Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Providing peripartum analgesia and anesthesia requires an understanding of the physiologic changes that occur during pregnancy and labor and the effects of anesthetic care on the mother, fetus, and neonate. It also demands an understanding of the course of labor and delivery; knowledge of high-risk maternal conditions; ability to provide a variety of analgesic and anesthetic techniques; and preparation for potential obstetric emergencies and complications requiring immediate intervention, such as fetal distress and maternal hemorrhage.
During pregnancy, labor, and delivery, women undergo significant changes in anatomy and physiology as a result of (1) altered hormonal activity; (2) biochemical changes associated with increasing metabolic demands of a growing fetus, placenta, and uterus; and (3) mechanical displacement by an enlarging uterus. ,
Changes in the cardiovascular system during pregnancy can be summarized as (1) an increase in intravascular fluid volume, (2) an increase in cardiac output, (3) a decrease in systemic vascular resistance, and (4) the presence of supine aortocaval compression ( Table 33.1 ).
| System Parameter | Value at Term Compared With Nonpregnant Value |
| Cardiovascular System | |
| Intravascular fluid volume | Increased 35%–45% |
| Plasma volume | Increased 45%–55% |
| Erythrocyte volume | Increased 20%–30% |
| Cardiac output | Increased 40%–50% |
| Stroke volume | Increased 25%–30% |
| Heart rate | Increased 15%–25% |
| Peripheral circulation | |
| Systemic vascular resistance | Decreased 20% |
| Pulmonary vascular resistance | Decreased 35% |
| Central venous pressure | No change |
| Pulmonary capillary wedge pressure | No change |
| Femoral venous pressure | Increased 15%–50% |
| Pulmonary System | |
| Minute ventilation | Increased 45%–50% |
| Tidal volume | Increased 40%–45% |
| Breathing frequency | Increased 0%–15% |
| Lung volumes | |
| Expiratory reserve volume | Decreased 20%–25% |
| Residual volume | Decreased 15%–20% |
| Functional residual capacity | Decreased 20% |
| Vital capacity | No change |
| Total lung capacity | Decreased 0%–5% |
| Arterial blood gases and pH | |
| Pa o 2 | Normal or slightly increased |
| Pa co 2 | Decreased 10 mm Hg |
| pH | No change or minimal alkalosis |
| Oxygen consumption | Increased 20% |
Maternal intravascular fluid volume begins to increase in the first trimester. At term, the plasma volume has increased about 50% above the nonpregnant state, whereas the erythrocyte volume has increased only about 25%. This disproportionate increase in plasma volume accounts for the relative anemia of pregnancy. The hemoglobin decreases but normally remains at 11 g/dL or greater. This expanded intravascular fluid volume of 1000 to 1500 mL at term offsets the 300 to 500 mL blood loss that accompanies vaginal delivery and the average 800 to 1000 mL blood loss from cesarean delivery. After delivery, the contracted uterus auto-transfuses about 500 mL of blood.
The total plasma protein concentration is decreased as a result of the dilutional effect of the increased intravascular fluid volume, and colloid osmotic pressure is reduced from about 27 mm Hg down to 22 mm Hg by term. Pregnancy is a hypercoagulable state with increases in factors I, VII, VIII, IX, X, and XII and von Willebrand factor and decreases in factors XI and XIII, antithrombin III, and protein S. This results in an approximately 20% decrease in prothrombin time (PT) and partial thromboplastin time (PTT). Platelet count may remain normal or decrease 10% by term, and leukocytosis is common.
Cardiac output increases by about 35% by the end of the first trimester and increases 40% to 50% above baseline by the third trimester. This augmentation of cardiac output is the result of increases in both stroke volume (25% to 30%) and heart rate (15% to 25%). The onset of labor is associated with further increases in cardiac output, with increases above prelabor values by 10% to 25% during the first stage and 40% in the second stage. The largest increase occurs immediately after delivery, when cardiac output is increased by as much as 80% above prelabor values. This presents a unique postpartum risk for patients with cardiac disease, such as fixed valvular stenosis or cardiomyopathy. Cardiac output decreases within the first hours after delivery and reaches prelabor values about 48 hours postpartum. By 2 weeks postpartum, it has decreased substantially toward pre-pregnant values.
Although cardiac output and plasma volume increase, systemic blood pressure decreases in an uncomplicated pregnancy secondary to a 20% reduction in systemic vascular resistance. Systolic, mean, and diastolic blood pressure may all decrease 5% to 20% by 20 weeks’ gestation and increase slightly towards pre-pregnant values as the pregnancy progresses. There is no change in central venous pressure during pregnancy despite the increased plasma volume because venous capacitance increases.
When supine, the gravid uterus can compress the aorta and vena cava. Compression of the vena cava can decrease preload, cardiac output, and systemic blood pressure ( Fig. 33.1 ). In the supine position, some vena caval compression can occur near the beginning of the second trimester. By term, full occlusion when supine is typical, with venous return of blood from the lower extremities through the epidural, azygos, and vertebral veins. In addition, significant aortoiliac artery compression occurs in 15% to 20% of pregnant women. Nearly 15% of pregnant women at term experience significant hypotension in the supine position. Diaphoresis, nausea, vomiting, and changes in cerebration often accompany the hypotension. This constellation of symptoms is termed supine hypotension syndrome. Vena cava compression decreases cardiac output 10% to 20% and may also contribute to lower extremity venous stasis and thereby result in ankle edema, varices, and increased risk of venous thrombosis.
