors, germ cell tumors | Nausea, vomiting, renal dysfunction, myelosuppression, ototoxicity, tetany, neurotoxicity, hemolytic-uremic syndrome, anaphylaxis | Therapeutic drug monitoring not available or indicated | Aminoglycosides may increase nephrotoxicity |
| Etoposide (VePesid) | Topoisomerase inhibitor | ALL, non-Hodgkin lymphoma, germ cell tumors | Nausea, vomiting, myelosuppression, secondary leukemia | Therapeutic drug monitoring not available or indicated | |
| Etretinate (Tegison) (vitamin A analog) and tretinoin | Enhances normal differentiation | Acute progranulocytic leukemia, neuroblastoma | Dry mouth, hair loss, pseudotumor cerebri, premature epiphyseal closure | Therapeutic drug monitoring not available or indicated |
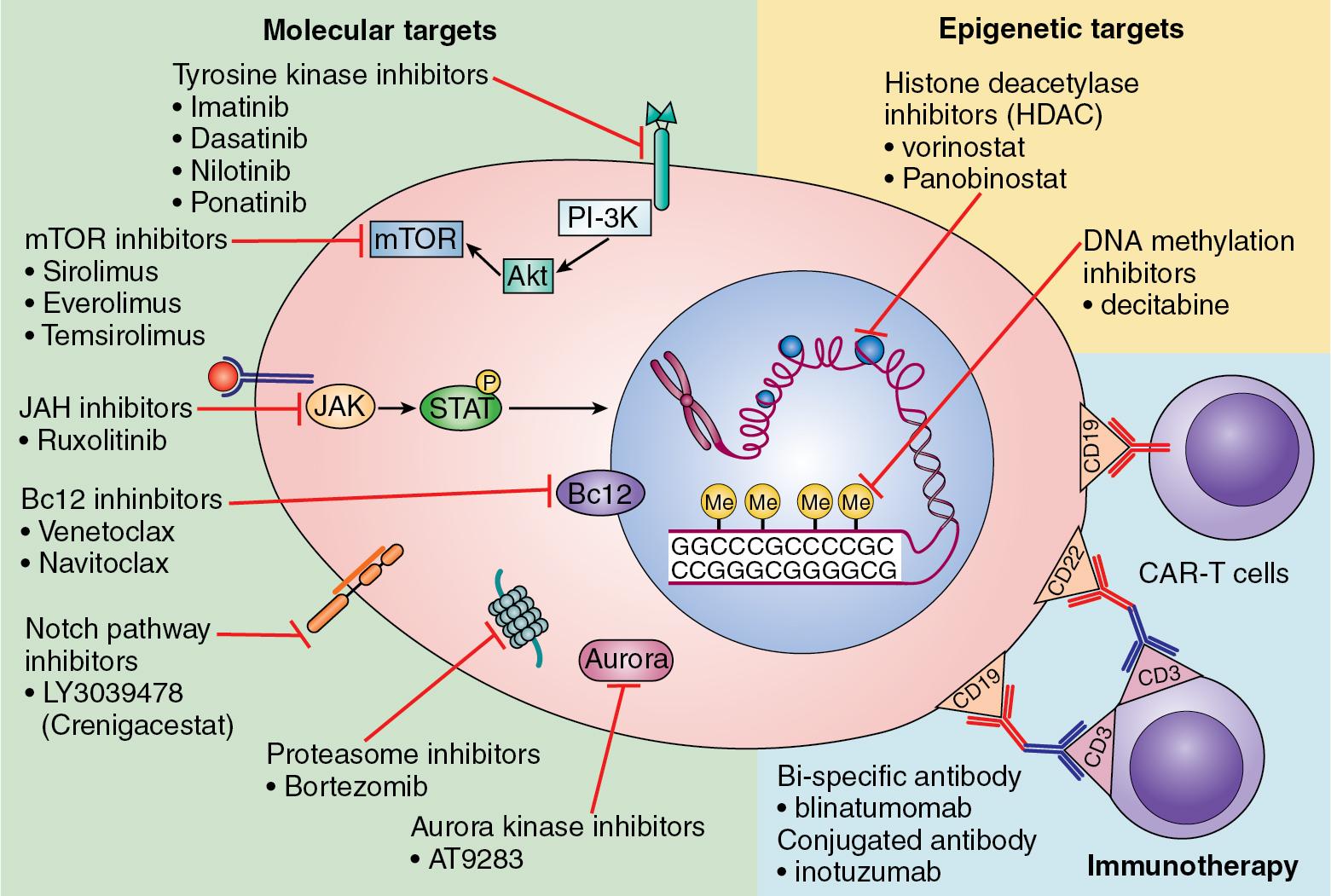
Next-generation sequencing (NGS) has revolutionized pediatric ALL treatment by revealing genetic alterations that are amenable to targeted therapy. To date, trials of promising targeted therapies have significantly improved outcomes for some high-risk groups. Novel immunotherapeutic approaches, including chimeric antigen receptor (CAR)–T-cell therapies, have cured a proportion of patients with highly refractory leukemia, including those who relapsed after HSCT ( Fig. 50.3 ) ( ).
