Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The massive burn is as much a metabolic insult as it is a tissue injury. Clearly resultant tissue defects represent the critical factor in long-term functional complications from massive burns. In the acute phase of burn care, a persistent, overwhelming metabolic response to thermal injury compromises the functional integrity of virtually every organ system. This response compromises wound healing capacity and the integrity of the body's defense mechanisms.
The massive burn represents a metabolic “double hit,” a nutritional challenge of both supply and demand for the injured patient. The burn wound creates drastically increased demand for anabolic metabolism for wound healing while simultaneously triggering a systemic catabolic state. The resulting mismatch between high anabolic demand and negative anabolic supply (catabolism) leads to a profound state of functional malnutrition which, in turn, facilitates rapid debilitation and immunosuppression that place patients at profound risk for infectious complications. Addressing these nutritional challenges is thus critical to effective care for the burn patient.
Following all forms of major trauma, inflammatory and hormonal responses are activated and greatly influence metabolic pathways and mechanisms. Nutrient intake, absorption, and substrate assimilation are all affected during the different stages of the stress response. Nutrient requirements will increase but become more difficult to predict, and enteral or parenteral feeding will often be necessary to meet vastly increased nutritional requirements.
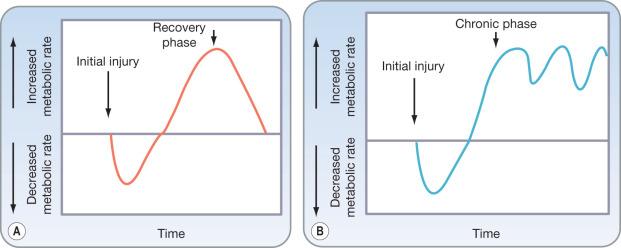
The physiologic response to trauma leads to activation of an array of processes, many of which increase immediate energy availability as part of the evolved flight-or-flight response. It is an evolved response to stress that assists the organism in overcoming a transient stress. However there is no adaptive response to the kind of sustained stress state seen with massive burns because these injuries were not survivable prior to the development of modern medicine. By supporting patients through what would otherwise be nonsurvivable states, modern medical care has created a novel physiologic state—a state of prolonged acute injury for which there is no adapted evolutionary response. In this state, powerfully adaptive responses to immediate stress become maladaptive when sustained for a timeline well beyond those encountered in the evolutionary context ( Fig. 28.1 ).
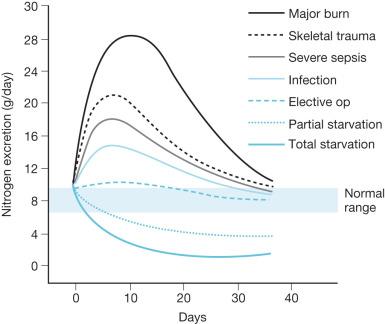
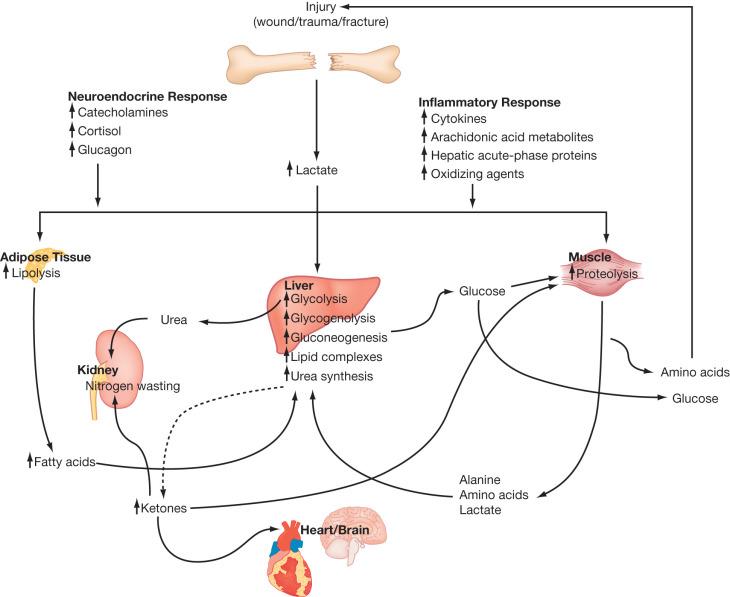
The hypermetabolic response to stress is particularly exaggerated in thermal injuries. The severity of the catabolic response to burn injury cannot be overemphasized. Decades of clinical and animal research have demonstrated with increasing depth and detail the ways in which burn injury profoundly alters the host organism's metabolism to upregulate catabolic processes and inhibit anabolic processes. The net result is a substantial net loss of lean body weight with a distinctly exaggerated effect on protein stores. The pathophysiology of this protein wasting is notable for a diverse array of endocrine and metabolic factors from the cellular to the organismal level ( Fig. 28.2 ).