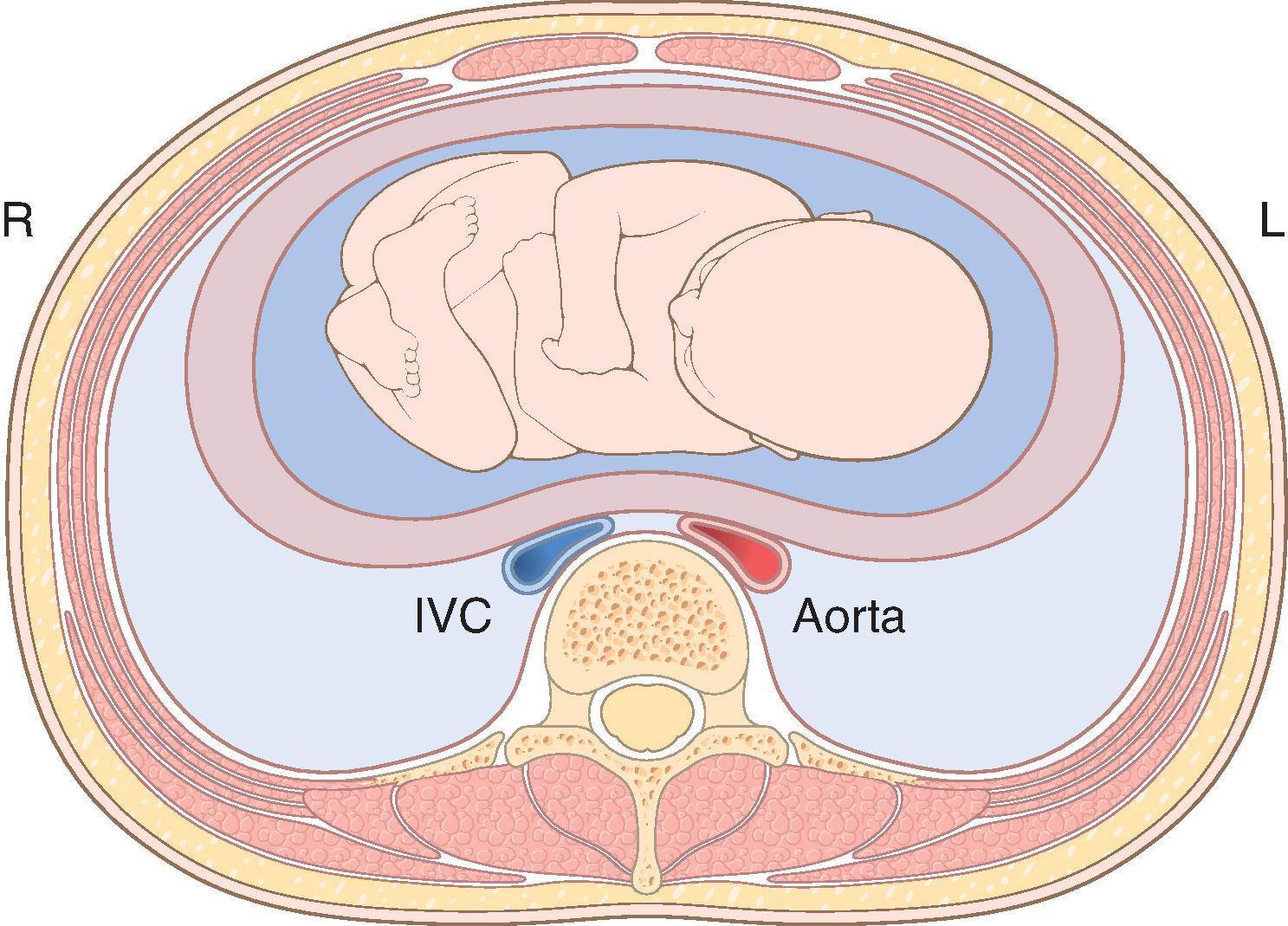
There are significant changes in echocardiography during pregnancy. The heart is displaced anteriorly and leftward. Right-sided chambers increase in size by 20% and left-sided chambers increase in size by 10% with an associated left ventricular (LV) eccentric hypertrophy. The right ventricular systolic pressure remains unchanged despite increases in intravascular volume. Mitral, tricuspid, and pulmonic valve annuli diameters increase. Tricuspid and pulmonic valve regurgitation is common, and about one in four women have mitral regurgitation. In addition, small insignificant pericardial effusions may be present.
Many pregnant women do not experience significant arterial hypotension when supine because they compensate for the reduction in preload with increases in systemic vascular resistance. This compensation is impaired by regional anesthetic techniques. Consequently, supine positioning is avoided during neuraxial anesthetic administration in the second and third trimesters. Significant lateral tilt (30 degrees) is frequently required to reduce hypotension and preserve fetal circulation by effectively displacing the gravid uterus off the inferior vena cava ( Fig. 33.2 ). Left uterine displacement can be accomplished by placing the patient in a left lateral position or by elevation of the right hip with a wedge or table tilt.