Pediatric anesthesiologists are most often involved during the treatment phase for central access (Broviac or Port-a-Cath placement), lumbar puncture, bone marrow biopsy/aspiration, and cranial irradiation. Anesthetic management is governed by the patient’s underlying state of health. Their immunocompromised state puts them at risk for infection.
The most common pediatric brain tumors are as follows: medulloblastoma, low-grade and high-grade glioma, diffuse intrinsic pontine glioma, and ependymoma (see Chapter 31 , “Anesthesia for Neurosurgery”). In the last 5 years, collaborative groups and institutional trials have advanced the survival of patients who have these tumors ( ).
Medulloblastoma is the most common malignant pediatric brain tumor, accounting for approximately 20% of all cases. It is a highly malignant embryonal tumor that was first described as a distinct CNS tumor in 1925. It occurs exclusively in the posterior fossa. It can occur in infancy, childhood, or adulthood. By using combined-modality therapy that includes surgical resection, risk-adjusted irradiation, and adjuvant chemotherapy, approximately 70% of children and adolescents with medulloblastoma can be cured. Craniospinal irradiation (CSI) and chemotherapy after surgical resection have transformed what was a universally fatal disease into one in which the cure rate is approximately 70%. Clinical risk stratification is based on the extent of tumor resection and the presence of metastatic disease. Children who are at least 3 years of age, have undergone gross total resection (1.5 cm 2 of residual tumor), have no metastatic disease, and who are histologically nonanaplastic are classified as standard-risk (SR) disease and have a long-term survival rate of 85%. The remainder of the patients are classified as high-risk (HR) disease and have a long-term survival rate of 70%. For children with SR disease, the introduction of chemotherapy (cisplatin, lomustine, vincristine, cyclophosphamide; see Table 50.1 ) has improved event-free survival (EFS). Patients 3 years of age and older with HR disease have a 5-year EFS between 60% and 70% after intensified chemotherapy (vincristine, lomustine, cisplatin, methylprednisolone, cyclophosphamide, cytarabine, etoposide, carboplatin, ifosfamide, methotrexate, and radiation; see Table 50.1 ).
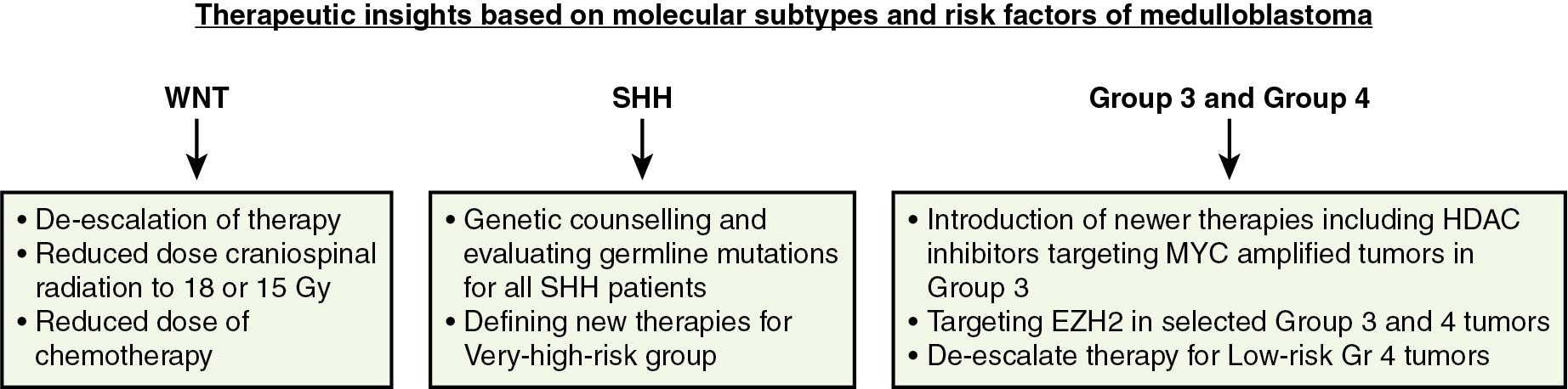
Over the last two decades, many clinical trials have been pursued with increased OS; however, long-term side effects are concerning. Side effects include neurocognitive deficits, hearing loss, endocrine dysfunction, and increased incidence of secondary malignancies. In addition, the uniform treatment approach based on histopathology and risk group has led to unpredictable relapse and heterogeneity in survival outcome. Over the last 10 years various transcriptional profiling studies have identified four molecular subgroups of medulloblastoma: wingless (WNT), sonic hedgehog (SHH), Group 3, and Group 4 ( ; ; ). In the new age of medulloblastoma genomics, these subgroups are now clearly identified with distinguishing features based on demographics, histology, chromosomal profile driver genes, and outcome ( Table 50.2 ). This has also now enabled risk stratification of medulloblastoma based on the molecular subtypes of these tumors and provides insight to newer therapies ( Fig. 50.4 ).
