Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Preterm birth is defined as a delivery that occurs at 20 weeks’ gestation or later but prior to 37 weeks’ gestation (from the first day of the last menstrual period). Although advancements in neonatology and obstetrical interventions have dramatically improved outcomes and reduced the burden associated with prematurity, the rate of preterm birth itself has not changed substantially over the past 40 years. From 1990-2006, the preterm birth rate increased to a peak of 12.6%, probably because of the increase in indicated preterm deliveries and early deliveries related to multiple gestations conceived with assisted reproductive techniques. This was followed by an encouraging drop in preterm birth rate to 9.57% in 2014. However, since then there has been a reverse trend with the 2016 preliminary birth data showing a preterm birth rate of 9.85%. On the other hand, the low birth weight rate declined slightly to 8.1% in 2015, and the infant mortality rate remained stagnant and was reported as 5.96 infant deaths per 1000 live births in 2013, compared with 6.15 deaths in 2010.
Prematurity remains a leading cause of neonatal morbidity and mortality worldwide, accounting for 60%-80% of deaths of infants without congenital anomalies. In 2013, 36.1% of all infant deaths in the United States were preterm related, second only to congenital malformations and chromosomal abnormalities among the leading causes of infant mortality. Maternal race and ethnicity have a particularly strong effect on preterm-related infant mortality, with 44% of non-Hispanic black infant deaths attributed to preterm-related causes, a 3 times higher rate compared with non-Hispanic white infants. However, although the preterm-related infant mortality rate was highest for non-Hispanic black women, they also experienced the largest declines since 2005, with the preterm-related infant mortality rates having declined by 22% for non-Hispanic black women, compared with a 14% decline for non-Hispanic white women.
There are few obstetric interventions that successfully delay or prevent spontaneous preterm birth. Interventions to reduce the morbidity and mortality of preterm birth can be primary (directed to all women), secondary (aimed at eliminating or reducing existing risk), or tertiary (intended to improve outcomes for preterm infants). Most efforts so far have been tertiary, including regionalized care and treatment with antenatal corticosteroids, tocolytic agents, magnesium sulfate for neuroprotection, and antibiotics. These measures have reduced perinatal morbidity and mortality but essentially have no effect on the incidence of preterm birth itself. Advances in primary and secondary care, following strategies used for other complex health problems such as cervical cancer, are necessary to truly move toward eradicating prematurity-related illness in infants and children.
Promising interventions such as progestin supplementation, cerclage placement, and cervical pessary insertion appear to be useful in the prevention of preterm birth in certain populations. The most pressing need is to better define the populations of pregnant women for whom these and other interventions are beneficial, and continued work to understand the complex pathophysiology of prematurity in an effort to design future interventions effective in reducing preterm birth.
Preterm birth prior to 37 weeks’ gestation may be divided into two major categories: (1) indicated preterm births and (2) spontaneous preterm births. Indicated preterm births include deliveries prompted by concerns regarding maternal or fetal well-being, processes that account for approximately 25% of all preterm births together. Common reasons for these indicated early deliveries include preeclampsia/eclampsia (see Chapter 17 ) and nonreassuring fetal status secondary to abnormal fetal heart rate, intrauterine growth restriction (see Chapter 15 ), or oligohydramnios (see Chapter 24 ).
Spontaneous preterm births include deliveries that follow either spontaneous labor or preterm premature rupture of membranes (PPROM). Spontaneous preterm births account for approximately 70% of all preterm deliveries, with 40%-50% of these early deliveries owing to preterm labor and 25% to PPROM.
The risk of neonatal mortality and morbidity is inversely related to the gestational age at the time of delivery. A recent review of studies from the United States and other developed countries showed survival to discharge of 23%-27% for births at 23 weeks, 42%-59% for births at 24 weeks, and 67%-76% for births at 25 weeks of gestation. Gestational-age-specific neonatal mortality rates are listed in Table 19.1 (see also Fig. 19.1 ). Survival by gender is presented in Fig. 19.2 . Attention has primarily been focused on prevention of early preterm births (23-32 weeks’ gestation), which represent less than 2% of all deliveries but contribute to 60% of perinatal mortality and nearly 50% of long-term neurologic morbidity. Perhaps the greatest benefit of this focus has been reaped by the extremely premature neonates. Although once considered to be nonviable, survival rates of 20%-40% have recently been noted in neonates delivered at 22-23 weeks’ gestation.
