Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In 1967, Sabiston and colleagues reported the first clinically successful coronary artery bypass graft (CABG) operation. Other early pioneers include Favaloro, who is credited with popularizing the use of autogenous saphenous vein, and Kolesov, who performed the first mammary artery to coronary artery anastomosis. Over the next several years, centers around the world began performing CABG procedures in large numbers. Subsequent refinements in cardiopulmonary bypass (CPB), the introduction of cardioplegia, the growing acceptance of the left internal mammary artery graft, and the development of the intra-aortic balloon pump (IABP) resulted in continued improvements in outcomes after CABG. By the early 1990s, more than 350,000 CABG procedures were being performed each year in the United States alone, with routine application in octogenarians, patients with severely impaired ventricular function, and patients with serious noncardiac morbidities.
As results continued to improve, and as mortality and major morbidity rates in the 2% range became commonplace, many surgeons turned their efforts toward decreasing the invasiveness of CABG and exploring alternative strategies of revascularization. Over the past several years, this effort has produced a number of new technologies, techniques, and variations on traditional CABG―all intended to avoid CPB, minimize aortic manipulation, improve graft patency, and reduce the trauma of surgical access. These variations include multivessel CABG without CPB, single-vessel CABG without CPB via a left mini-thoracotomy, videoscopic single-vessel CABG using surgical robotics, and multivessel CABG via a left mini-thoracotomy using peripheral cannulation and CPB. Other new procedures include transmyocardial revascularization, reoperative CABG through alternative incisions, and grafting strategies and devices that minimize or eliminate aortic manipulation, facilitate construction of the distal anastomosis, and perhaps improve long-term graft patency. This chapter describes these developments, discusses their relative merits, and summarizes current clinical results.
The development of the heart-lung machine by Gibbon and its early application by Kirklin initiated the era of open heart surgery, which eventually made CABG possible. CPB and the subsequent introduction of cardioplegia provided a motionless, blood-free surgical field, which enabled the precision and reproducibility necessary for direct coronary anastomosis. Despite the obvious benefits of CPB, however, there is an increasing awareness of its potential deleterious effects. As the formed elements of the blood contact the various components of the heart-lung machine, the blood elements are activated, resulting in a number of hematologic and ultimately systemic changes. Typical sequelae include activation of complement, release of endotoxin, activation of leukocytes, increased expression of adhesion molecules, and release of inflammatory mediators, including cytokines, arachidonic acid metabolites, free radicals, and other components. These sequelae occasionally manifest as coagulopathy, third-space fluid retention, and subtle end-organ dysfunction, including neurocognitive changes. The magnitude of this systemic inflammatory response is generally modest but can be quite severe, especially when the CPB time is prolonged. Furthermore, a small percentage of patients with preoperative end-organ dysfunction sustain significant morbidity when subjected to even a moderate systemic inflammatory response.
Ongoing efforts have been directed at reducing the magnitude of the systemic inflammatory response associated with CPB. These efforts include decreasing the surface area and modifying the surface composition of the CPB circuit to minimize surface activation, reducing hemodilution by decreasing the volume of crystalloid prime needed to institute CPB, avoiding the use of a cardiotomy sucker, and pretreating patients with various anti-inflammatory medications, such as aprotinin. Despite progress in these areas, performing CABG without CPB remains attractive.
Off-pump coronary artery bypass (OPCAB) grafting is as old as coronary surgery itself. Large case series dating back to the 1970s document its feasibility. Nevertheless, OPCAB only recently gained widespread acceptance and entered the mainstream of clinical practice, propelled by a greater awareness of the potential morbidity of CPB and aortic manipulation and facilitated by improvements in surgical tools and techniques. OPCAB is part of the procedural armamentarium of a growing proportion of surgeons worldwide, and it can be performed in virtually any patient requiring coronary revascularization.
The introduction of self-retaining coronary stabilizers and the development of techniques for their use are key factors that have led to resurgent interest in OPCAB. When properly used, coronary stabilizers provide a relatively motionless and blood-free field, similar to that provided by CPB. The subsequent introduction of self-retaining cardiac positioning devices and the evolution of surgical techniques for rotating the heart enabled precise anastomoses to be constructed on the inferior, posterior, and lateral walls of the beating heart while maintaining adequate hemodynamic values without CPB.
