Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Current recommendations for the provision of parenteral and enteral nutrition to the infant born prematurely are based on the goal of approximating the rate and composition of weight gain of a normal fetus at the same postmenstrual age. For a number of reasons this goal is seldom achieved, and postnatal growth failure still remains a significant complication of extreme prematurity. Infants who experience one or more major morbidities, such as bronchopulmonary dysplasia, necrotizing enterocolitis (NEC), or late-onset sepsis in the neonatal period, demonstrate slower growth than their counterparts who do not experience these conditions. Of particular concern is the significant association between suboptimal postnatal growth and short-term morbidities as well as adverse long-term neurodevelopmental outcomes. This relationship persists even after adjustments for severity of illness, including the perception of illness, which may alter nutritional delivery in high-risk infants. These findings reinforce the principle that nutritional delivery is critical in modifying disease risk in preterm infants. To emphasize the importance of a combined approach to optimize outcomes in the premature neonate, this chapter will first describe overall nutrient requirements and then provision of intense nutritional support with both early parenteral and enteral nutrition.
In the neonatal intensive care unit, birth-weight-derived intrauterine growth curves (such as the Fenton or Olsen) are typically employed to monitor postnatal growth. It is important to recognize the inherent limitation of such curves, namely that these curves were generated by measuring infants following premature birth, which is likely not the same physiologic state for the mother and/or fetus as if the infant had remained in utero until term. The revised Fenton growth curves include an international cohort of approximately 4 million infants from six population-based studies and are available online ( http://www.biomedcentral.com/1471-2431/13/59 ) and have been incorporated into many electronic medical record platforms. The Olsen curves include more than 250,000 infants born in the United States between 22 and 41 weeks’ gestation and when combined with the WHO-CDC growth charts ( http://www.pediatrix.com/workfiles/NICUGrowthCurves7.30.pdf ) can be used to monitor postnatal growth through 50 weeks’ corrected age. The number of infants born at 22-24 weeks’ gestation is very low (less than 1% of the total cohort) in both of these growth charts, limiting their generalizability as the number of infants in this gestational age range increases in neonatal intensive care units. The Vermont Oxford Network (VON) database recently published growth curves derived from birth measurements in a cohort of 156,000 infants from 852 centers in the United States. The VON curves do include more infants born in the periviable period than previously published growth curves (2000 infants born at 22 weeks’ gestation and 9000 infants born at 23 weeks’ gestation). Growth curves based on serial intrauterine measurements such as those generated by the INTERGROWTH-21 st Project may change the standard for the assessment of postnatal growth and may provide a more appropriate means to examine the relationship between growth and outcomes of infants born prematurely.
Postnatal growth failure has commonly been defined as weight less than the 10th percentile for gestational age. At the time of birth, approximately 20% of very low birth weight (VLBW) infants are small for gestational age (defined as weight less than the 10th percentile). After a stay in the neonatal intensive care unit, however, many of these infants will have experienced poor growth and weigh less than the 10th percentile at 36 weeks’ postmenstrual age. The incidence of postnatal growth failure at 36 weeks’ postmenstrual age was 79% in a large cohort of VLBW infants born between 2003 and 2007 at centers participating in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network. Improved growth outcomes in the past decade may reflect widespread use of early parenteral nutrition with protein and lipid delivery at most neonatal intensive care units as well as a decline in the use of postnatal corticosteroids.
The association between weight gain in the neonatal intensive care unit and neurodevelopmental outcomes was firmly established in a cohort of 600 extremely low birth weight (ELBW) infants reported by Ehrenkranz and colleagues. Infants were divided into quartiles based on in-hospital growth velocity; infants in the highest quartile gained an average of 21 g/kg per day, and those in the lowest quartile gained 12 g/kg per day. Compared with infants in the highest quartile of in-hospital weight gain, the odds of cerebral palsy, Bayley Scales of Infant Development (BSID) II mental developmental index (MDI) less than 70, and neurodevelopmental impairment were significantly higher in infants in the lowest quartile of in-hospital weight gain. Similar findings were also observed with in-hospital head growth. Since this time, multiple studies continue to demonstrate these relationships with recent evaluations using a more contemporary population, and the new edition of the Bayley (BSID III) has reported findings consistent with the earlier work by Ehrenkranz. Other investigators have described the association between poor linear growth and neurodevelopmental outcomes. As evidence continues to accumulate that early nutritional inadequacies have long-term consequences, optimizing provision of both parenteral and enteral nutrition to high-risk neonates is crucial to ensure the best possible outcomes.