| Gestational Age, Weeks | Survival by Gestational Age | |||
|---|---|---|---|---|
| Japan, % | Sweden, % (CI) | NRN, % (Range) | EPICure, % | |
| 23 | 55 | 53 (44-63) | 26 (2-53) | 17-28 |
| 24 | 76 | 67 (59-75) | 55 (20-100) | 40-60 |
| 25 | 85 | 82 (76-87) | 72 (56-90) | 66-75 |
| 26 | 90 | 85 (81-90) | 88 (60-100) | 77-83 |
| 27 | 93 | 85 (81-90) | 91 (75-100) | 95 |
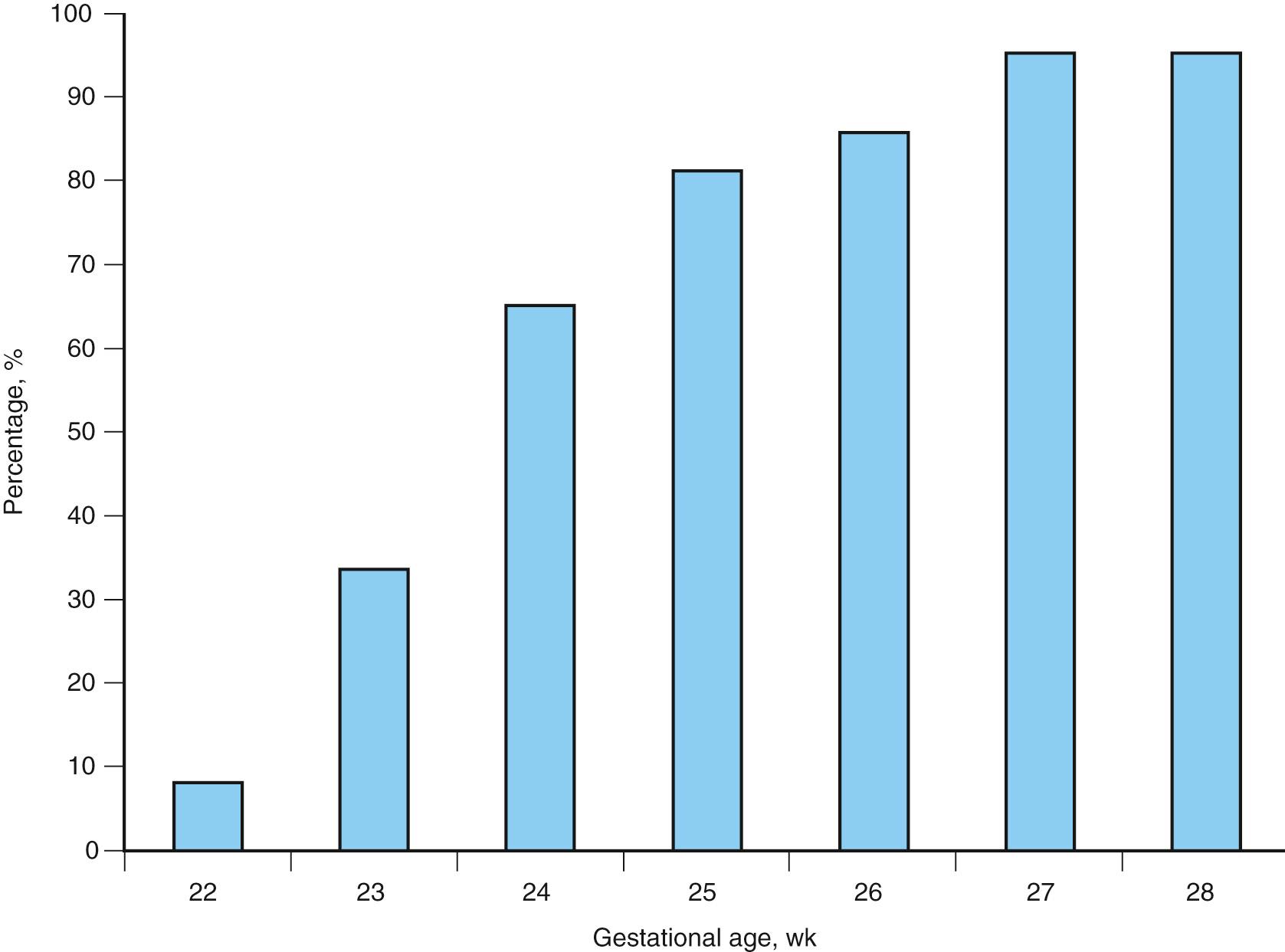
Stoll et al. reported that survival increased between 2009 and 2012 for infants at 23 weeks’ gestation (27%-33%; adjusted RR 1.09 [95% CI, 1.05-1.14]) and 24 weeks (63%-65%; adjusted RR 1.05; 95% CI, 1.03-1.07), with smaller relative increases for infants at 25 and 27 weeks’ gestation and no change for infants at 22, 26, and 28 weeks’ gestation. Survival without major morbidity increased approximately 2% per year for infants at 25-28 weeks’ gestation, with no change for infants at 22-24 weeks’ gestation.