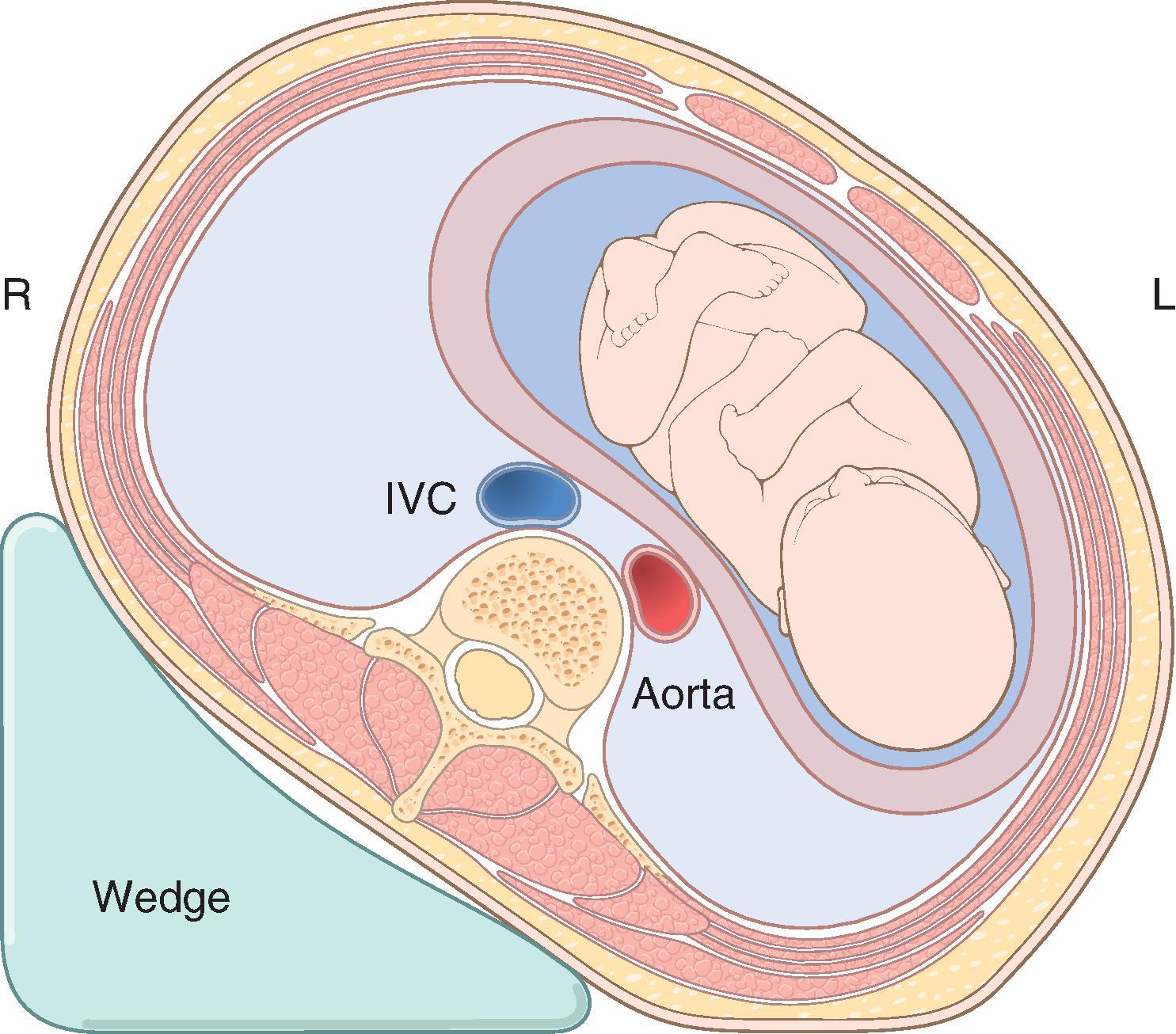
The gravid uterus can also compress the lower abdominal aorta. Such compression leads to arterial hypotension in the lower extremities, but decreases in systemic blood pressure measured in the arms may not reflect this decrease. The significance of aortocaval compression is the associated decrease in uterine and placental blood flow. Even with a healthy uteroplacental unit, prolonged maternal hypotension (more than a 25% decrease for an average patient) for longer than 10 to 15 minutes can significantly decrease uterine blood flow (UBF) and lead to progressive fetal acidosis.
The increased venous pressure distal to the level of vena caval compression diverts blood return from the lower half of the body via the paravertebral venous plexuses to the azygos vein. Flow from the azygos vein enters the superior vena cava and returns to the heart. Dilation of the epidural veins with pregnancy may increase the rate of unintentional intravascular placement of an epidural catheter and accidental intravascular injection of the local anesthetic solution. A “test dose” is therefore employed before dosing an epidural catheter in order to decrease the likelihood of an unintended intravascular placement before initiating neuraxial blockade. This technique is described in the section “Neuraxial Dosing and Delivery Techniques."
The most significant changes in the pulmonary system during pregnancy include alterations in (1) the upper airway, (2) minute ventilation, (3) arterial oxygenation, and (4) lung volumes (see Table 33.1 ).
During pregnancy there is significant capillary engorgement of the mucosal lining of the upper respiratory tract and increased tissue edema and friability. Additional care is needed during suctioning and placement of airways (e.g., avoid nasal instrumentation if possible) to prevent tissue bleeding with instrumentation. Mask ventilation, laryngoscopy, and intubation are all more challenging. A smaller cuffed tracheal tube (6.0 to 6.5 mm internal diameter) is typically selected because the vocal cords and arytenoids are often edematous. The presence of preeclampsia, upper respiratory tract infections, and active pushing with associated increased venous pressure further exacerbate airway tissue edema, making both ventilation and intubation more problematic. In addition, the weight gain associated with pregnancy, particularly in women of short stature or with coexisting obesity, can result in difficulty placing the laryngoscope because of a shorter neck and increased breast tissue.