Adrenal hyperactivity in burn patients was well documented more than 50 years ago, with massive surges in serum and urinary catecholamine levels seen within hours of burn. Inappropriate catecholamine and cortisol production has since been found to persist for weeks to months following massive burns, with end organ effects reaching the metabolic, cardiovascular, musculoskeletal, and immune systems. A sustained surge of catecholamines and inflammatory cytokines, along with massive wounds and profound dysregulation of thermal homeostasis, all combine and interact to yield enormous increases in resting energy expenditure.
Distorted thermal regulation plays a significant role in the increase in resting energy expenditures seen in burn patients. Destruction of a large portion of the primary thermoregulatory organ, the skin, leads to an ongoing drain of body heat via decreased insulation and the facilitation of evaporative heat loss. The primary central hyperthermia associated with the burn response compounds this energy drain. Alterations in thalamic temperature set-point, catecholamine-induced hypermetabolism, and shunting of mitochondrial energy to heat-producing pathways all drive the hyperthermia seen within 24 h of burn injury. As Wilmore and Pruitt et al. described in 1974, “Burn patients are internally warm, not [just] externally cold.” Thus, the burn patient's metabolism is taxed with the challenge of generating sufficient heat to maintain a higher body temperature with less heat-conserving resources.
Thermal injury triggers a marked increase in global energy expenditure, driven by a maelstrom of catecholamines, corticosteroids, and inflammatory cytokines. In an earlier era of burn care—prior to the advent of early excision, pharmacologic interventions for hypermetabolism, and modern critical care—measurements of energy expenditure in patients with large burns were reported to range from 1.5 to 2.5 times those found in nonburn controls. While modern burn care has made significant progress in countering this hypermetabolic response, contemporary studies still report an average resting energy expenditure from 1.3 to 1.5 times those found in nonburn controls.
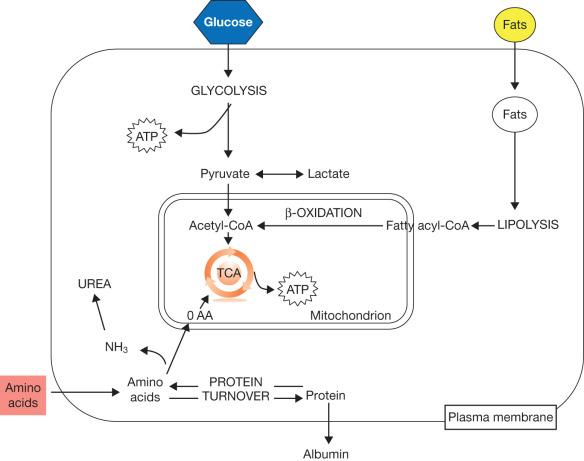
See Fig. 28.3 for a simplified overview of the metabolic pathways involved in massive burn trauma.
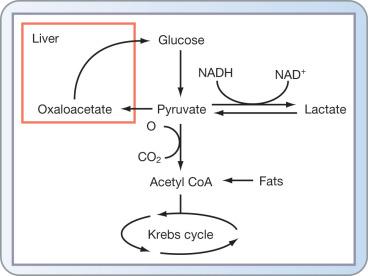
The acute metabolic response to burn injury significantly alters carbohydrate metabolism and demands. Glucose/carbohydrate metabolism presents the burn physician with a rather vexing conundrum. On the one hand, failure to provide adequate carbohydrates results in additional compensatory protein catabolism. And yet, on the other hand, the human body has a finite capacity for glucose oxidation. Multiple studies have demonstrated a limit to the amount of carbohydrate calories a burn patient can assimilate—approximately 5 g/kg per day in adult and 7 g/kg per day in children. Providing glucose in excess of these limits will thus result in increasing hyperglycemia with resultant lactic acidosis ( Fig. 28.4 ).
Unfortunately, with the enormous global energy expenditure of the massive burn patients, even a diet with a reasonably low percentage of carbohydrate-based calories can yield total carbohydrate loads well in excess of these limits when titrated up to meet full caloric requirements. Unfortunately administration of carbohydrates in excess of the patient's capacity for glucose oxidation will only result in increasing degrees of hyperglycemia, glucosuria, and hypertriglyceridemia. In such cases, the patient's nutritional demands simply reach a point where they exceed that patient's metabolic capacity.
Guidelines typically recommend providing 50–60% of calories as carbohydrates in the setting of trauma or burn injury. However these same guidelines also recommend that total carbohydrate provisions not exceed 5 g/kg per day (or 7 g/kg per day in children). Unfortunately providing 50–60% of calories as carbohydrate in the context of a nutritional therapy that meets the appropriately calculated total caloric needs of a burn patient will often result in doses of carbohydrates well in excess of these recommended limits. There is no simple, clinically vetted solution to this challenge. A reasonable approach to this dilemma is to start by titrating enteral formula delivery up to a rate that delivers 5–7 g/kg per day of carbohydrates and then provide all further calories in the form of protein supplementation.
