Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Patients in need of treatment for head and neck or oral tumors, whether benign or malignant, require a multidisciplinary team approach, including the head and neck surgeon, prosthodontist, dental oncologist, speech and language pathologist, radiation oncologist, medical oncologist, highly skilled nursing, dieticians, psychiatrists/psychologists, and nurse navigators to coordinate their care. The expertise and collaboration of these individuals can greatly contribute to quality of life issues, such as eliminating or decreasing the intensity of dental disease and/or complications of cancer treatment, along with restoration of oral defects and facial deformities. Maxillofacial prostheses can rehabilitate compromised function after surgical resection of structures essential for speech, mastication, and swallowing nearly to a presurgical level, thus restoring the patient's life. Similarly, prostheses can effectively improve the facial appearance of patients who have undergone major resections such as a rhinectomy or an orbital exenteration, allowing patients to face their family, friends, and the working world with confidence that life after cancer is no different than before the diagnosis.
Patients planned for nonsurgical treatment, such as radiation and/or chemotherapy, should be evaluated by a dental professional before treatment to screen for potential sources of dental infection. Such sources of dental infection are extensive dental caries, periapical pathology, and periodontal disease, which should be treated before initiating cytotoxic treatment. Ideally, a period of at least 2 weeks should be permitted for soft-tissue healing following dentoalveolar surgery so that mucosal integrity is restored before commencement of oncologic treatment. It must be emphasized that dental extraction in the proximity of the tumor opens up a route for invasion through the bony cortex and thus should be avoided. Additionally, subsequent bleeding through this route can be challenging to manage and should therefore be avoided unless a management strategy has been determined.
The initial dental evaluation of the patient is focused to establish a baseline against which subsequent examinations can be compared. Measures to enhance and maintain oral hygiene are explained to the patient and must be reinforced during and after treatment ( Table 18.1 ). It is critical that the patient fully understand that their hygiene from this point on must be impeccable, and the use of fluoride supplementation, either through dentifrice or otherwise, and other anticariogenic supplements is crucial to the maintenance of the health of their teeth. Additionally, these measures are critical to avoid rampant caries in a hyposalivating patient following radiotherapy. This is equally true for patients taking bisphosphonates, bevacizumab, denosumab, or other bone-stabilizing drugs. Failure to comply with these treatment recommendations increase the likelihood of the patient experiencing undesirable treatment complications, which may include osteonecrosis of the jaw secondary to dentoalveolar surgery (e.g., dental extractions, gingival surgery, dental implants, etc.). The patient's awareness and compliance, along with appropriate counseling and motivation, are crucial to the success of preventive dental strategies and should be reiterated by the entire team of caregivers and documented well for the protection of all involved.
|
The mucosal surfaces of the oral cavity are exposed to significant doses of radiation therapy during treatment of most tumors except those of the larynx, hypopharynx, and thyroid. Inclusion of the upper neck (i.e., levels I and II) in the radiation portals also places the oral cavity at risk. When examining patients before radiation therapy, whether as single modality or in the postoperative setting, the age, diagnosis, fields, and prescription of radiation should be taken into consideration before making dental treatment recommendations. The effects of radiation on the dentition, periodontium, and mucosal surfaces are listed in Table 18.2 . Indirect effects on these structures also can result from exposure of the parotid and submandibular glands to radiation, with consequent alteration of saliva production. Lastly, it is important to remember that patients who are planned for radioactive iodine (RAI) treatment should be seen for dental screening before therapy as well. RAI treatments can have a profound effect on the salivary glands, and the secondary effect of this xerostomia can be devastating to the teeth.
| ACUTE SIDE EFFECTS | LONG-TERM SIDE EFFECTS |
|---|---|
| Mucositis Dysgeusia Dysphagia Ropey saliva |
Xerostomia Caries Periodontal disease Trismus Osteoradionecrosis |
Patients who have extensive metallic restorations, including restorations made of zirconia that appear tooth colored, require custom mouthguards to reduce scatter that causes mucositis in the surrounding tissues ( Fig. 18.1 ). These guards should be fabricated in time for them to be delivered before the radiation simulation appointment. Table 18.3 lists the indications for dental extraction in patients who are scheduled to undergo radiation therapy. As previously mentioned, all extractions should be completed in time for mucosal healing to occur. If possible, the mucosa of the gingiva at the extraction site should be closed with sutures.