Minute ventilation increases about 50% above pre-pregnant levels during the first trimester and is maintained for the remainder of the pregnancy. This increased minute ventilation is achieved primarily by a greater tidal volume, with small increases in the respiratory rate (see Table 33.1 ). Elevated circulating levels of progesterone and increased CO 2 production are presumed to be the stimulus for the increased minute ventilation. Resting maternal Pa co 2 decreases from 40 mm Hg to approximately 30 mm Hg during the first trimester as a reflection of the increased minute ventilation. Arterial pH, however, remains only mildly alkalotic (7.42 to 7.44) because of increased renal excretion of bicarbonate ions (HCO 3 − is approximately 20 to 21 mEq/L).
Early in gestation, maternal Pa o 2 while breathing room air is normally above 100 mm Hg because of the presence of hyperventilation and the associated decrease in alveolar CO 2 . Later, Pa o 2 will decrease towards pre-pregnancy values, likely reflecting airway closure and intrapulmonary shunt. Maternal hemoglobin (Hgb) is right-shifted, with the oxygen partial pressure associated with 50% Hgb saturation (P 50 ) increasing from 27 mm Hg up to approximately 30 mm Hg. This right-shift combined with the left-shifted fetal Hgb (a P 50 of approximately 18 mm Hg) facilitates oxygen delivery across the placenta.
At term, maternal oxygen consumption is increased by 20%. The added work of labor results in further increases in both minute ventilation and oxygen consumption. During labor, oxygen consumption increases above prelabor rates by 40% during the first stage and 75% during the second stage.
The expiratory reserve volume (ERV) and residual lung volume (RV) do not begin to change until about the third month of pregnancy (see Table 33.1 ). With enlargement of the uterus, the diaphragm is forced cephalad, which is primarily responsible for the 20% decrease in functional residual capacity (FRC) present by term. This change is created by approximately equal decreases in the ERV and RV. As a result, FRC can be less than closing capacity for many small airways and may give rise to atelectasis in the supine position. Vital capacity is not significantly changed with pregnancy. The combination of increased minute ventilation and decreased FRC results in a greater rate at which changes in the alveolar concentration of inhaled anesthetics can be achieved. Respiratory measures of forced expiratory volume in 1 second (FEV 1 ), FEV 1 /FVC (forced vital capacity), and closing capacity do not change significantly with pregnancy.
During induction of general anesthesia in a pregnant patient, Pa o 2 decreases much more rapidly than in a nonpregnant patient because of decreased oxygen reserve (decreased FRC) and increased oxygen uptake (increased metabolic rate). For these reasons, the administration of supplemental oxygen, or “preoxygenation,” before general anesthesia is critical for patient safety. The pregnant patient should breathe oxygen for 3 minutes before any anticipated period of apnea (such as induction) or take four maximal breaths over the 30 seconds just before induction if emergent general anesthesia is needed. In addition, the increased airway edema makes both ventilation and intubation more difficult and further increases the potential for complications and morbidity. Optimal positioning should be obtained and backup airway equipment readily available before induction of general anesthesia. See “Anesthesia for Cesarean Delivery.”
Gastrointestinal changes during pregnancy make women beyond 20 weeks’ gestation vulnerable to aspiration of gastric contents and the development of pneumonitis. Displacement of the stomach and pylorus cephalad by the enlarged uterus repositions the intraabdominal portion of the esophagus into the thorax and decreases the competence of the lower esophageal sphincter. Higher progesterone and estrogen levels of pregnancy further reduce esophageal sphincter tone. During vaginal delivery, gastric pressure is increased by both the gravid uterus and the lithotomy position. Gastrin, which is secreted by the placenta, stimulates gastric hydrogen ion secretion such that the pH of gastric fluid is predictably low in pregnant women. For these reasons, gastric fluid reflux into the esophagus with subsequent esophagitis (heartburn) is common and increases with the pregnancy gestational age. In addition, gastric emptying is delayed with the onset of labor or administration of opioids, further increasing the risk of aspiration.
Regardless of the time interval since the ingestion of food, women in labor are treated as having a full stomach and an increased risk for pulmonary aspiration. This includes the routine use of nonparticulate antacids, rapid-sequence induction, cricoid pressure, and cuffed intubation as part of the general anesthesia induction sequence in a pregnant woman after approximately 20 weeks gestational age. Pain, anxiety, and opioids administered during labor can further slow gastric emptying beyond an already prolonged transit time. Epidural analgesia using local anesthetics with low-dose fentanyl does not delay gastric emptying, but administering epidural boluses of fentanyl does. The low pH of aspirated gastric fluid is important in the production and severity of acid pneumonitis and is the basis for the administration of antacids to pregnant women before induction of anesthesia. Current American Society of Anesthesiologists (ASA) guidelines recommend the “timely administration of oral nonparticulate antacids, IV H2-receptor antagonists, and/or metoclopramide for aspiration prophylaxis” before the induction of anesthesia in pregnant women. Nonparticulate antacids such as sodium citrate (30 mL) work rapidly. Metoclopramide can significantly decrease gastric volume in as little as 15 minutes, although gastric hypomotility associated with prior opioid administration reduces the effectiveness of metoclopramide. H2-receptor antagonists increase gastric fluid pH in pregnant women approximately 1 hour after administration without producing adverse effects. There is some evidence that antacids plus H2 antagonists are better than antacids alone in decreasing gastric acidity. The physiologic effects of pregnancy on gastric function return to normal approximately 24 hours after delivery.