These babies are at risk for a wide array of complications, though, including long-term neurologic impairment. Moore noted from the EPICure studies that looked at extremely preterm infants that survival of babies admitted for neonatal care increased from 39% (35%-43%) in 1995 to 52% (49%-55%) in 2006, an increase of 13% (8%-18%), and that survival without disability increased from 23% (20%-26%) in 1995 to 34% (31%-37%) in 2006, an increase of 11% (6%-16%). A recent cohort from the Neonatal Research Network of non-anomalous infants born between 2006 and 2011 before 27 weeks showed similar patterns with overall rates of survival and intact survival (without severe impairment) of 5.1% (IQR 0-10.6) and 3.4% (IQR 0-6.9) respectively among children born at 22 weeks to 81.4% (IQR 78.2-84) and 75.6% (IQR 69.5-80) respectively among those born at 26 weeks’ gestation.
While extremely premature neonates contribute the bulk of perinatal mortality and morbidity, those born as late preterm (between 34 weeks 0 days and 36 weeks 6 days; 6.87% in 2015) make up the majority of preterm infants, and recent evidence has emerged showing that they have increased mortality and various morbidities, including transient tachypnea of the newborn, respiratory distress syndrome (RDS), persistent pulmonary hypertension, respiratory failure, temperature instability, jaundice, hypoglycemia, feeding difficulties, and prolonged neonatal intensive care unit (NICU) stay compared with their term counterparts ( Table 19.2 ). These late preterm infants may also manifest long-term neurodevelopmental consequences. It is, therefore, important to remember that although morbidity is inversely related to gestational age, there is no gestational age, including term, that is wholly exempt from adverse outcomes. The best outcomes remain for babies delivered at 39 weeks.
| Variable | Late Preterm Infants (%) | Full-Term Infants (%) | ||
|---|---|---|---|---|
| 34 Weeks | 35 Weeks | 36 Weeks | ||
| Neonatal mortality | 0.57% | 0.34% | 0.23% | 0.06% |
| Mechanical ventilation or endotracheal intubation | 3.6% | 1.7% | 0.79% | 0.35% |
| Nasal CPAP | 8.8% | 5.3% | 2.1% | 0.26% |
| Use of surfactant | 7.4% | 4.35 | 2.2% | 0.23% |
| Use of nasal oxygen | 9.6% | 6.5% | 3.3% | 0.5% |
| RDS | 10.6% | 6% | 2.7% | 0.36% |
| Sepsis work-up | 31.2% | 22% | 14.8% | 11.8% |
| Sepsis (culture proven) | 0.64% | 0.33% | 0.22% | 0.13% |
| Hypoglycemia | 11.5% | 5.3% | 4.4% | 0.26% |
| Feeding problems | 51% | 34% | 22% | 5.3% |
| Jaundice requiring phototherapy | 10.3% | 6% | 2.4% | 1.3% |
While gestational age remains the major determinant of morbidity and mortality, other factors affect these rates. For example, female infants demonstrate better survival rates than male infants at any gestational age, and black neonates tend to do better than white neonates. Neonatal survival rates also increase as infant birth weight increases, with 55% survival at 501-750 g, 88% at 751-1000 g, 94% at 1001-1250 g, and 96% at 1251-1500 g. In addition, differences in hospital practices regarding the initiation of active treatment in infants born between 22 and 24 weeks’ gestation may affect overall and intact survival among these patients. The best outcomes at the limits of viability continue to be reported from Japan.
Neonatal morbidities related to prematurity also remain a significant clinical problem, including RDS, intraventricular hemorrhage, periventricular leukomalacia, necrotizing enterocolitis, bronchopulmonary dysplasia, sepsis, patent ductus arteriosus, jaundice, growth failure, cerebral palsy, disorders of cognition, and retinopathy of prematurity. The risk for these morbidities is inversely related to both gestational age at delivery and birth weight, with Table 19.3 depicting the morbidity rates according to gestational age. Use of antenatal corticosteroids (betamethasone or dexamethasone) has been shown to reduce the incidence or severity of RDS, intraventricular hemorrhage, and necrotizing enterocolitis, but rates of other adverse outcomes remain unchanged.