Among potential causes of postnatal growth failure are significant protein and energy deficits that occur in the early neonatal period and are difficult to recoup in extremely premature infants. The cumulative nutritional deficits described in the sentinel work by Embleton and colleagues underscore the urgency in avoiding early deficits in both energy and protein intake as a means of achieving optimal growth outcomes.
Duplicating rates of in utero protein accretion remains a difficult clinical challenge. Failure to provide adequate protein, either in quantity or in quality, can significantly impact the long-term outcome of extremely premature infants. A variety of methods have been used to quantitate protein requirements in human infants: fetal accretion rates, nitrogen balance studies, plasma amino acid concentrations, and stable isotope studies investigating the kinetics of labeled essential amino acids. Clearly, the gold standard needs to be that which safely optimizes growth and neurodevelopment.
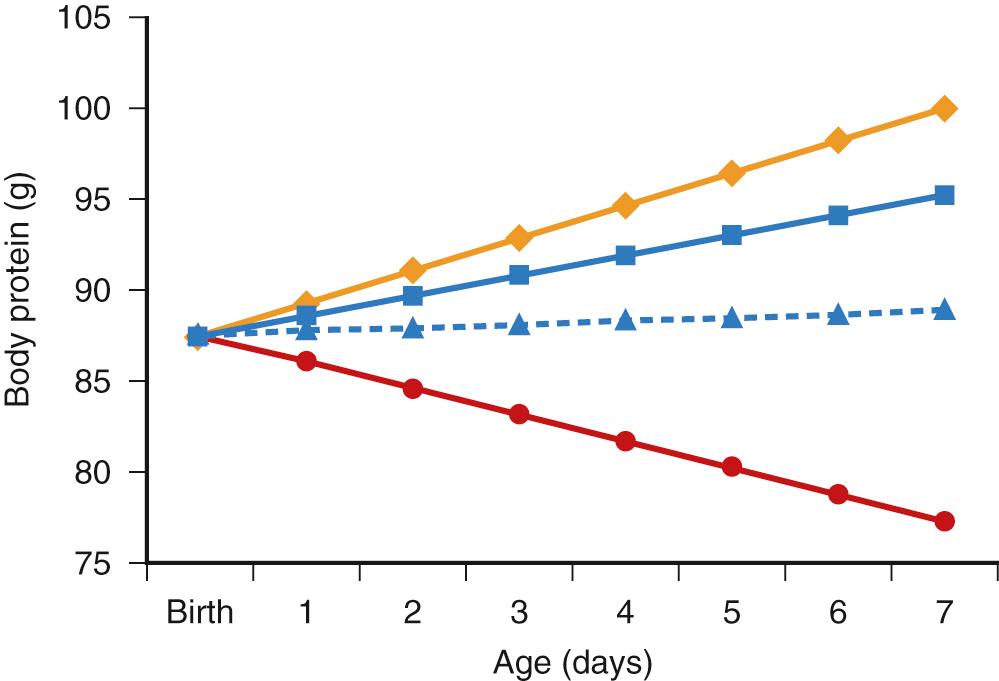
Normal human fetal development is characterized by rapid rates of growth and accretion of protein. In fact, the greatest rate of relative protein gain throughout life occurs prior to birth. At 26 weeks’ gestation, the human fetus gains approximately 1.8-2.2 g of body protein per day, with the placenta supplying about 3.5 g/kg per day of amino acids to the developing fetus. The placental supply of amino acids to the fetus is in excess of that needed for accretion of protein. The extra amino acids are oxidized by the fetus and contribute significantly to fetal energy production. In contrast to the high rate of protein gain in utero, protein losses in extremely premature infants are approximately twofold higher than in term infants. In the absence of intravenous amino acids, extremely premature infants lose approximately 1.2 g/kg of protein each day, which corresponds to a daily loss of 1%-2% of total endogenous body protein stores ( Fig. 41.1 ).