Volatile anesthetic requirements (minimum alveolar concentration [MAC]) decrease up to 40% during pregnancy in animal studies and 28% in humans within the first trimester of pregnancy. However, an electroencephalographic study suggests that anesthetic effects of sevoflurane are similar in pregnant and nonpregnant women. Consequently, the degree of anesthetic reduction and mechanism remains uncertain, but progesterone activity may be partially responsible. A clinical implication of this decreased MAC is that alveolar anesthetic concentrations that would not routinely produce unconsciousness may approximate anesthetizing concentrations in pregnant women. Judicious administration of agents that depress the central nervous system (CNS) is required to prevent unintended impairment of upper airway reflexes and increased risk of pulmonary aspiration that is already elevated secondary to gastrointestinal changes detailed previously. Notably, rates of intraoperative awareness under general anesthesia are increased for cesarean delivery, and reducing standard anesthetic levels in stable pregnant patients is not advised.
Pregnant patients are more sensitive to the local anesthetics used during neuraxial blockade. There is a decrease in local anesthetic dose needed for epidural or spinal anesthesia in pregnant women at term. The observation of decreased neuraxial local anesthetic doses as early as the first trimester suggests a role for biochemical changes causing increased nerve sensitivity. Although this increased sensitivity is likely based on hormonal changes, there may be some role for mechanical changes as well. Engorgement of epidural veins as intraabdominal pressure increases with progressive enlargement of the uterus results in a decrease in both the size of the epidural space and volume of cerebrospinal fluid (CSF) in the subarachnoid space. The decreased volume of these spaces facilitates the spread of local anesthetics. However, CSF pressure itself does not increase with pregnancy.
Renal blood flow and the glomerular filtration rate are increased about 50% to 60% by the third month of pregnancy and do not return to pre-pregnant levels until 3 months postpartum. Therefore, the normal upper limits of blood urea nitrogen and serum creatinine concentrations in pregnant women are only 50% of those in nonpregnant women. There is decreased tubular resorption of both protein and glucose, and excretion of these in the urine is common. A 24-hour urine collection of less than 300 mg protein or 10 grams glucose is considered the upper limit of normal in pregnancy.
Liver blood flow does not change significantly with pregnancy. Plasma protein concentrations are reduced during pregnancy, and decreased serum albumin levels can result in elevated free blood levels of highly protein-bound drugs. Slightly elevated liver function tests are common in the third trimester. Plasma cholinesterase (pseudocholinesterase) activity is decreased about 25% to 30% from the tenth week of gestation up to 6 weeks postpartum. This decreased activity is unlikely to be associated with significant prolongation of the neuromuscular blockade of succinylcholine, but return of muscle strength should always be verified. In addition, incomplete gallbladder emptying and changes in bile composition increase the risk of gallbladder disease during pregnancy. Even without underlying pathology, alkaline phosphate levels double during pregnancy from placental production.
The placenta is the interface of maternal and fetal tissue for the purpose of physiologic exchange. Maternal blood is delivered to the uterus and placenta by two uterine arteries. On the fetal side, nutrient-rich and waste-free blood is returned from the placenta to the fetus through a single umbilical vein, and fetal blood returns to interface with the maternal circulation via two umbilical arteries.
UBF increases throughout gestation from about 100 mL/min before pregnancy to 700 mL/min (about 10% of cardiac output) at term gestation. About 80% of UBF perfuses the intervillous space (placenta) and 20% supports the myometrium. The uterine vasculature has limited autoregulation and remains essentially maximally dilated under normal pregnancy conditions. UBF can decrease from any cause of reduced uterine perfusion pressure such as systemic hypotension secondary to hypovolemia, aortocaval compression, or decreased systemic resistance from either general or neuraxial anesthesia. UBF can also decrease with increased uterine venous pressure. This can result from vena caval compression (supine position), prolonged or frequent uterine contractions, significant abdominal musculature contraction (Valsalva maneuver during pushing), or extreme hypocapnia (Pa co 2 <20 mm Hg) associated with hyperventilation secondary to labor pain.
Epidural or spinal anesthesia does not significantly alter UBF as long as maternal hypotension is avoided. Although certain types and higher doses of vasopressors can increase uterine artery resistance and decrease UBF, phenylephrine, ephedrine, and norepinephrine can all be used safely to treat hypotension in standard clinical doses, See “Hypotension” section below.
Transfer of oxygen from the mother to the fetus is dependent on a variety of factors, including the ratio of maternal UBF to fetal umbilical blood flow, the oxygen partial pressure gradient, the respective hemoglobin concentrations and affinities, the placental diffusing capacity, and the acid–base status of the fetal and maternal blood (Bohr effect). The fetal oxyhemoglobin dissociation curve is left-shifted (greater oxygen affinity), whereas the maternal hemoglobin binding curve is right-shifted (decreased oxygen affinity), resulting in facilitated oxygen transfer to the fetus. The fetal Pa o 2 is normally 20 to 40 mm Hg, but can reach 60 mm Hg if the mother is breathing 100% oxygen. This is because the placental exchange to the fetus from the mother represents more of a venous rather than arterial blood. CO 2 readily crosses the placenta and is not limited by diffusion, but rather flow.
Placental exchange of most drugs and other substances less than 1000 Daltons occurs principally by diffusion from the maternal circulation to the fetus and vice versa. Diffusion of a substance across the placenta to the fetus depends on maternal-to-fetal concentration gradients, maternal protein binding, molecular weight, lipid solubility, and the degree of ionization of that substance. Minimizing the maternal blood concentration of a drug is the most important method of limiting the amount that ultimately reaches the fetus.