| N | Gestational Age (Weeks) | |||||
|---|---|---|---|---|---|---|
| 23 | 24 | 25 | 26 | 27 | 28 | |
| 496 | 1223 | 1426 | 1530 | 1811 | 1967 | |
| Morbidity | ||||||
| Respiratory distress syndrome * | 97 | 95 | 90 | 86 | 78 | 65 |
| Surfactant therapy | 95 | 90 | 89 | 84 | 78 | 67 |
| Bronchopulmonary dysplasia | ||||||
| Mild | 26 | 26 | 37 | 35 | 28 | 16 |
| Moderate | 35 | 34 | 29 | 26 | 20 | 15 |
| Severe | 38 | 37 | 26 | 17 | 13 | 8 |
| Patent ductus arteriosus | 54 | 60 | 55 | 48 | 42 | 32 |
| Grade III-IV intraventricular hemorrhage | 36 | 26 | 21 | 14 | 11 | 7 |
| Necrotizing enterocolitis (proven) | 12 | 15 | 13 | 9 | 10 | 7 |
| Late-onset septicemia | 62 | 55 | 46 | 35 | 27 | 20 |
* An infant was determined to have respiratory distress syndrome if each of the following was true: required oxygen at 6 hours of life, continuing to age 24 hours; demonstrated clinical features up to age 24 hours; needed respiratory support to age 24 hours; had an abnormal chest radiograph up to age 24 hours.
Cerebral palsy is an umbrella term encompassing disorders of movement and posture, attributed to non-progressive disturbances occurring in the developing fetal or infant brain. Cerebral palsy complicates approximately 2 per 1000 live births. Although the majority of cases are associated with term deliveries, the relative risk for an early preterm infant developing cerebral palsy is nearly 40 times that of a term infant. The risk of cerebral palsy is particularly high in children with extremely low birth weight (less than 1000 g), a population that also has substantially higher rates of cognitive disorders, hearing and visual disabilities, neurobehavioral dysfunction, and poor school performance (see Chapter 60 ). The high risk of cerebral palsy in this population may be associated with the increased rate of intrauterine infection and inflammation in extreme preterm patients. Intrauterine infection and postnatal sepsis appear to play an important role in the pathophysiology of this condition. Intrauterine infection and inflammatory cytokines appear to substantially increase the risk of cell death, resulting in periventricular leukomalacia and intraventricular hemorrhage, and ultimately contributing to development of cerebral palsy. Recently, obstetric professional societies have advocated for the use of magnesium sulfate for fetal neuroprotection. Research has shown that administration of this therapy to women at risk for imminent delivery of a fetus less than 32 weeks’ gestation may reduce the risk of moderate or severe cerebral palsy by up to 45%.
Despite exhaustive research attempts, a complete understanding of the pathogenesis of preterm labor remains elusive. It is becoming increasingly clear that the factors that lead to the development of preterm labor are distinct from those that occur with term labor and thus represent a pathologic rather than a physiologic process. To add to the complexity, preterm labor appears to involve a number of processes that all lead to the common pathway of spontaneous preterm birth.
To understand the pathophysiologic processes of preterm labor, one must be familiar with the pathway leading to normal term labor. Three main biologic events appear to be involved with the onset of spontaneous labor: cervical ripening, formation and expression of myometrial oxytocin receptors, and myometrial gap junction formation. Prostaglandins E 2 and F 2α (PGE 2 and PGF 2α ) are believed to be important factors involved in these events. Levels of these specific prostaglandins increase in amniotic fluid, maternal plasma, and urine during labor, wherein they have been shown to facilitate cervical ripening and to promote myometrial gap junction formation. To that end, exogenous administration of prostaglandins facilitates cervical ripening and induction of labor at any point in gestation, while administration of prostaglandin synthetase inhibitors arrests preterm labor. Although prostaglandins are clearly an important component of the parturition process, the mechanism by which this cascade of events begins is not fully understood. Several theories exist regarding the initiation of parturition, including (1) progesterone withdrawal, (2) oxytocin initiation, and (3) decidual activation.
The progesterone withdrawal theory stems primarily from studies of sheep. Endogenous progesterone is known to inhibit decidual prostaglandin formation and release. As parturition nears, the fetal adrenal axis becomes more sensitive to adrenocorticotropic hormone, which incites an increased secretion of cortisol. Fetal cortisol then stimulates trophoblast 17α-hydroxylase activity, which decreases progesterone secretion and leads to a subsequent increase in estrogen production. This reverse in the estrogen-to-progesterone ratio then results in increased prostaglandin formation, thus leading to parturition. However, although this mechanism is well established in sheep, it does not appear to be the primary initiator of parturition in humans. Despite this, it is thought that premature activation of this axis can lead to preterm birth, primarily as triggered by increased maternal physical or psychological stress or fetal uteroplacental vasculopathy (i.e., as seen with preeclampsia or intrauterine growth restriction). Supporting this theory are the increased fetal adrenal zone size and elevated corticotropic hormone levels associated with preterm delivery, particularly late preterm births.