A number of coronary artery stabilizers are available for clinical use. Current stabilizers can be categorized as compression, suction, or capture devices, depending on their design. When used properly, each type provides excellent immobility.
Compression-type stabilizers are generally two-pronged forks that are placed lightly on the epicardial surface so that the prongs are parallel to, and flanking, the coronary artery at the intended anastomotic site. The undersurface of the prongs provides traction to avoid slippage. The fork is attached to the retractor via an articulating arm and can be locked tightly in position once the desired position has been achieved. Generally, the best stability occurs when only light downward force is exerted on the surface of the heart. Paradoxically, an increase in motion occurs when excessive downward force is applied. One should use the exposure techniques described later in this chapter to present the target artery before positioning the stabilizer rather than relying on the stabilizer to retract the heart. Compression-type stabilizers are advantageous in that they may be set up quickly and simply and have an extremely low profile that allows unrestricted access to the coronary artery. For some aspects of the heart, however, compression stabilizers may require slightly more downward pressure to avoid slippage ( Fig. 89-1 ).

Suction-type stabilizers also are generally two-pronged forks attached to the retractor by means of an articulating arm. These stabilizers, however, are constructed with a series of ports on the undersurface of the prongs, which are connected by sterile tubing to a −100 to −300 mm Hg wall-suction unit. The epicardium and epicardial fat that flank the vessel are sucked into these ports, allowing the stabilizer to grip the surface of the heart. Thus, less downward force is required at the anastomotic site. Suction stabilizers are set up quickly but require a regulated vacuum line. They have the added advantage of providing traction and countertraction of the fat surrounding the coronary artery, so they are well suited for arteries deep within the fat. Suction stabilizers tend to have a slightly higher profile than compression types, but access to the coronary artery is not generally problematic ( Fig. 89-2 ).