| WNT (10%) | SHH (30%) | Group 3 (25%) | Group 4 (35%) | |
|---|---|---|---|---|
| Clinical Features | ||||
| Age | Older children and adults (median age ≈10 years) | Bimodal: <5 years and >16 years; less common in children aged 5–16 years | Infants and young children | Children (median age ∼9 years); can occur in all age groups |
| Gender ratio (M:F) | ≈1:1 | ≈1.5:1 | ≈2:1 | ≈3:1 |
| Histology | Classic; rarely LC/A | Classic > desmoplastic nodular > LC/A > MBEN | Classic > LC/A | Classic; rarely LC/A |
| Proposed cell of origin | Lower rhombic lip progenitor cells | CGNPs of the EGL and cochlear nucleus; neural stem cells of the subventricular zone | Prominin 1 + /lineage − neural stem cells; CGNPs of the EGL | Deep cerebellar nuclei in the nuclear transitory zone; upper rhombic lip progenitor cells |
| Location of tumor | Fourth ventricle; infiltrating brainstem | Cerebellar hemispheres; rarely midline | Fourth ventricle; midline | Fourth ventricle; midline |
| Metastasis at diagnosis | ≈5%–10% | ≈15%–20% | ≈40%–45% | ≈35%–40% |
| Prognosis (5-year survival) | ≈95% | ≈75% | ≈50% | ≈75% |
| Genomic Features | ||||
| Cytogenetics | Monosomy 6 | Loss of 9q, 10q, and 17p; gain of 3q and 9p | Loss of 10q, 16q, and 17p; gain of 1q, 7, 17q, and 18 | Loss of 8, 10, 11, and 17p; gain of 4, 7, 17q, and 18 |
| Driver genes (most prevalent) | CTNNB1 ; DDX3X ; SMARCA4 ; KMT2D ( MLL2 ); TP53 | PTCH1 ; TP53 ; KMT2D (MLL2) ; DDX3X ; MYCN amplification; BCOR ; LDB1 ; TCF4 ; GL12 amplification | MYC amplification; PVT1 amplification; SMARCA4 ; OTX2 amplification; CTDNEP1 ; LRP1B ; KMT2D (MLL2) | KDM6A ; SNCAIP gain; MYCN amplification; KMT2C (MLL3); CDK6 amplification; ZMYM3 |
| Expression signature | WNT signaling | SHH signaling | MYC signature; photoreceptor GABAergic signature | Neuronal signature; glutamatergic signature |
Neuroblastoma is a cancer of primordial neural crest cells that give rise to sympathetic neural ganglia and the adrenal medulla. It is the most common extracranial solid tumor of childhood, with most patients diagnosed before 5 years of age, with a median age of patients at diagnosis of 19 months and less than 5% diagnosed at older than 10 years of age. It is also the most common cancer diagnosed in infants. Neuroblastoma accounts for approximately 10% of all pediatric cancers and up to 15% of cancer-related deaths in children ( ; ) (see Chapter 33 , “Anesthesia for General Abdominal, Urologic Surgery”).