The second parturition theory involves oxytocin, a substance known to cause uterine contractions and to promote prostaglandin release, as an initiator of labor. The number of myometrial oxytocin receptors increases substantially as women near term. Despite this, oxytocin levels themselves do not rise before labor, and the involvement of this hormone in parturition likely represents a final common pathway instead of an inciting event.
The final and most likely theory regarding preterm parturition involves premature decidual activation through processes such as inflammation and/or hemorrhage. There is a large body of evidence establishing a strong link between inflammation, such as seen in the presence of genitourinary pathogens or even with systemic infections, and spontaneous preterm delivery. Colonization or infection of the upper genital tract results in inflammation and disruption of the choriodecidual interface, initiating a cascade of events ultimately resulting in spontaneous labor. Pathogens may have a direct role in this process, as evidenced by the finding of bacteria (usually organisms that are hard to culture using standard techniques) within the amniotic fluid of up to two-thirds of women with preterm birth. More importantly, however, pathogens lead to inflammation that then drives the process of preterm parturition. The evidence for these events is well supported by the biochemical changes that have been observed within the amniotic fluid, trophoblast, and decidua of patients with spontaneous preterm labor. Further support for this hypothesis comes from studies of mid-trimester amniotic fluid, obtained at the time of genetic amniocentesis, which demonstrate that elevated interleukin (IL)-6 levels are often associated with subsequent spontaneous abortion, fetal death, or preterm labor. In addition, amniotic fluid levels of the proinflammatory cytokines IL-1β and tumor necrosis factor-α increase in association with preterm labor. These specific cytokines appear to enhance prostaglandin production in the amnion and decidua while also triggering expression of matrix metalloproteinases that subsequently cause the breakdown of the cervical-chorionic-decidual extracellular matrix. These processes then lead to cervical ripening, separation of the chorion from the decidua, and possible membrane rupture.
Hemorrhage, as with overt placental abruption or even more subtle bleeding, can also lead to decidual activation. Multiple studies have linked the occurrence of vaginal bleeding to an approximately fourfold increase in the risk of spontaneous preterm birth. It is likely that inflammatory and coagulation pathways converge to result in this association. Not only can inflammation or infection lead to hemorrhage secondarily, but vaginal bleeding may also be the inciting event itself, triggering thrombin production that then generates pro-inflammatory cytokines.
The strong association between inflammation and preterm birth represents a series of complex, interconnected pathways. Recognition of this association is an important advance in our understanding of the mechanisms involved in spontaneous preterm delivery and represents a potential target for therapeutic intervention.
The identification and management of preterm labor have been directed at defining various epidemiologic, clinical, and environmental risk factors that are related to spontaneous preterm birth. Early recognition of these risk factors ( Box 19.1 ) may allow modification of the traditional approaches to prenatal care and ultimately may reduce the rate of preterm deliveries.
Age
Race
Socioeconomic status
Smoking
Substance abuse
Poor nutrition
Absent or inadequate prenatal care
Poor obstetric history
Uterine or cervical malformations (short cervix)
Myomas
Exposure to diethylstilbestrol
Hypertension
Diabetes
Other medical conditions
Multiple gestation
Excess or decreased amniotic fluid
Vaginal bleeding
Low body mass index (<19.8 kg/m 2 )
Fetal anomalies
Abdominal surgery
Infection (systemic or local)
In the United States, race is one of the most significant risk factors for preterm delivery. In 2015 birth data, black women have a prematurity rate of about 13.4%, in comparison with 8.9% for white women. The rate of preterm birth less than 32 weeks was more than double in non-Hispanic black women (3.09%) compared with non-Hispanic white women (1.27%). Similarly, the rate of very low birth weight neonates (less than 1500 g), which are associated with the greatest risk of neonatal morbidity and death, is more than doubled in non-Hispanic black women (2.89%) compared with non-Hispanic whites (1.09%). Other risk factors including extremes of maternal age, less education, and lower socioeconomic status, are associated with increased risk of preterm delivery. However, even when these factors are controlled for, black women still have higher rates of preterm delivery.
In addition to race, various behavioral factors increase the risk of preterm birth. Nutritional status, low maternal prepregnancy weight and BMI, and poor gestational weight gain are associated with increased risk. Smoking is associated with several poor pregnancy outcomes including placental abruption, intrauterine growth restriction, and preterm birth, with 10% to 20% of all preterm births attributed to maternal smoking. Substance use in pregnancy, especially cocaine, opiates, and alcohol, are other behavioral factors. Whether the pathophysiology of substance use is direct or through concurrent exposure to other lifestyle-related risks is to be determined.