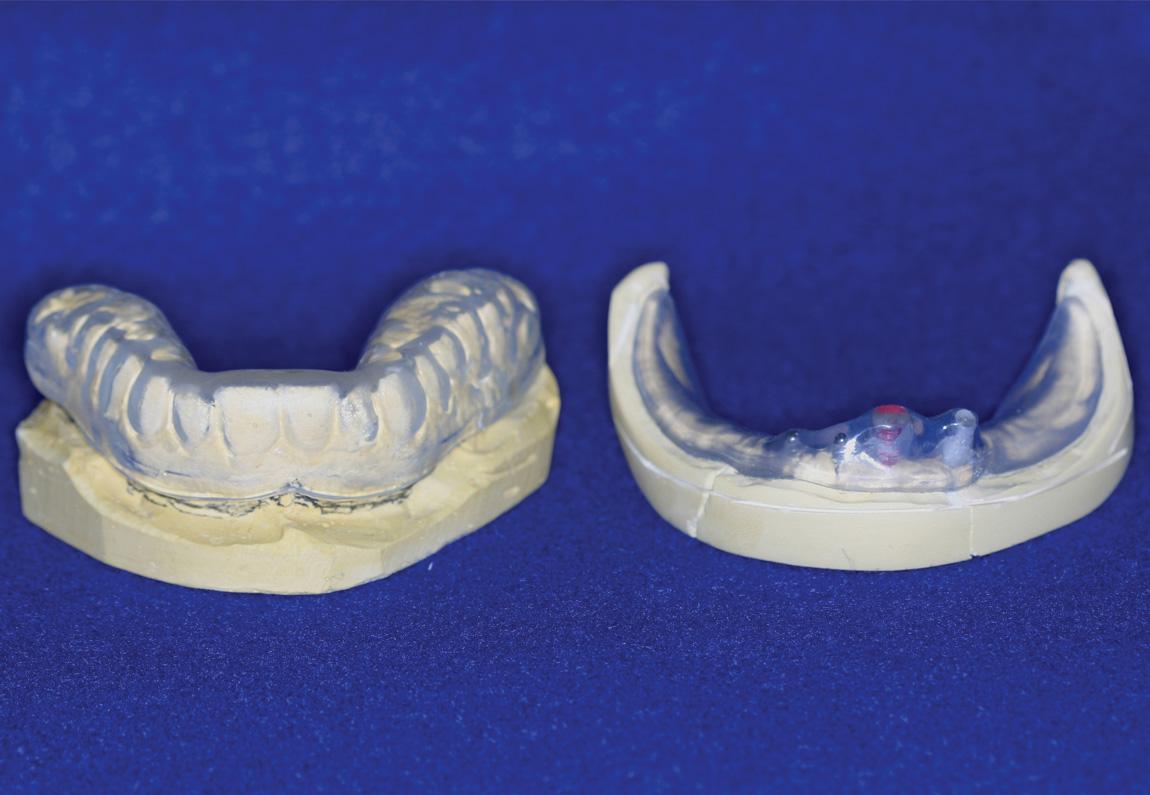
|
The general practicing dentist and hygienist can use the interval between the initial screening appointment and commencement of radiation therapy to complete oral hygiene procedures such as scaling, polishing, subgingival root planning, and curettage. Overhanging and faulty restorations can be removed and replaced as needed, and ill-fitting dentures should be corrected.
Home care should include effective daily plaque removal and use of soft toothbrushes with application of a fluoridated dentifrice. Factors such as the type of fluoride used or the modality of its application are less important to outcome than is daily compliance in using high-potency fluoride for the remainder of the patient's life. Patients are instructed to floss daily and brush their teeth after every meal, which includes liquid supplements, because they contain cariogenic carbohydrates. A toothpaste or gel that contains neutral 1% sodium fluoride is preferred over stannous fluoride, which has an unpleasant taste and adverse effects such as sensitivity of the teeth and gingiva. The toothpaste can be used with a brush every night for 2 minutes; the patient may expectorate as much as desired but should not rinse, thus leaving a thin film of fluoride on the teeth while sleeping.