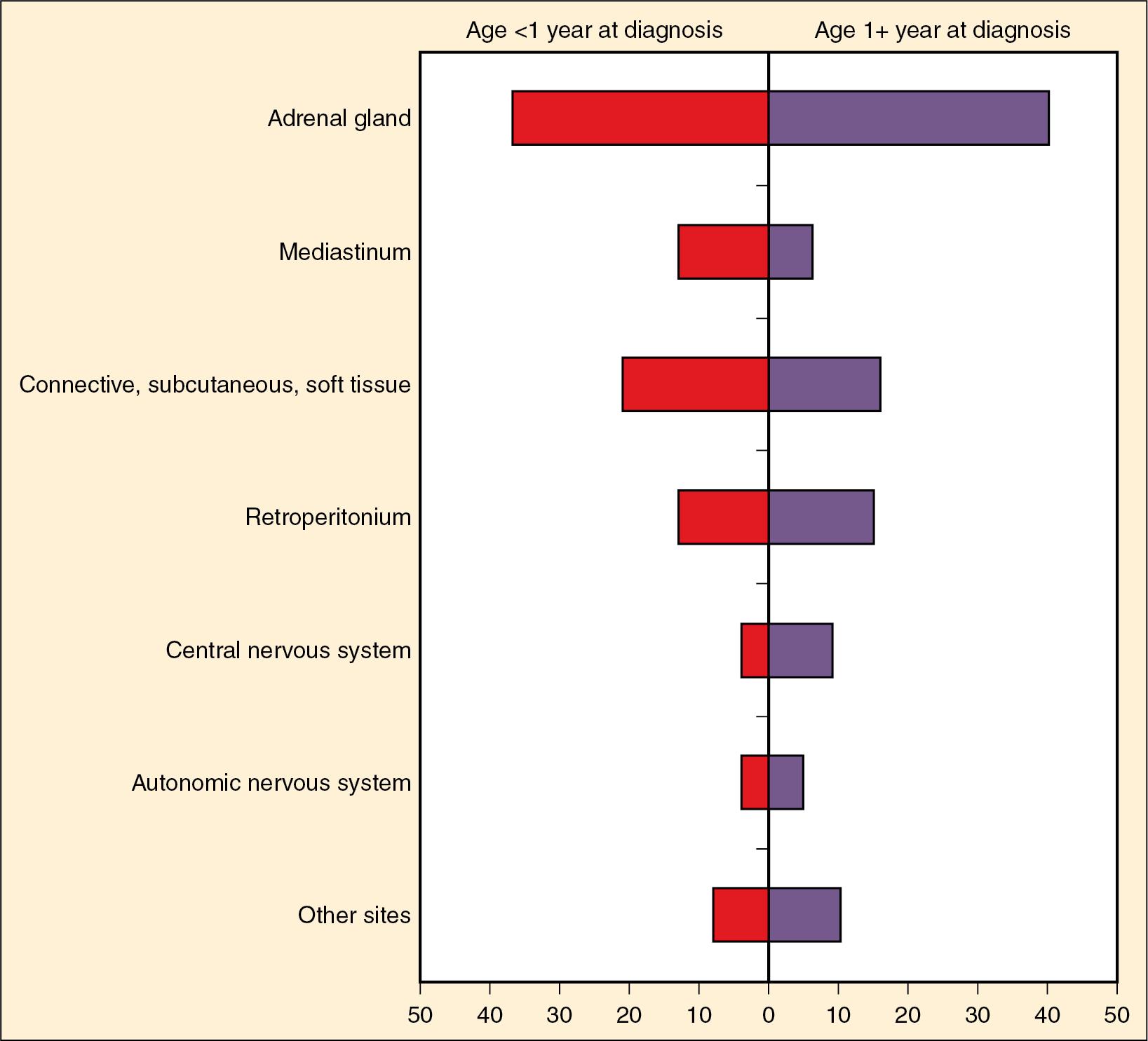
An interesting feature of neuroblastoma is the unique clinical and biological heterogeneity of neuroblastoma tumors, with some children having tumors that regress completely or that spontaneously differentiate without treatment, whereas other children have widespread metastatic tumors with poor outcomes despite aggressive multimodal therapy ( ; ). Neuroblastoma presents anywhere along the sympathetic chain ( Fig. 50.5 ). Most neuroblastomas (65%) arise in the abdomen in the adrenal gland. Diagnosis is confirmed either by (1) tumor biopsy and histopathology or (2) detection of neuroblastoma tumor cells in the bone marrow coupled with elevated urine or serum catecholamine or catecholamine metabolites (dopamine, vanillylmandelic acid, and homovanillic acid). Evaluation for extent of disease includes computed tomography (CT) or magnetic resonance imaging (MRI), bilateral bone marrow aspirates and biopsies, and radioiodine-labeled metaiodobenzylguanidine (MIBG). MIBG is a norepinephrine analog that selectively concentrates in sympathetic nervous tissue and is used as a marker to detect primary tumors and metastatic sites. A positron emission tomography (PET) scan can also be used to detect tumors that are not MIBG avid ( ).