The high molecular weight and poor lipid solubility of nondepolarizing neuromuscular blocking drugs result in limited ability of these drugs to cross the placenta. Succinylcholine has a low molecular weight but is highly ionized and therefore does not readily cross the placenta. Thus, during administration of a typical general anesthetic for cesarean delivery, the fetus/neonate is not paralyzed. Additionally, heparin, insulin, and glycopyrrolate have significantly limited placental transfer. Placental transfer of benzodiazepines, volatile anesthetics, local anesthetics, and opioids is facilitated by the relatively low molecular weights of these substances. In general, drugs that readily cross the blood–brain barrier also cross the placenta.
Fetal uptake of a substance that crosses the placenta is affected by the lower pH (0.1 unit) of fetal blood compared with maternal. The lower fetal pH means that weakly basic drugs (e.g., local anesthetics) that cross the placenta in the nonionized form will become ionized in the fetal circulation. Because an ionized drug cannot readily cross the placenta and return to the maternal circulation, this drug will accumulate in the fetal blood against a concentration gradient. Therefore, in an acidotic fetus, higher concentrations of local anesthetic can accumulate (ion trapping), especially during periods of fetal distress. Increased concentrations of local anesthetics in the fetus can result in decreased neonatal neuromuscular tone. If direct maternal intravascular local anesthetic injection occurs, significant fetal toxicity can result in bradycardia, ventricular arrhythmia, acidosis, and severe cardiac depression. Placental transfer and fetal uptake of specific analgesic and anesthetic agents are detailed in the upcoming sections on “Methods of Labor Analgesia” and “Anesthesia for Cesarean Delivery.”
The fetal circulation helps protect vital fetal organs from exposure to high concentrations of drugs initially present in umbilical venous blood. For example, about 75% of umbilical venous blood initially passes through the fetal liver such that significant portions of drugs are metabolized before reaching the fetal arterial circulation for delivery to the heart and brain. Despite decreased liver enzyme activity in comparison with adults, fetal/neonatal enzyme systems are adequately developed to metabolize most drugs. Moreover, drugs in the portion of umbilical venous blood that enter the inferior vena cava via the ductus venosus will be diluted by drug-free blood returning from the lower extremities and pelvic viscera of the fetus. These circulatory characteristics decrease the fetal plasma drug concentrations compared with maternal after an intravenous drug bolus.
The anesthesia provider may be consulted at any time to aid in a safe delivery. The labor course, mode of delivery, and maternal comorbidities should all be considered in determining which analgesic or anesthetic technique is most appropriate. Understanding the stages of labor and common labor patterns is important to appreciate when labor is not progressing and obstetric intervention may be required. Progression of labor is reliably unpredictable. Retrospective reviews of the maternal labor course have led to a change of definitions for what constitutes normal labor progress. Ideally, this change will decrease cesarean deliveries in the first stage of labor (active-stage arrest).
The course of labor is divided into three stages. The first stage begins with maternal perception of uterine contractions (latent phase) and continues with significant acceleration of cervical dilation (active phase), until the cervix is fully dilated. The latent phase can persist for hours to days. The active phase begins when the rate of cervical dilation increases (usually after 5 to 6 cm dilation). The second stage of labor begins with full cervical dilation and ends with neonatal birth. This stage is referred to as the “pushing and expulsion” stage. Once the neonate is born, the third stage begins and is completed when the placenta is delivered. Concern for abnormal labor and obstetric interventions may be indicated if progression through the stages of labor is halted or delayed.
If dilation slows or stops in the active phase of labor despite pharmacologic interventions, this is considered active-phase arrest and cesarean delivery will likely be considered by the obstetrician. Arrest of descent occurs during the second stage of labor, when the neonate cannot be born vaginally. The mode of delivery in this case depends on the location of the neonatal head when arrest of descent occurs. If the neonate is low enough in the pelvis, the obstetrician can perform an instrumented vaginal delivery (also known as an operative vaginal delivery ) via vacuum or forceps. If the neonate remains too high in the pelvis, then the woman will likely need to undergo a cesarean delivery. Additionally, the fetal condition can dictate a change in delivery mode based on the fetal heart rate tracing.
Uterine contractions, cervical dilation, and perineal distention cause pain during labor and delivery. Afferent somatic and visceral sensory fibers from the uterus and cervix travel to the spinal cord with sympathetic nerve fibers ( Fig. 33.3 ). Most painful stimuli in the first stage of labor are the result of afferent nerve impulses from the lower uterine segment and cervix, with visceral pain arising from the uterine body. Nerve fibers from the uterus and cervix course with the hypogastric nerves and sympathetic chain to the dorsal root ganglia of levels T10–L1. In the second stage of labor, additional somatic pain is transmitted from afferent nerves innervating the vagina and perineum and results from distension, ischemia, and tissue injury of these areas as the fetus descends into the pelvis and delivers. These afferent impulses travel primarily via the pudendal nerve to dorsal root ganglia of levels S2–S4. Neuraxial analgesic techniques must block levels T10–L1 to provide pain relief during the first stage of labor and also include S2–S4 for efficacy during the second stage of labor.