Although commonly used chemotherapeutic agents such as cisplatin, 5-fluorouracil, and methotrexate have the potential to cause acute mucositis on their own, their combination with each other and/or with radiation therapy can result in treatment-limiting stomatitis. Current regimens for the treatment of head and neck cancer in general result in less severe oral complications than those seen with treatment of hematologic disease. Chemotherapy-related adverse effects can include mucositis and ulceration, oral candidiasis, and bacterial and/or viral infections of the oral mucosa. In addition to their direct effects, these problems can cause a severe reduction in dietary intake with all the sequelae of malnutrition. As with patients scheduled to have radiation therapy, these patients also should be evaluated at least 2 weeks before chemotherapy is begun. The review should include a discussion of the hematologic status, that is, total white blood cell and neutrophil count, platelet counts, and clotting parameters. The patient is carefully screened for potential sources of bacteremia such as periodontal disease, and appropriate therapy is undertaken if needed. Endodontic treatment or dental extractions are considered for symptomatic teeth with periapical lesions, and third-molar pathology is addressed. As previously described, patients are instructed regarding home care hygiene and caries control. Oral hygiene during treatment should include frequent rinsing with a salt and baking soda solution and brushing of the teeth.
Patients with very advanced cancer may be managed less aggressively, keeping their overall status and prognosis in mind. Asymptomatic teeth may be retained and managed conservatively even in the presence of active chronic dental disease. Abutment teeth for a removable prosthesis should be preserved if possible. Definitive restorations with crowns, fixed bridges, and onlays for control of carious teeth may be delayed until completion of treatment and provisionally restored with use of intermediate restorative material.
Xerostomia is probably the most common long-term sequela of radiating the oral cavity. The severity of xerostomia depends on the total dose of radiation, the source, the fractionation, and the portals of delivery. Radiation results in atrophy of salivary glandular elements, causing cessation of saliva production. The problem is compounded if the patient receives chemotherapy concomitantly with radiation. Anecdotally, patients who have undergone RAI can have profound xerostomia and should thus be evaluated and managed as appropriate.
Apart from the adverse impact on quality of life because of difficulty with eating, oral comfort, fit of prostheses, and speech, xerostomia also increases the risk of caries, periodontal disease, and ultimately osteoradionecrosis (ORN) if the extent of tooth breakdown leads to extraction. Pharmacologic agents such as pilocarpine and amifostine have been investigated with mixed results for relief of xerostomia. However, most patients obtain symptomatic relief through frequent use of water.
Other long-term sequelae are related to effects on soft tissue, bone, or blood vessels. Irradiation of the muscles of mastication can produce fibrosis that leads to trismus over time. Regular stretching exercises of these muscles can mitigate the effects of this complication. Patients should begin to perform these exercises before radiation therapy commences, and if the exercises are stopped during treatment, they must be resumed as soon as the acute side effects of radiation therapy have subsided. The exercises should be performed in multiple cycles throughout every day and can be assisted with appliances such as an acrylic resin corkscrew, tongue depressors taped together, or a commercially available device ( Fig. 18.2 ). Alternatively, patients may be instructed to use their thumb and fingers to forcefully open the mouth to the point of tolerance. It is crucial for the clinician to reinforce the importance of preventing trismus at every opportunity, because difficulty in opening the mouth not only affects quality of life but also interferes with adequate posttreatment monitoring for recurrent or new cancer and providing routine dental care.