Criteria for diagnosis and staging of neuroblastoma were based on the surgical-pathologic International Neuroblastoma Staging System (INSS) until 2009 ( Box 50.1 ). However, cooperative groups from different regions of the world have not consistently used the same markers to classify patient risk and treatment strategies, making it difficult to compare the results of risk-based clinical trials. To address this concern, the International Neuroblastoma Risk Group Staging System (INRGSS) was developed ( Box 50.2 ). Seven clinically relevant and statistically significant factors were incorporated into the INRGSS, allowing the International Neuroblastoma Risk Group (INRG) to risk-stratify neuroblastoma into low-risk, intermediate-risk, and high-risk groups. It is currently in use in multiple national and international clinical trials.
Stage 1: Localized tumor confined to the area of origin.
Stage 2A: Unilateral tumor with incomplete gross resection; identifiable ipsilateral and contralateral lymph node negative for tumor.
Stage 2B: Unilateral tumor with complete or incomplete gross resection; with ipsilateral lymph node positive for tumor; identifiable contralateral lymph node negative for tumor.
Stage 3: Tumor infiltrating across midline with or without regional lymph node involvement; or unilateral tumor with contralateral lymph node involvement; or midline tumor with bilateral lymph node involvement.
Stage 4: Dissemination of tumor to distant lymph nodes, bone marrow, bone, liver, or other organs except as defined by stage 4S.
Stage 4S: Younger than 1 year of age with localized primary tumor as defined in stage 1 or 2, with dissemination limited to liver, skin, or bone marrow (less than 10% of nucleated bone marrow cells are tumors).
Stage L1: Localized disease without image-defined risk factors
Stage L2: Localized disease with image-defined risk factors
Stage M: Metastatic disease
Stage MS: Metastatic disease “special” where MS is equivalent to stage 4S
Treatment strategies based on risk group are summarized in Table 50.3 . OS rates for patients with low-risk neuroblastoma are excellent with surgery alone, and rare recurrences can often be cured with salvage chemotherapy. Intermediate-risk classification encompasses a wide spectrum of disease for which surgical resection and moderate-dose multiagent chemotherapy are the backbone of most regimens. Survival after surgical resection and moderate-dose chemotherapy (carboplatin or cisplatin, doxorubicin, etoposide, and cyclophosphamide; see Table 50.1 ) is greater than 90% for children whose tumors exhibit favorable characteristics. Overall, outcomes for patients with low- or intermediate-risk neuroblastoma continue to remain excellent despite significant reductions in therapy in recent years ( ). The outcome of high-risk patients, although improved, continues to be poor and remains one of the most challenging to treat. Children with high-risk neuroblastoma account for approximately half of all patients diagnosed with neuroblastoma. Despite evolving treatment regimens over the last 10 years, standard high-risk therapy continues to have four main components: (1) induction chemotherapy, (2) local control, (3) consolidation, and (4) maintenance. Standard North American induction regimens (Children’s Oncology Group [COG]) include combinations of anthracyclines, alkylators, platinum compounds, and topoisomerase II inhibitors. Optimal local control is achieved with a combination of aggressive surgical resection and external beam radiotherapy to the primary tumor. Neuroblastoma is one of the most radiosensitive pediatric solid tumors. Consolidation therapy in most neuroblastoma treatment regimens includes myeloablative chemotherapy and autologous stem cell rescue (ASCR) ( ). A meta-analysis from 2015 revealed a statistically significant improvement in EFS when myeloablative therapy combined with ASCR was used for patients ( ). Although many children achieve complete clinical remission with this multimodal approach, relapse is common, suggesting that minimal residual disease is an important factor in neuroblastoma relapse.
| LOW RISK (40%) | INTERMEDIATE RISK (20%) | HIGH RISK (40%) | |
|---|---|---|---|
| Event-Free Survival | > 95 | 80–95 | 40–50 |
| Patient/tumor characteristics |
|
|
|
| Treatment | Observation or surgery; chemotherapy only for symptoms (e.g., stage 4S or cord compression) | Chemotherapy (2–8 cycles based on biology), surgery | Chemotherapy, surgery, radiation, myeloablative therapy with autologous stem cell rescue, immunotherapy, and biological agents (isotretinoin) |
* Summarized are general treatment strategies and characteristics for each risk group based on recent Children’s Oncology Group (COG) trials. This chart includes the most common characteristics for each group and overall treatment strategies. These treatments may vary across different cooperative groups internationally and change based on ongoing and future clinical trials. The approximate relative proportion of patients in each risk group is based on data from the COG ANBL00B1 Biology Study (since 2001).