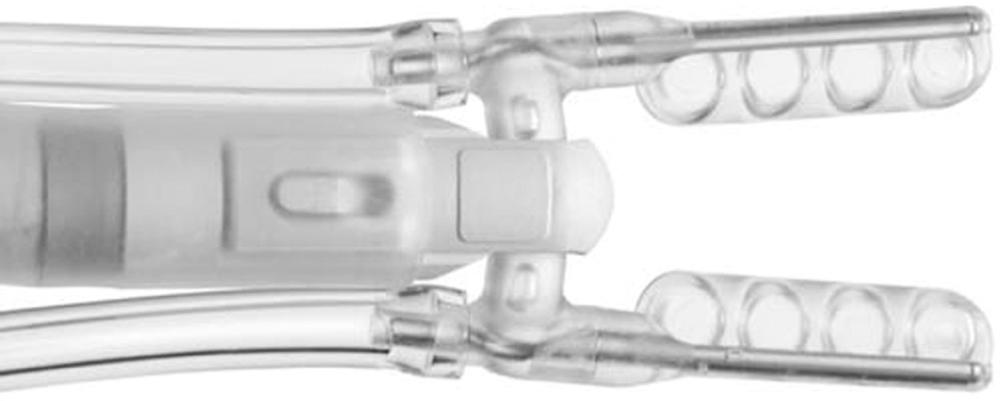
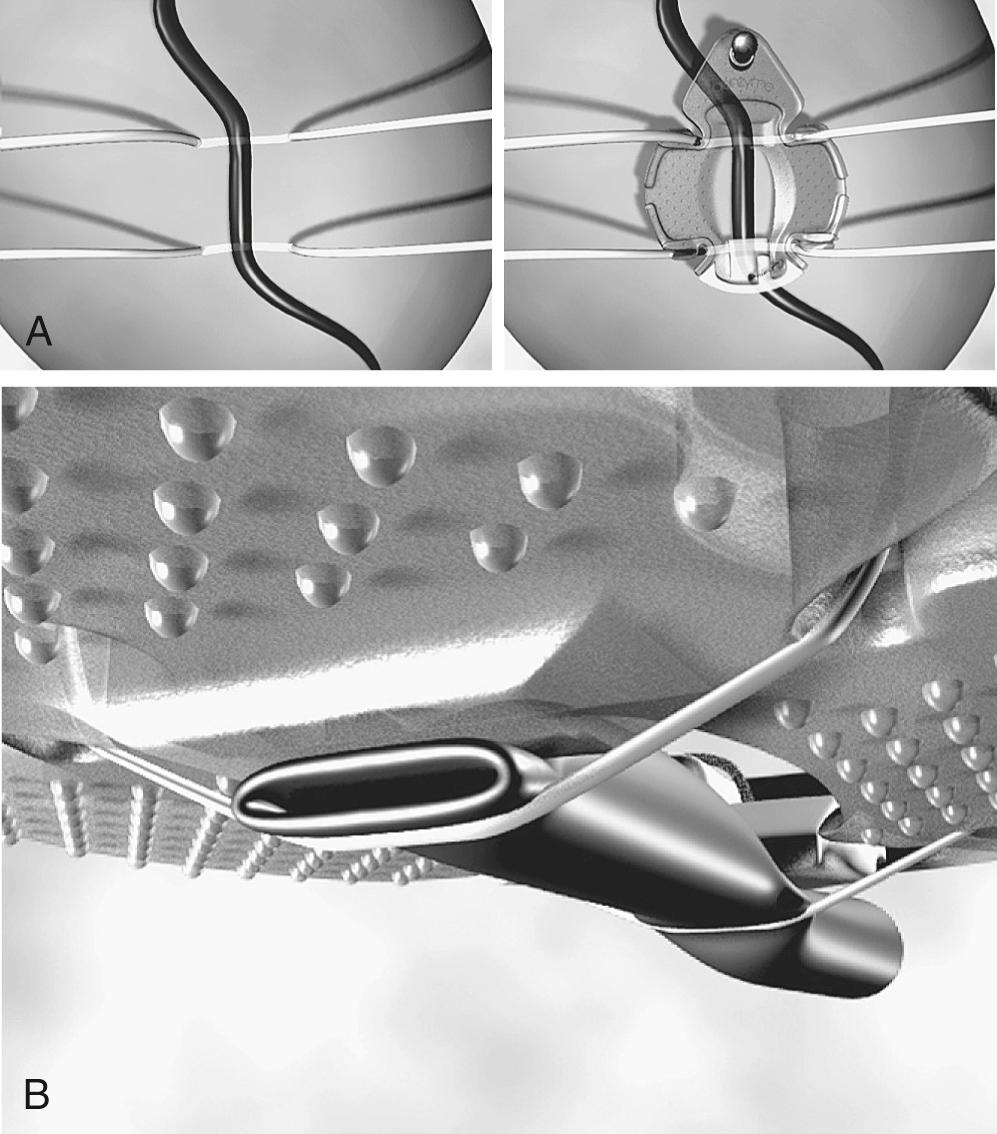
Capture-type stabilizers are usually fenestrated platforms that frame the intended anastomotic site. Silicone elastic tapes are passed deep under the coronary and locked to the platform under tension, pulling the epicardium up against the platform's undersurface ( Fig. 89-3 A ). Like suction, this effect reduces the demand for downward force to prevent slippage. Capture stabilizers, by virtue of their circumferential effect, provide superior anastomotic stability. Additionally, the silicone elastic tapes compress the coronary against the platform's posterior aspect, providing integrated biplane coronary occlusion (see Fig. 89-3 B ). Capture stabilizers are arguably more complex to position, however, and require proper spacing and alignment of the silicone elastic tapes to ensure adequate hemostasis. Furthermore, because of their larger size, they may not be well suited for use during construction of tightly spaced sequential anastomoses.
One of the greatest challenges of the early OPCAB procedures was to maintain hemodynamic stability while grafting coronaries on the lateral and posterolateral aspects of the heart. This challenge was greatest in patients with poor ventricular function and marginally compensated ischemia. The problem has been largely eliminated by advances in surgical technique, including placement of deep pericardial retraction sutures, right vertical pericardiotomy and pleurotomy, and right hemisternal elevation, as well as the introduction of cardiac positioning devices and advances in anesthetic management. To understand how these tools and techniques minimize the adverse effects of cardiac manipulation, it is important to understand the causes of potential hemodynamic compromise during OPCAB.