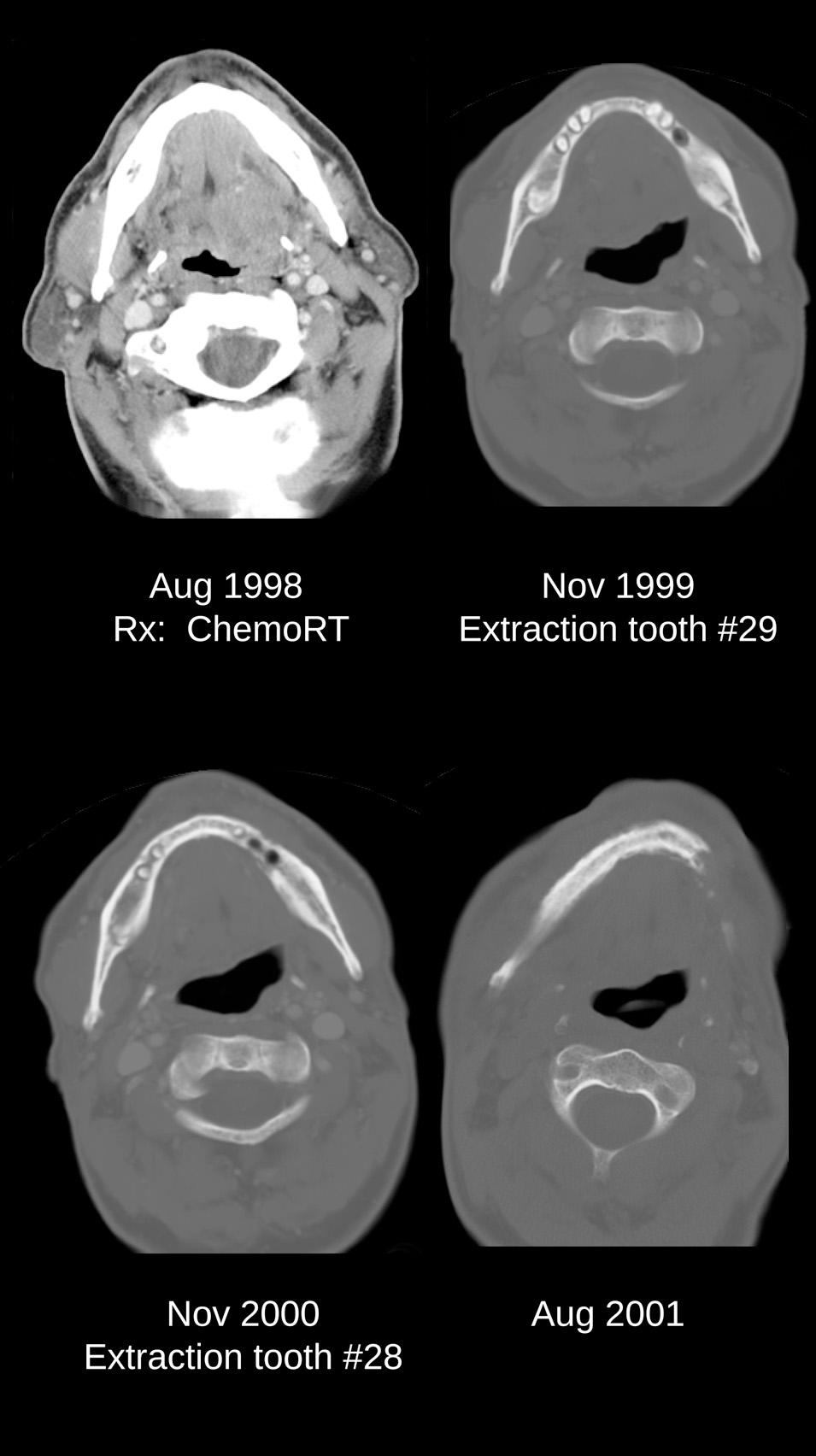
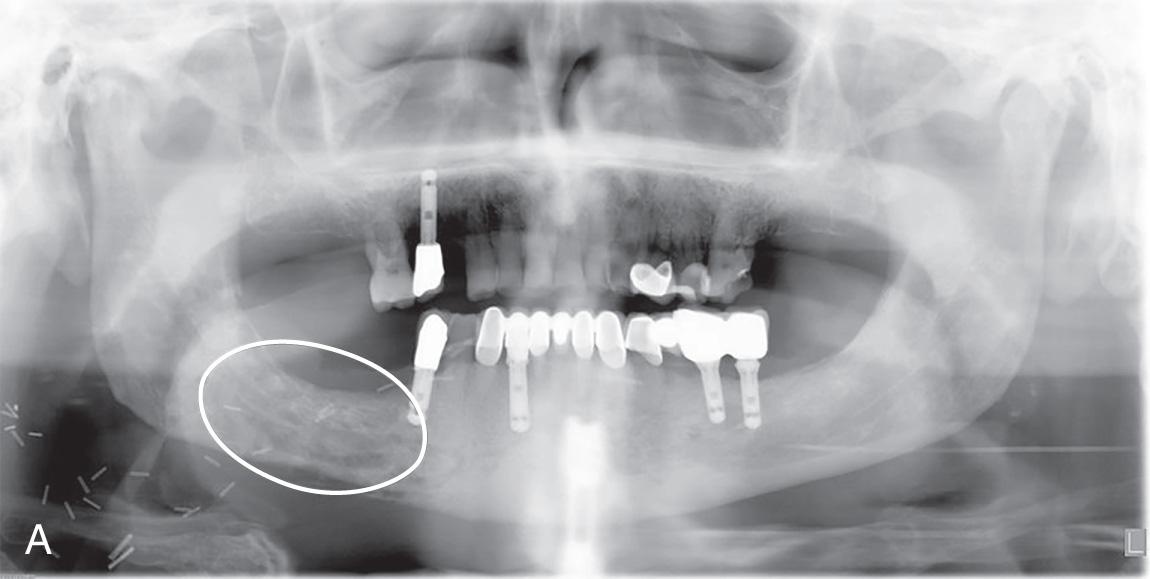
The risk for ORN of the jaw increases if the bone is irradiated with more than 55 Gy. The mandible is more commonly affected because of endothelial damage to the relatively sparse intraosseous blood vessels. The maxilla has a richer blood supply, and therefore ORN of this bone is less common. The process can take many months or even years to develop, and patients are at risk for the development of ORN for the remainder of their lives. Bone exposure or exposed retained roots are a common cause for initiation of ORN ( Fig. 18.3 ). Dental caries and periodontal disease leading to extractions are the most common inciting factors. A series of computed tomography (CT) images of a patient who experienced ORN of the mandible after dental extraction in a previously irradiated mandible is shown in Fig. 18.4 . However, a third of ORN cases occur spontaneously. Prevention of this complication by rigorous oral hygiene is crucial, because conservative measures such as antibiotics, debridement, and curettage are effective only in early disease. Advanced ORN of the mandible with exposed intraoral bone generally requires segmental resection and reconstruction.

The role of hyperbaric oxygen (HBO) in the management of ORN is controversial. It is important for the clinician to realize that HBO cannot revitalize dead, necrotic bone, for which the only definitive remedy is a sequestrectomy. The major concern in using HBO for ORN revolves around whether replenishing oxygen in the affected tissues can lead to activation of cancer cells that otherwise were dormant. Although no systematic study of this question and other questions regarding the use of HBO in patients with ORN has been conducted, anecdotal instances of the reactivation of a tumor in this setting certainly exist. Therefore HBO should be used with caution, especially if any doubt exists about the cancer status of the patient. There are increasing reports of the successful use of pentoxifylline and alpha-tocopherol for management of both ORN and MRONJ (medication-related osteonecrosis of the jaws). Pentoxifylline has been long used for the treatment of intermittent claudication. Pentoxifylline increases blood flow to the affected microcirculation. Alpha tocopherol is used for its antioxidant properties. Although the precise mechanism of action is not well defined, blood viscosity is lowered, erythrocyte flexibility is increased, leukocyte deformability is increased, and neutrophil adhesion and activation are decreased. Overall, tissue oxygenation is significantly increased. Because of its interaction with antiplatelet and anticoagulant medications, the use of pentoxifylline in patients taking such drugs should be discussed with the patient's physician. Although there have not been large-scale studies on the effectiveness of pentoxifylline/alpha-tocopherol in the treatment of ORN/MRONJ, current reports appear promising ( Fig. 18.5 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here