In the 1980s and 1990s, clinical trials testing antibodies directed against GD2 ganglioside—a cell-surface marker expressed on the surface of neuroblastoma tumor cells—demonstrated promising results as a postconsolidation treatment option for children with neuroblastoma. A recent randomized-controlled phase III trial demonstrated that the addition of the anti-GD2 chimeric monoclonal antibody ch14.18 [(dinutuximab (Unituxin)] in conjunction with cytokines, granulocyte-macrophage colony-stimulating factor (GM-CSF), and interleukin-2 (IL-2) to standard isotretinoin maintenance therapy significantly improved short-term survival in high-risk patients, with both higher EFS and OS rates compared with standard therapy after 2 years of follow-up ( ). This led to Food and Drug Administration (FDA) approval of dinutuximab for use in children with high-risk neuroblastoma ( ). Additional studies have shown efficacy for different anti-GD2 regimens at diagnosis and recurrence. However, anti-GD2 regimens have a number of significant side effects, including allergic reactions, fever, hypotension, capillary leak syndrome, extended infusion times, and pain ( ; ). Pain caused by cross-reactivity with GD2 expressed on peripheral nerve cells is significant and neuropathic in nature. Treatment with gabapentin pre- and posttreatment course, patient-controlled anesthesia (PCA), or nurse-controlled analgesia (NCA) with opioids (morphine or Dilaudid) and low-dose ketamine infusion (2 to 7 mcg/kg/min) are frequently necessary during the course of these treatments. Overall, outcomes for patients with high-risk neuroblastoma have been improving over the past several years with the addition of immunotherapy into postconsolidation/maintenance therapy regimens ( ).
HSCT represents the most common and effective form of immunotherapy for pediatric malignant and nonmalignant diseases. Autologous HSCT involves infusion of the patient’s own hematopoietic cells after conditioning with chemotherapy with or without radiation. Autologous transplant has traditionally been performed for children with high-risk or relapsed solid tumors and lymphoma ( ). Allogeneic HSCT refers to the transfer of hematopoietic stem cells from one individual to another (related or unrelated) with the intent to obtain lifelong engraftment of the administered cells (see Chapter 38 , “Solid Organ Transplantation”). Allogeneic transplant offers the advantages of being free of contaminating malignant cells in the setting of cancer and of providing the missing or dysfunctional hematopoietic elements in children with immunodeficiencies, nonmalignant hematologic diseases, and disorders of metabolism. Disadvantages of allogeneic transplantation are higher risks of transplant-related morbidity and mortality, graft failure, and the potential for graft-versus-host disease (GVHD) ( ). The use of allogeneic HSCT as a cellular immunotherapy for acute leukemia first became feasible in the early 1960s. In the 1970s, Thomas and colleagues cured several patients with end-stage leukemia by using human leukocyte antigen (HLA)–identical siblings after ablating the recipient marrow with total body irradiation (TBI) combined with cyclophosphamide ( ).
The outcome and efficacy of HSCT in malignancies is influenced by several factors, including the underlying disorder, level of residual tumor, donor source, HLA matching, degree of graft-versus-leukemia/tumor effect (GVL/T), and the toxicities associated with the preparative chemotherapy regimens. Various allogeneic graft sources have the potential to produce a potent antineoplastic GVL/T effect to sustain complete remission of malignant disease. Donor sources for HSCT in children include cells from bone marrow, umbilical cord blood, or mobilized peripheral blood from related or unrelated donors ( ). Bone marrow was the first stem cell source for transplantation and is the standard to which other sources are compared. Peripheral blood stem cells (PBSCs) are not a preferred stem cell source in pediatrics. PBSC transplants have a greater risk of GVHD, and in pediatric studies, this has resulted in greater overall mortality. Umbilical cord blood has a lower risk of GVHD compared with equally HLA-matched marrow ( ). The most important selection criterion for a donor source is HLA matching. HLA-identical sibling donors are considered the preferred stem cell source for allogeneic HSCT. HLA-identical sibling donors produce less transplantation-related mortality, less acute GVHD and chronic GVHD, and better disease-free and OS rates than unrelated donors ( ).