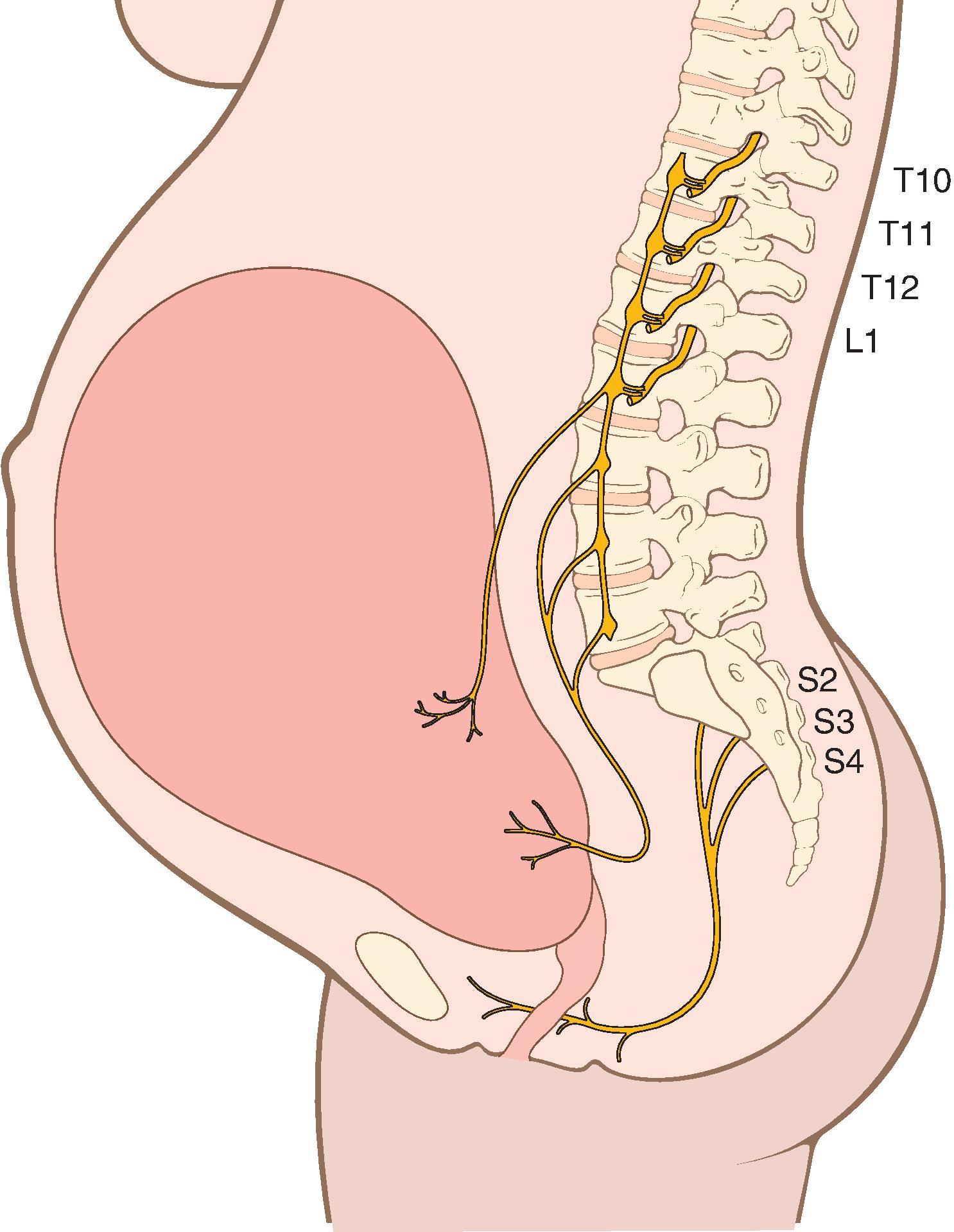
The mother, fetus, and labor course can all be affected by the physiologic effects of labor pain. Pain stimulates the sympathetic nervous system, elevates plasma catecholamine levels, creates reflex maternal tachycardia and hypertension, and can decrease UBF. In addition, changes in uterine activity can occur with the rapid decrease in plasma epinephrine concentrations associated with the onset of neuraxial analgesia. Variations in epinephrine levels can result in a range of uterine effects, including a transient period of uterine hyperstimulation (tachysystole), a period of uterine quiescence, or conversion of a dysfunctional uterine activity pattern to a more regular pattern. There is variation in the severity of pain reported by women during labor and delivery. However, the intensity of pain during labor increases with cervical dilation. In the absence of medical contraindications to labor pain relief options, maternal request is a sufficient indication for treatment of pain during labor.
There are many nonpharmacologic techniques for labor analgesia. Three theoretical models describe how these techniques work by their effects on endogenous labor pain pathways. Hydrotherapy, massage, position changes, and transcutaneous electrical nerve stimulation (TENS) inhibit pain fiber transmission by providing tactile stimulation to an area of pain (gate control model). Acupuncture/acupressure, sterile water injections, and TENS cause pain at sites remote from labor pain, which stimulates the endorphin system, leading to decreased overall pain (diffuse noxious inhibitory control model). The CNS control model focuses on attention divergence from labor pain using Lamaze, meditation, hypnosis, relaxation, expectation management, and music. Though data for analgesic benefit with these techniques are limited and modest, acupuncture, acupressure, TENS, relaxation, and massage techniques offer some element of patient control and increased patient satisfaction. Hypnosis and intradermal water injection techniques show no significant analgesic benefit beyond control populations. Nonetheless, perceived patient autonomy and patient control appear to substantially contribute to a woman's satisfaction with labor and delivery in comparison with analgesic efficacy. Additionally, a meta-analysis reviewing the effectiveness of a support individual (e.g., doula, family member) noted that women with a support individual used fewer pharmacologic analgesia methods, had a decreased length of labor, were more likely to have a vaginal birth, and were less likely to have negative feelings about childbirth.