While lipids are unquestionably critical to effective basic homeostasis and wound healing, the amount of fat required to prevent essential fatty acid deficiency is remarkably small in patients with intact fat stores. Excess fat administration, on the other hand, can cause significant harm. The hypermetabolic response to thermal trauma upregulates lipolysis of circulating lipids. This surfeit of circulating free fatty acids is countered by increased hepatic esterification, with close to 70% of the free fatty acids accumulating in the liver. To minimize hepatic steatosis, the percentage of dietary calories from fat should be limited and carefully monitored. Multiple studies have demonstrated improved outcomes with lower fat nutrition therapy in burn patients. Fat content should make up 3–15% of total calories. Patients receiving total parenteral nutrition (TPN) for a short period of time (e.g., less than 10–14 days) can generally be spared lipid infusion. In those requiring sustained TPN, 0.5–1 g/kg one to two times per week is sufficient. It should be noted that nondietary fat calories need to be taken into considerations as well: for example 1% propofol solution carries a proportional fat load equivalent to that found in a 10% intralipid emulsion.
It should be noted that the preceding discussion of the role of fat alimentation in nutritional therapy reflects a literature that largely predates the application of alternative fat sources (e.g., fish oil, olive oil, borage oil, etc.). In the United States, the current standard of care for both enteral and parenteral fat administration features significant components of fats that have been shown to encourage inflammation, most notably omega-6 fatty acids. Alternative fat sources have become the standard of care in Europe and are anticipated to receive U.S. Food and Drug Administration (FDA) approval in this country shortly. While there are still insufficient data to declare these alternatives superior to the standard of care, there is certainly sufficient evidence to warrant future study. As our understanding of the unique advantages and disadvantages of different fat sources matures, a reexamination of the role of fat alimentation in burn nutrition is sure to come due.
Proteolysis is the metabolic hallmark of the hypermetabolic response to thermal injury. As much as 150 g of skeletal muscle is lost per day in the absence of adequate nutritional support. If not attenuated, ongoing systemic proteolysis leads to immune dysfunction and retarded wound healing. Protein requirements of 1.5–2 g/kg per day in burned adults and 2.5–4 g/kg per day in burned children is well accepted in clinical practice. Unfortunately there are limits to the rate of effective protein assimilation as well. Increasing protein intake above these values will often increase urea load and azotemia, with limited additional impact on muscle wasting.
Amino acids alanine, arginine, and glutamine play a key role in wound healing in burn patients. Glutamine plays an important role in the function of enterocytes and lymphocytes, and low plasma glutamine concentrations have been associated with increased bowel permeability and increased rates of infection. Copious studies in animal models of trauma and shock have shown distinct roles for these particular amino acids in recovering enteral and immunologic competence. Glutamine supplementation and other “immunotrition” formulas featuring these key amino acids have been associated with decreased incidents of infection, shorter hospital stays, and decreased mortality in the setting of traumatic injury. While the appropriateness and even safety of glutamine supplementation has recently been called into question by the results of a large, international randomized controlled trial in critically ill patients, showing an increased mortality risk in patients randomized to receive high-dose parenteral glutamine supplements, how these results should be interpreted remains in question ( Fig. 28.5 ). (See further discussion in the section on formulas.)
In the face of the metabolic storm associated with acute burn injuries, provision of effective nutritional support is critical to wound healing, avoidance of complications and, ultimately, survival. Failure to provide sufficient calories superimposes starvation over a state of hypercatabolism, resulting in devastating loss of lean body mass and depletion of the protein-energy pool available to meet the pressing needs of wound healing, mucosal integrity, and basic immunologic defenses.
In addition to serving as a means for systemic delivery of nutrients, enteral nutrition (EN) performs a critical function in supporting the alimentary tract itself. Enteral feeds provide direct high-concentration nutrients (e.g., glutamine, alanine), stimulate enteric blood flow, maintain barrier function by preserving tight-junction integrity, and induce production and release of mucosal immunoglobulin and critical endogenous growth factors. These functions are not replaced with parenteral nutrition (PN). After dietary ingestion, polysaccharides such as fiber and starch undergo bacterial fermentation in the colonic lumen. Bacterial fermentation provides two functions crucial to intestinal health: (1) it supports the normal flora of the gut lumen, which in turn prevents colonization and subsequent infection (e.g., Clostridium difficile ) and (2) it produces acetoacetate, propionate, and butyrate (a short-chain fatty acid). Butyrate appears to be the preferred fuel of colonic mucosa cells and is therefore essential for mucosal integrity. Both animal and human studies show that the presence of enteral feeds (in addition to or in place of PN) is associated with increased mucosal mass, mucosal oxygenation, brush-border enzyme synthesis, and villus height when compared to PN alone. Clinically enteral feeding has frequently been found to associate with improved clinical outcomes both in terms of gastrointestinal and total patient morbidity and mortality.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here