Several factors contribute to the outcome of HSCT. In particular, the preparative chemotherapy regimen is a major determinant. The goal of preparative or conditioning regimens in patients undergoing HSCT for malignant diseases is to provide significant immune ablation to prevent graft rejection, as well as reduce the tumor burden. Myeloablative conditioning regimens are used for this purpose and consist of alkylating agents with or without TBI. High-dose TBI is used as part of myeloablative regimens. TBI reduces the risk for relapse, but TBI-based regimens have significant late effects in children. TBI complications include gastrointestinal, hepatic, and pulmonary toxicities; cataracts; endocrinopathies; second malignancies; impaired growth; and delayed cognitive development. The addition of immunotherapy with antibodies that target T cells, such as antithymocyte globulin (ATG) and alemtuzumab (Campath1H), to conditioning regimens is also used to decrease the incidence of graft rejection and prevent GVHD ( ).
Relapsed ALL is the most common malignant childhood disease for which an HSCT is offered, particularly in those who experience bone marrow relapse with treatment or within 18 months of discontinuing therapy. The use of HSCT for patients with AML has evolved significantly in the last 3 decades, and in chronic myeloid leukemia, a relatively rare hematopoietic malignancy in the pediatric and adolescent population, allogeneic HSCT offers the only proven curative approach. Myelodysplastic syndrome (MDS) is a clonal disorder of hematopoiesis with variable bone marrow dysplasia and cellularity, progressive cytopenias, and susceptibility for transformation to AML. MDS in children has a poor prognosis, and the curative treatment of choice is allogeneic HSCT ( ).
For most patients undergoing allogeneic HSCT, the major causes of morbidity and mortality are related to disease relapse, acute GVHD and chronic GVHD, infection, regimen-related toxicity, and graft failure. Acute GVHD is an immune response stimulated and accentuated by injury resulting from the preparative regimen used before transplantation. The incidence ranges from 20% to 80%. A postulated mechanism of acute GVHD involves the activation of toll-like receptors on various cells, which then leads to the release of inflammatory cytokines. Activation of toll-like receptors blocks the suppressive effects of regulatory T cells, thus permitting the activated alloreactive cytotoxic T cells to enter the circulation and damage organs such as the gut, liver, and skin, resulting in acute GVHD. Standard acute GVHD prophylaxis regimens usually combine a calcineurin inhibitor (tacrolimus or cyclosporine) with short-course methotrexate ( ).
The pathogenesis of chronic GVHD is not completely understood, but it is believed to involve autoimmune and alloimmune dysregulation, leading to disordered immunologic reactivity against autologous (self) and allogeneic (donor) antigens. Chronic GVHD can potentially affect any organ of the body, although the skin, eyes, oral cavity, gastrointestinal tract, genitourinary system, liver, and lungs are most commonly affected ( Table 50.4 ).
| System | Features |
|---|---|
| Systemic | Recurrent infections with immunodeficiency, weight loss, sicca syndrome, debility |
| Skin | Lichen planus, scleroderma, hyperpigmentation or hypopigmentation, dry scale, ulcerated, freckling, flexion contractures |
| Hair | Alopecia |
| Mouth | Sicca syndrome, depapillation of tongue with variegations scalloping of lateral margins, lichen planus and ulcer, angular tightness, salivary gland inflammation, fibrosis |
| Joints | Decreased range of motion, diffuse myositis/tendonitis |
| Eyes | Decreased tearing, injected sclerae, conjunctivae |
| Liver | Cholestasis, cirrhosis |
| Gastrointestinal | Failure to thrive, esophageal strictures, malabsorption, chronic diarrhea |
| Lung | Bronchiolitis obliterans can manifest as dyspnea, cough, wheezing with normal CT scan and marked obstructive ventilatory defects, pneumothorax, chronic sinopulmonary symptoms, and/or infections |
| Heart | Bradycardia, chest pain |
Advances in allogeneic HSCT have led to improved patient survival, and long-term complications have increased in importance. Long-term effects may involve the cardiovascular, pulmonary, renal, CNS, and endocrine systems. Second malignancies can also occur. In addition, the psychosocial implications of survivorship have lasting effects. Allogeneic HSCT represents the most common and effective form of immunotherapy in childhood leukemia. Over the past several decades, clinical studies have revealed that the effectiveness of graft-versus-leukemia (GVL) in eradicating malignant disease is linked closely to the activity of immunoreactive cells in the graft, particularly the T cells and natural killer (NK) cells from allogeneic bone marrow, PBSCs, or umbilical cord blood. Comparison of relapse rates seen in patients who develop no GVHD, acute GVHD, chronic GVHD, or both acute GVHD and chronic GVHD continues to reveal that the GVL effect is strongly associated with the development of GVHD. The GVL effect is affected by several factors, the primary one being MRD status before transplant, resulting in a higher risk for relapse after HSCT in ALL ( ).