Systemic analgesics can be beneficial for women in the early stages of spontaneous or induced labor. However, use of these medications is typically limited by both dose and timing. These limits are dictated by the potential for maternal sedation, respiratory compromise, loss of airway protection, and proximity to time of delivery because of concern for adverse neonatal effects. Whereas systemic opioid analgesics are commonly used for labor, use of sedatives, anxiolytics, and dissociative agents is rare.
All opioids readily cross the placental barrier and can cause neonatal effects, including decreased fetal heart rate variability and neonatal dose-related respiratory depression. Additionally, maternal side effects are common and include nausea, vomiting, pruritus, and delayed gastric emptying.
Fentanyl has a short duration of action and no active metabolites, which makes it a preferable analgesic for labor. When administered in small intravenous (IV) bolus doses of 50 to 100 mcg per hour, there are no significant differences in neonatal Apgar scores and respiratory effort compared with newborns of mothers not receiving fentanyl.
Meperidine has a longer duration of action (maternal half-life 2 to 3 hours) and is metabolized to an active metabolite (normeperidine). Meperidine can be administered IV (12.5 to 25 mg) or intramuscularly (IM) (25 to 50 mg). The fetal and neonatal half-life of meperidine is prolonged (13 to 23 hours) and highly variable. Increased levels of neonatal normeperidine have been associated with decreased Apgar scores, increased time to sustained respiration, lower fetal oxygen saturation levels, abnormal neurobehavior, and difficulty initiating breastfeeding. , Despite these undesirable side effects, it remains a commonly used labor analgesic worldwide, likely because of cost, ease of administration, and availability.
Morphine, like meperidine, also has an active metabolite (morphine-6-glucuronide) and a longer duration of action. Notably, morphine causes profound maternal sedation, has a prolonged half-life in neonates, and is no longer commonly used. It remains occasionally administered IM in combination with promethazine to provide latent labor analgesia (for 2.5 to 6 hours) and rest. This technique is termed morphine sleep and based on a prospective cohort study does not affect maternal or neonatal outcomes.
Remifentanil has a rapid onset and fast elimination by plasma and tissue esterases with significant tissue metabolism. The primary benefit of remifentanil over other opioid labor analgesics is the minimization of neonatal side effects because of its rapid metabolism. Although remifentanil patient-controlled analgesia (PCA) does not provide superior pain relief compared with epidural labor analgesia, remifentanil provides better pain relief with improved patient satisfaction compared with other opioids and does not appear to adversely affect neonatal outcomes. A retrospective comparison of remifentanil and fentanyl PCA for labor analgesia found more transient maternal desaturation with remifentanil, but fentanyl resulted in greater need for assisted neonatal ventilation and supplemental oxygen. Because of maternal safety concerns (specifically related to respiratory effects), remifentanil use in labor is typically reserved for women with contraindications to neuraxial techniques. When remifentanil is used for labor analgesia, continuous pulse oximetry, end-tidal CO 2 monitoring, oxygen supplementation, and 1:1 nursing are recommended.
Use of inhaled nitrous oxide (N 2 O) for labor analgesia began in the 1880s, with increased use in the 1930s after invention of a portable device for administration. Although many countries have high utilization rates (>50%), it is only over the past decade that it has been widely available in the United States as an option for labor analgesia. The mechanisms of N 2 O are not well understood, and there are few high-quality studies examining its efficacy, optimization, and potential adverse impacts. N 2 O is an N -methyl-d-aspartate (NMDA) antagonist, but may also modulate pain perception via alpha-2 receptors in the spinal cord dorsal horn and release endogenous opioids in the brain. N 2 O possesses many desirable qualities for labor analgesia, including rapid onset, rapid elimination, and no effect on uterine contractility. It can be used in all stages of labor, with no studies to suggest a detrimental effect of N 2 O on the fetus or neonate. Although the Food and Drug Administration (FDA) issued a 2016 communication warning against lengthy or repeated use of 11 anesthetic agents in children less than 3 and pregnant women during the third trimester, N 2 O was not included. Side effects are well tolerated, most commonly nausea, dysphoria, and dizziness. Although N 2 O provides less analgesic effect than epidural analgesia, it offers a safe and satisfactory analgesic option for many women that allows mobility and control of analgesic administration during labor. It is self-administered in a fixed mixture of 50% N 2 O with 50% oxygen via facemask. Out of concern for maternal respiratory depression and loss of airway reflexes with deeper levels of sedation, coadministration of systemic opioids or other sedating medications is not recommended. Emissions of this greenhouse gas are significantly reduced in the labor setting with use of the demand valve, and occupational exposure risk is negligible when N 2 O is administered with proper scavenging equipment (also see Chapter 49 ).
In the United States, neuraxial analgesia (e.g., epidural, spinal, dural puncture epidural [DPE], combined spinal epidural [CSE]) is the most widely used method for labor analgesia. Neuraxial analgesia typically involves the administration of local anesthetics in combination with opioid analgesics into the epidural and/or intrathecal space. Adjuvant drugs such as epinephrine and clonidine have been shown to decrease the dose of local anesthetics or opioids required for analgesia. The FDA has issued a “black box” warning regarding increased risk of hypotension and bradycardia with neuraxial clonidine in obstetrics, and caution should be used.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here