Yet another behavioral factor related to preterm birth rates is the degree of physical activity and stress during pregnancy. Several studies have evaluated the effects of employment on preterm delivery, with disparate results ranging from an increase to an actual decrease in the risk of preterm birth in the working group. These variable results are likely related to the fact that physical activity levels probably impact the rate of preterm birth more than simple employment statistics. For example, activity in the standing position has been shown to increase uterine irritability, likely owing to uterine compression of pelvic vessels resulting in a decreased venous blood return to the heart, a phenomenon that may be temporarily relieved via contractive activity. Maternal stress also plays a role in the association between work activity and preterm birth. It seems, therefore, reasonably clear that women who engage in hard, physical work for long hours under increased stress are at a greater risk of preterm birth than inactive women or those with less physically demanding jobs.
A history of a prior preterm delivery is one of the most significant risk factors. The recurrence risk of preterm birth ranges from 12%-57%, depending on the number of prior preterm deliveries and the gestational age at which those deliveries occurred. For example, whereas women with one prior preterm birth have a threefold increased risk for preterm delivery in comparison with women with no such history, a six- to tenfold increased risk is seen in women with two previous preterm births. Furthermore, the risk of recurrent preterm birth is increased in women with prior second-trimester losses, induced abortions, prior twin preterm birth, and prior indicated preterm birth.
Patients with congenital Müllerian anomalies have an increased risk of preterm delivery. Approximately 3%-16% of all preterm births are associated with a uterine malformation. The incidence of preterm labor varies greatly depending on the type of uterine anomaly with unicornuate, didelphic, and bicornuate abnormalities having higher preterm labor rates (approximately 35%) compared with septate uterus (approximately 15%). In addition, these patients are at increased risk of placental abruption and PPROM.
Uterine leiomyomata have also been associated with an increased risk of preterm delivery, primarily owing to an increased incidence of antepartum bleeding and PPROM. Of the various types of myomata, submucosal and subplacental myomata appear to be most strongly associated with preterm delivery.
Cervical incompetence is another important risk factor for preterm delivery. The classic clinical description of cervical incompetence involves a history of painless cervical dilation between 12 and 20 weeks. A history of a second-trimester pregnancy loss has been the cornerstone of the diagnosis, but distinguishing between cervical incompetence and preterm labor can at times be difficult, and many times may not help in the clinical management of these patients.
Intrauterine exposure to diethylstilbestrol (DES) is a significant factor associated with congenital causes of cervical incompetence and both upper and lower genital tract structural abnormalities. An estimated 1-1.5 million women were exposed in utero to DES between the late 1940s and 1971. These women have an increased risk of preterm delivery ranging from 15% to 28%, with an increased risk of spontaneous abortion of 20%-40%. Women exposed to DES who have associated anomalies, such as T-shaped uterus, cervical incompetence, or vaginal structural anomalies, have a greater risk of preterm delivery than those who do not demonstrate these structural abnormalities. Because most women exposed to DES in utero are now older than 40, this risk factor is becoming less and less of an issue.
In addition, cervical procedures performed for the diagnosis and treatment of cervical intraepithelial neoplasia, including cold knife conization, or loop electrosurgical excision, have been associated with increased risk of preterm birth in subsequent pregnancies. A greater depth of excision was suggested to lead to greater risk of PPROM and preterm birth. However, a recent meta-analysis showed that the risk of preterm birth was only increased when these patients were compared to women without any history of cervical dysplasia and not when compared to those with history of abnormal cytology. This suggests other factors like high-risk HPV virus infection as the link toward increased preterm birth risk rather than the procedure itself.
Multiple gestations carry a sixfold increased risk of preterm delivery compared with singleton pregnancies. In the United States, 15%-20% of all preterm births are in multifetal pregnancies (only 3% of all pregnancies). Approximately 50% of twin and nearly all of higher-multiple gestations deliver prior to 37 completed weeks, with an average length of gestation of 35.2 weeks for twins, 32.1 weeks for triplets, and 29.7 weeks for quadruplets. Owing to artificial reproductive techniques, the prevalence of multiple gestations has increased in the United States. Multiple gestations are discussed further in Chapter 21 .
Vaginal bleeding is also associated with an elevated risk of preterm delivery. This is particularly true in the setting of placenta previa and placental abruption, but first- or second-trimester vaginal bleeding in the absence of these conditions is also associated with an increased risk of preterm delivery. The mechanism responsible for the development of preterm labor in the setting of vaginal bleeding appears to be related to thrombin deposition with subsequent production of prostaglandins and plasminogen activators that stimulate an array of degradative enzymes leading to destruction of the extracellular matrix.