When a coronary stabilizer is positioned by using excessive downward force, the stabilized aspect of the heart is constrained, leading to diastolic dysfunction and a decreased left ventricular end-diastolic volume (LVEDV), stroke volume, and cardiac output. Whereas volume loading and Trendelenburg positioning attenuate these effects by elevating left ventricular (LV) filling pressures, recourse to these methods carries a risk of exacerbating intraoperative third-space fluid sequestration and associated morbidity. Similarly, the use of short-acting α-adrenergic agents can effectively maintain perfusion pressure in this setting, but they may be associated with an increased risk of perioperative mesenteric ischemia. β-Adrenergic agents should similarly be avoided in the setting of yet-to-be-grafted coronary arteries. There are also anecdotal reports of severe mitral regurgitation during exposure of the lateral wall, possibly related to deformation of subvalvular structures in combination with coronary insufficiency. Exposure techniques that minimize or eliminate LV deformation are therefore desirable.
Exposure of the lateral LV wall displaces the heart toward the right, compressing the right ventricle against the pericardium and right side of the sternum. The relatively low pressure in the right ventricle makes that chamber particularly vulnerable to deformation and reduction of the right ventricular end-diastolic volume (RVEDV). The decreased right ventricular (RV) stroke volume leads to poor LV filling and a decrease in cardiac output. Volume loading and use of the Trendelenburg position can compensate for this effect, as noted in the previous paragraph, but not without potential risk. RV compromise is thought to be the dominant cause in many cases of hemodynamic instability that occurs during exposure of the lateral wall. Various groups have proposed the use of RV assistance as palliation for RV compromise during multivessel OPCAB. Clearly, however, exposure techniques that minimize RV deformation are desirable.
Intraoperative myocardial ischemia, secondary to decompensated coronary disease, transient coronary snaring during grafting, or poor perfusion pressure during heart manipulation, is another mechanism that leads to hemodynamic instability. This complication is best prevented by close communication with the anesthesiologist, a revascularization strategy that minimizes myocardial ischemia, and exposure techniques that maintain hemodynamic stability. Whereas each patient should be managed on an individual basis, useful axioms exist. Patients with significant left main stenosis and marginally compensated ischemia may benefit from completion of the left internal mammary artery (LIMA) to left anterior descending (LAD) graft before manipulation of the heart. Similarly, initial revascularization of occluded, collateralized coronary arteries should be carried out before one transiently occludes the vessels that supply collaterals to those arteries. Selective use of intracoronary shunts, aortocoronary shunts, or assisted coronary perfusion devices can be essential when grafting a moderately stenosed right coronary artery (RCA) proximal to the origin of the posterior descending branch, or when grafting the proximal portion of a moderately stenosed LAD ( Fig. 89-4 ). The judicious use of an intra-aortic balloon pump can occasionally prove invaluable.

Depending on the size and shape of the heart and the dimensions of the chest cavity, visualization of the proximal obtuse marginal and posterolateral branches of the circumflex system may be obstructed by the left hemisternum. Although intuitive attempts at improving exposure may involve applying additional rightward force to that aspect of the heart with the stabilizer, this maneuver frequently leads to hemodynamic compromise and paradoxically poor stabilization. Maximal access and stability at the anastomotic site with minimal compromise of cardiac performance is most readily achieved when the stabilizer is applied with little or no pressure; thus, this device should be used only to stabilize the anastomotic field rather than to assist in retracting the heart. In many patients with relatively normal hearts and readily accessible coronaries, little attention to exposure techniques is required. In contrast, during multivessel OPCAB on patients with large, poorly contracting hearts, attention to these techniques is essential. In these patients, exposure of the lateral cardiac wall is best achieved with deep pericardial sutures, a right pleurotomy and pericardiotomy, right hemisternal elevation, or apical suction devices, as described next.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here