Protein requirements and recommendations for protein intake in premature infants have been made based on several different approaches. Ziegler has quantified protein and energy requirements in relation to body weight using the factorial approach. In the factorial approach, nutrient requirements are determined as the sum of the needs for growth plus needs for replacement of losses. The disadvantage of this approach is that it does not account for nutrient requirements for catch-up growth. It is important to note that protein requirements are inversely related to body weight. Based on the factorial method, protein requirements for infants who weigh less than 1200 grams are estimated at 4.0 g/kg per day. Other investigators have utilized empirical approaches to estimate the amount of protein intake required to duplicate fetal growth. The European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) recommendation for protein intake in infants weighing up to 1000 g is 4.0-4.5 g/kg per day and 3.5-4.0 g/kg per day for infants weighing 1000-1800 g. This recommendation takes into account the need to make up for accumulated protein deficits observed in nearly all extremely premature infants. The ESPGHAN committee further commented that protein intake can be reduced toward discharge if the infant's growth pattern allows. An international consensus panel has also recommended 3.5-4.5 g of protein per kg per day for very low birth weight infants receiving full enteral feedings.
The protein content and composition of human milk changes throughout lactation; the concentration diminishes from about 2 g/dL at birth to about 1 g/dL for mature milk. Qualitative changes also occur during lactation, resulting in a whey-casein ratio of 80:20 at the beginning of lactation, changing to 55:45 in mature milk. Although the levels of casein, α-lactalbumin, albumin, and lysozyme remain constant, the levels of secretory immunoglobulin A and lactoferrin decrease. Because these different protein fractions have different amino acid profiles, the content of the individual amino acids is also affected.
Casein-predominant cow milk formulas have the same whey-casein ratio as cow milk—that is, 18:82. Adding bovine whey makes whey-predominant formulas, such that the whey-casein ratio becomes similar to that of human milk (60:40). Nevertheless, the protein and amino acid profile remains very different from that of human milk. Compared with human milk, whey-predominant formulas have higher levels of methionine, threonine, lysine, and branched amino acids. These differences in amino acids have not resulted in any apparent clinical consequences in either term or preterm infants. Currently, most commercially available preterm formulas are whey predominant.
Because the supply of glucose to the fetus depends solely on maternal glucose, cord clamping at the time of birth requires that a number of events occur to maintain glucose homeostasis in the newborn. Fetal glucose use in utero matches umbilical glucose uptake, implying that glycogenolysis and gluconeogenesis are minimal in the fetus. Several factors promote glycogen deposition in utero: blunted pancreatic β-cell regulation of insulin secretion, high insulin receptor density, and relative glucagon resistance. In late gestation, the fetus begins to prepare for the transition to postnatal life by increasing hepatic glycogen stores and brown fat deposits. Hepatic glycogen synthesis increases in response to increases in adrenal corticosteroid production, also characteristic of late gestation. At the time of delivery, glucagon levels rise and insulin levels fall. Higher levels of plasma catecholamines, both epinephrine and norepinephrine, directly stimulate increases in hepatic glucose output. The increased levels of epinephrine and glucagon stimulate lipolysis and the activity of phosphorylase, a key enzyme in glycolysis. The increased level of glucagon also results in increased activity of phosphoenolpyruvate carboxykinase, a rate-limiting enzyme in gluconeogenesis. The newborn must be able to initiate gluconeogenesis, because glycogen stores can sustain glucose production only for several hours after birth. All of these changes act together to preserve glucose homeostasis after the infant's maternal source of glucose is removed with cord clamping.
Because neural tissue makes up a greater proportion of body weight, newborns have higher rates of glucose oxidation than adults, with glucose being the primary energy substrate for the brain. The rate of glucose production in term newborns is approximately 3-5 mg/kg per minute, whereas extremely premature infants have an even higher rate of glucose production of approximately 8-9 mg/kg per minute. Historically, glucose intolerance in infants with extremely low birth weights was attributed to persistent endogenous hepatic glucose production in the face of increased exogenous supply, insufficient insulin production, or tissue insensitivity to insulin. However, ELBW infants are able to suppress endogenous glucose production when given parenteral glucose.