Infections of the decidua, fetal membranes, and amniotic fluid have been associated with preterm delivery (see Chapter 25 ). For example, although intra-amniotic infection or chorioamnionitis complicates 1%-5% of term pregnancies, the contribution of infections to preterm birth has been estimated to be at least 25%-40% (see also Chapter 25 ).
There are data suggesting a link between occult upper genital tract infection and spontaneous preterm delivery even in the absence of clinical features of intra-amniotic infection. Moreover, the likelihood of a positive amniotic fluid culture or colonization of the chorioamnion in women with spontaneous preterm labor is inversely proportional to the gestational age at delivery. Typical organisms associated with histologic chorioamnionitis include Ureaplasma , Mycoplasma , Gardnerella , Bacteroides , and Mobiluncus species.
Numerous theories exist regarding the underlying pathogenesis of intra-amniotic infection: (1) ascending infection from the vagina and cervix, (2) transplacental passage through hematogenous dissemination, (3) retrograde seeding from the peritoneal cavity through the fallopian tubes, and (4) iatrogenic means as a result of intrauterine procedures such as amniocentesis and chorionic villus sampling. There is some evidence to support each of these theories, but ascending infection is the most well-accepted theory.
The mechanism of ascending infection appears to start with excessive overgrowth of certain organisms within the vagina and cervical canal. These microorganisms then gain access to the intrauterine cavity by infecting the decidua, the chorion, the amnion, and then finally the amniotic cavity itself. In this manner, pathogens are able to cross intact membranes. The fetus then becomes infected by aspirating or swallowing infected amniotic fluid or by direct contact with the organism within the fluid, leading to localized infections such as pneumonitis, otitis, or conjunctivitis. Seeding of these areas can lead, in turn, to fetal inflammatory response syndrome and sepsis. Alternatively, sepsis may result from maternal bacteremia, leading to placental infection with subsequent spread of organisms through the umbilical cord to the fetus.
Intra-amniotic infection can also lead to preterm labor through less direct methods, namely stimulation of prostaglandin. Microorganisms, probably through the release of endotoxins (in gram-negative organisms) such as phospholipase A 2 and C or lipopolysaccharide, activate pattern-recognition receptors such as Toll-like receptors that lead to release of inflammatory cytokines such as IL-8, IL-1β, and TNF-α, which in turn stimulate the production of prostaglandins and matrix-degrading enzymes by the amnion and decidua. The amniotic fluid concentrations of prostaglandins PGF 2α and PGE 2 , as well as their metabolites, are increased in patients with preterm labor, preterm premature rupture of membranes, and intra-amniotic infection.
Group B streptococcus (GBS) has been associated with an increased risk of preterm delivery, but more importantly, GBS colonization plays a major role in neonatal morbidity and death. Between 10% and 30% of pregnant women are colonized with GBS. Pregnant women colonized with GBS have higher incidence of preterm birth, PPROM, and low birth weight. Patients with urinary colonization also have positive results on cervical and vaginal cultures, possibly indicating that the presence of GBS in the urine may be a marker of more severe forms of genital tract colonization. Unfortunately, treatment of GBS genital tract colonization has never been shown to decrease the risk of preterm delivery or PPROM.
In 2010, the Centers for Disease Control and Prevention (CDC), in conjunction with the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics, revised their guidelines for the prevention of perinatal GBS disease to confirm universal screening at 35-37 weeks of gestation and intrapartum antibiotic prophylaxis for culture-positive and high-risk women. The revised guidelines also included new recommendations for laboratory methods for identification of GBS colonization during pregnancy, algorithms for screening, and intrapartum prophylaxis for women with preterm labor and premature rupture of membranes, as well as clarification of the colony count threshold required for reporting GBS detected in urine of pregnant women, updated prophylaxis recommendations for women with a penicillin allergy, and a revised algorithm for the care of newborn infants.
Intrapartum GBS prophylaxis is indicated in women with: (1) prior infant with invasive GBS disease, (2) GBS bacteriuria any time during pregnancy, (3) GBS carriers through prenatal screening cultures collected at 35-37 weeks’ gestation, and (4) unknown GBS culture at the onset of labor in women with any of the following: (i) preterm labor at less than 37 weeks’ gestation, (ii) membranes ruptured for 18 hours or longer, (iii) intrapartum maternal fever of 38°C or higher, and (iv) intrapartum nucleic acid amplification test (NAAT) GBS positive.