The anesthetic management of patients with cancer is influenced by the type of cancer, surgery, chemotherapeutic agents, amount of radiation, and the patient’s underlying medical condition. The diagnosis of cancer can illicit visceral emotions in both the patient and family members. Hospital treatments can extend over years, and treatments can create unwanted physical changes (e.g., hair loss) in addition to emotional effects. Patients and families deal with the diagnosis and treatment of cancer in various ways. Anesthesia personnel must be cognizant of the stress that both patients and families undergo and be supportive of their needs. For cancer patients, the concern for cancer is a lifelong issue, even when they have been cured. Many of these patients require frequent anesthetics, and anesthesia providers need to determine what makes the procedure easier and safe for each patient. In addition to their emotional health, anesthetic concerns need to be directed to patients’ medical issues. Acute and delayed toxicities result from the administration of chemotherapy. Acute toxicities common to most chemotherapy agents include myelosuppression, alopecia, nausea, vomiting, mucositis, liver dysfunction, infection, and adrenal suppression (see Table 50.1 and Table 50.5 ).
| Risk Factor | Description |
|---|---|
| Cumulative dose | Risk <1% for doses <300 mg/m 2 |
| 5%–10% for doses 350–450 mg/m 2 ; 30% for doses >550 mg/m 2 | |
| Schedule of administration | Greatest risk with bolus administration |
| Less risk with continuous infusion | |
| Less risk with dexrazoxane | |
| Mediastinal irradiation | Strong association with increasing risk |
| Cardiac disease | Preexisting coronary artery disease, valvular heart disease, hypertension |
| Age | Young children |
| Adults older than 70 years of age |
Pediatric patients with oncologic disease present for diagnostic and therapeutic procedures in all areas of the hospital. In addition to the operating room, they require sedation and general anesthesia in procedure rooms, radiology (CT, nuclear medicine, and MRI), interventional radiology, and radiation therapy. General principles for the sedation and anesthesia of these patients are outlined in Box 50.3 and Box 50.4 .
Aseptic techniques: Strict hand washing and central line access cleaning
Preoperative evaluation
Evaluate emotional state of patient and family
Premedication as necessary
Review medications and use of steroids
Assess cardiovascular and pulmonary status
Determine ANC level and platelet count
Consider continuing antihypertension medications
Sedation and anesthetic induction and maintenance
Sedation with propofol and/or dexmedetomidine and/or midazolam and/or ketamine (see Box 50.4 )
| Sedation Type | N(%) New ALL |
|---|---|
| General anesthesia | 115(6) |
| Propofol alone | 1152(57) |
| Propofol with opioid and/or midazolam | 641(32) |
| Midazolam and an opioid | 70(4) |
| Other a | 40(2) |
Other types of sedation included (a) ketamine and propofol, (b) ketamine/midazolam or fentanyl/midazolam, and (c) opioid/midazolam or ketamine.
Inhaled or intravenous agents for induction and maintenance
Standard monitors, minimize Fi o 2 , avoid rectal temperatures
Regional anesthesia as clinically indicated
Anesthetic adjuncts
^ Determine whether patient on preexisting granisetron (Kytril).
* Steroids should be avoided if diagnosis and protocol treatment are not decided because steroid administration may preclude entry into some cancer protocols.
Radiation therapy presents unique challenges because of the repeated procedures, immobilization devices (head and face), and distance required between the anesthesia team and the patient (remote monitoring). Historically, sedation and general anesthesia with volatile anesthetics have been used for these patients. Propofol anesthesia with a natural airway has become a popular anesthetic hypnotic for these procedures and is well described. Many centers use a protocol consisting of an intravenous bolus (1 to 2 mg/kg) followed by an infusion (200 to 250 mcg/kg/min) ( ). The safety of propofol-based anesthesia for radiation therapy has been established. Complications can occur, and these appear to be related to medication error (programming the infusion pump incorrectly) and airway obstruction (positioning, apnea, and laryngospasm) ( ). These findings highlight the need to double-check infusion pumps and be vigilant with airway management and assessment, including continuous monitoring with pulse oximetry during transportation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here