Preterm infants have very low energy reserves, owing to limited body fat and glycogen stores in the liver. Maintaining these limited energy stores requires an energy intake that approximates energy expenditure. Energy expenditure in premature infants is thought to be in the 50-60 kcal/kg per day range, but it must be noted that data for ventilated infants and infants with extremely low birth weights are limited. Thermal stress can substantially increase energy expenditure. Conversely, activity contributes relatively little to energy expenditure in premature infants. The energy cost of growth in these infants has been estimated at about 5 kcal/g. To achieve the equivalent of the estimated third-trimester in utero weight gain of 14-18 g/kg per day, theoretically, an additional energy intake of about 70 kcal/kg per day is necessary.
Energy balance is a delicate equilibrium between energy intake and energy loss plus storage. Positive energy balance is achieved when exogenous metabolizable energy intake is greater than energy expenditure. Growth is then possible, with the excess energy stored as new tissue, usually fat. If exogenous energy intake is less than expenditure, energy balance is negative, and body energy stores must be mobilized to meet ongoing needs. During the acute phase of disease, the primary goal is avoidance of catabolism. This is difficult for infants with very low birth weights, owing to their higher maintenance energy requirements, lower energy stores, and often reduced intake.
Energy is lost either by excretion or by expenditure. Energy excreted is lost primarily as fecal fat. In preterm infants receiving full enteral feeding, approximately 90% of energy intake is absorbed. Usual measurements of total energy expenditure include the energy used to maintain basal metabolic rate (BMR), as well as the postprandial increase in energy expenditure (diet-induced thermogenesis), physical activity, and energy for the synthesis of new tissue. The BMR is the largest component of energy expenditure and includes energy requirements for basic cellular and tissue processes. In a critically ill patient, it also includes a “disease factor.” Little is known about this component in neonates, but it may contribute significantly in neonates with fever, sepsis, and chronic hypoxia. Because the BMR can be measured only after overnight fasting, the resting metabolic rate (RMR) has been accepted as an alternative. The RMR of preterm infants on a per kilogram basis is higher than that of term infants, and the nutritional requirements on a per kilogram basis are correspondingly greater. The energy required for thermoregulation can be minimized by keeping the infant in a thermoneutral environment and limiting stimulation. For example, energy requirements for thermoregulation are negligible under thermoneutral conditions, but routine nursing procedures can increase oxygen consumption or energy expenditure by as much as 10% in stable preterm infants. Both of these components may be of considerable magnitude in a critically ill infant.
The level of energy intake and diet composition determine the magnitude of diet-induced thermogenesis, which represents the energy required for transport, metabolism, and conversion of nutrients into stored energy. Estimates of diet-induced thermogenesis in enterally fed preterm infants vary considerably, but this component of expenditure is probably small.
Total energy expenditure is affected by several factors, assuming a thermoneutral environment and minimal interference from nursing procedures. Increases in metabolic rate with postnatal age are influenced primarily by energy intake and weight gain. Energy expenditure increases with increases in metabolizable energy intake, indicating increased substrate oxidation or tissue synthesis. If the preterm infant is growing at the same rate as the fetus during the third trimester—that is, gaining approximately 15 g/kg per day—then about 15% of the total energy intake is used for synthesis of new tissue.
Energy storage is a linear function of metabolizable intake, and the accretion rate for energy is related more to the level of metabolizable energy intake than to diet composition. Energy requirements for energy storage are difficult to predict. The increase in tissue mass during growth includes the energy stored as protein, carbohydrate (usually less than 1% of body weight), and fat. Therefore, the energy stored can be assumed to equal the sum of the cost of protein plus fat gain. The energy storage component of the energy balance equation is a function of the composition of weight gain, which in turn is a function of protein and energy intake and is likely to be quite variable. Therefore, the energy intake required to produce a specific rate of weight gain cannot be predicted without specifying the composition of that weight gain. From the point of view of energy storage, protein is a poor material, because a small quantity of energy is stored per gram of weight gain. Approximately the same amount of energy is deposited in 1 g of fat tissue as in about 8 g of lean tissue.