For women who are culture positive for GBS, intrapartum chemoprophylaxis with intravenous penicillin G (5 million units initially and then 2.5 million units every 4 hours) is recommended until delivery. Intravenous ampicillin (2 g initially, and then 1 g every 4 hours) is an acceptable alternative to penicillin G. Because of emerging resistance of GBS to macrolides, guidelines have recently been modified for women who are allergic to penicillins. In penicillin-allergic women who are not at high risk for anaphylaxis, intravenous cefazolin (2 g initially, and then 1 g every 8 hours until delivery) is recommended. In penicillin-allergic women who are at high risk for anaphylaxis, clindamycin and erythromycin susceptibility testing of the GBS isolate is recommended. If the isolate is sensitive to both clindamycin and erythromycin, intravenous treatment with clindamycin 900 mg every 8 hours is recommended. If the GBS isolate is resistant to either agent or susceptibility is unknown, treatment with intravenous vancomycin (1 g every 12 hours) is recommended. Currently, 70%-80% of cases of neonatal GBS infection in the United States occur in term infants; however, the case fatality rate and the burden of disease is much higher in those born preterm. In addition, half the cases are usually early in onset (within the first week of life). In addition, 10%-15% of neonatal infection cases were attributed to missed screening among mothers, and many were born to women who had tested negative for GBS before delivery. Further improvement toward eradication of GBS colonization and disease may involve universal screening in conjunction with rapid diagnostic technologies or other novel approaches, including DNA techniques for the identification of GBS. Given the complications and potential limitations associated with maternal intrapartum prophylaxis, however, vaccines are being considered to prevent neonatal GBS disease. Developing a universal vaccine has proved to be a daunting task, though, because of the variability of serotypes in diverse populations and geographic locations.
Bacterial vaginosis (BV) is yet another infection associated with an increased risk for preterm labor and delivery. It is a common lower genital tract infection found in approximately 20%-40% of African-American women and 10%-15% of white women. This condition is a clinical syndrome characterized by a decrease in the normal vaginal lactobacilli-dominant microflora and a compensatory predominance of bacteria such as Gardnerella vaginalis , Prevotella , Bacteroides , Peptostreptococcus , Mobiluncus , Mycoplasma hominis , and Ureaplasma urealyticum . Characteristically, patients with symptomatic BV complain of a watery, homogeneous grayish discharge with a fishy amine odor.
As above, BV has been associated with increased risk for preterm birth, especially among African-American women in whom up to 40% of early spontaneous preterm births may be associated with BV. While some randomized clinical trials demonstrated that treating BV in high-risk patients resulted in reductions in preterm birth rates, other trials did not confirm these findings. The majority of women with BV never manifest any signs or adverse outcomes related to the infection, and it is likely that BV is only a surrogate marker for a more important, presently unrecognized condition that may be the cause of the preterm birth.
Many other maternal infections or colonizations have been reportedly associated with preterm birth. One of the difficult questions to address is whether these relationships are causal or associative. Gonorrhea, chlamydia, trichomonas, syphilis, and other genital pathogens are more frequently found in women who have a spontaneous preterm birth. For example, gonorrhea, chlamydia, and syphilis have all been associated with a twofold increased risk for preterm delivery, and trichomonas has been associated with a 1.3-fold increased risk. Furthermore, many other sexually transmitted infections (e.g., human immunodeficiency virus, human papilloma virus, hepatitis B, and genital herpes simplex virus) have been associated with an increased risk for spontaneous preterm birth in some, but not most, studies. Importantly, the women affected by these infections often have other risk factors for preterm birth (e.g., low socioeconomic status, malnutrition, smoking, substance abuse, bacterial vaginosis). These confounding variables make it difficult to establish causality.
Despite evidence that genetic factors contribute to the duration of gestation and the risk of preterm birth, robust associations with genetic variants have not until recently been identified. Zhange used large data sets that included the gestational duration to determine possible genetic associations. They performed a genome-wide association study in a discovery set of samples obtained from 43,568 women of European ancestry using gestational duration as a continuous trait and term or preterm (<37 weeks) birth as a dichotomous outcome. In the discovery and replication data sets, four loci (EBF1, EEFSEC, AGTR2, and WNT4) were significantly associated with gestational duration. Functional analysis showed that an implicated variant in WNT4 alters the binding of the estrogen receptor. Common variants in EBF1, EEFSEC, and AGTR2 showed association with preterm birth with genome-wide significance. An analysis of mother-infant dyads suggested that these variants act at the level of the maternal genome. Previously established roles of these genes in uterine development, maternal nutrition, and vascular control support their mechanistic involvement.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here