Lactose is the predominant carbohydrate in human milk (6.2-7.2 g/dL) and supplies 40%-50% of the caloric content. Lactose is hydrolyzed to glucose and galactose in the small intestine by β-galactosidase (lactase). Despite low lactase activities in premature infants, lactose is well tolerated by premature infants, and stable isotope data suggest efficient lactose digestion. However, most premature infant formulas include glucose polymers as a significant source of carbohydrate; these glucose polymers are digested by α-glucosidases, which achieve 70% of adult activity between 26 and 34 weeks’ gestation. In addition, salivary and mammary amylases may contribute to glucose polymer digestion. Glucose polymers have the advantage of increased caloric density without a rise in osmolality, and they may also enhance gastric emptying.
Overall, the current recommendation for energy intake for an enterally fed VLBW infant is 110–130 kcal per kg/day. It is important to recognize that this recommendation assumes that the infant is also receiving adequate protein intake. Infants who are small for gestational age or infants with diseases that increase energy requirements may need higher intakes to achieve the same growth rates. Infants with growth restriction often require an increased caloric intake for growth because of both higher maintenance energy needs and higher energy costs of new tissue synthesis.
In humans, linoleic and linolenic acids cannot be endogenously synthesized and are, therefore, essential fatty acids. Biochemical evidence of essential fatty acid deficiency can develop in preterm infants within 72 hours. To meet energy requirements, additional intravenous lipid is required in early postnatal life.
Fat provides the major source of energy for growing preterm infants. At birth, the digestive function of premature infants is not fully developed; preterm infants have decreased gut absorption of lipids because of low levels of pancreatic lipase, bile acids, and lingual lipase. The fact that term and preterm infants absorb fat reasonably well is due to the development of alternative mechanisms for the digestion of dietary fat. One important mechanism is intragastric lipolysis, in which lingual and gastric lipases compensate for the low pancreatic lipase concentration. By 25 weeks’ gestation, lingual lipase is secreted by the serous glands of the tongue, and gastric lipase is secreted from gastric glands. The fatty acids and monoglycerides resulting from intragastric lipolysis compensate for low bile acid concentration by emulsifying lipid mixtures. Lingual lipase can also penetrate the core of the human milk lipid globule and hydrolyze the triglyceride core without disrupting the globule membrane. Human milk provides lipoprotein lipase, bile salt stimulated esterase, and nonactivated lipase to further aid lipolysis.
Lipid digestion and absorption are also affected by dietary fat composition. Fatty acid absorption increases with decreasing chain length and with the degree of unsaturation, meaning that medium chain triglycerides (MCT) with chain lengths of 6-12 carbons are hydrolyzed more readily than long chain triglycerides (LCT), and that fatty acids with more double bonds are absorbed more efficiently. In an attempt to increase the fat absorption of premature infants, the fat in commercial formulas contains relatively high levels of MCTs that can be absorbed without the need for lipase or bile salts. Standard commercial formulas for healthy term infants do not contain MCTs, and human milk typically contains 8%-12% of fat as MCTs. Unlike LCTs, MCTs are readily hydrolyzed in the gut, and the released fatty acids are transported across the gut barrier without the need for bile acids. Then MCTs are transported directly to the liver via the portal vein as nonesterified fatty acids. In addition, MCTs can enter mitochondria and be oxidized without the need for carnitine-mediated transport through mitochondrial membranes. However, inclusion of MCTs in infant formula remains controversial, because the available data do not support the assertion of improved fat absorption or improved growth in preterm infants.
In human milk, fat is transported in globules consisting of a membrane composed of a polar mixture of proteins, phospholipids, triglycerides, cholesterol, glycoproteins, and enzymes surrounding a triglyceride core containing 98% of the fat in milk. The milk fat globules are among the largest structural components of milk, having a diameter of 4 mm in mature milk. The size of the globules increases with both length of lactation and length of gestation, with colostrum having smaller globules (especially in milk of women who deliver prematurely) than mature milk. After birth, as the total fat content of human milk increases, the percentage of cholesterol and phospholipids, both of which reside primarily in the milk fat globule membrane, decreases; in addition, the total phospholipid content decreases as lactation progresses. During the first weeks of lactation, preterm milk is also richer in membranous material compared with term or mature milk, resulting in a higher content of cholesterol, phospholipids, and very-long-chain polyunsaturated fatty acids (PUFA) with chain lengths of 20-22 carbons (C20-C22). Because these membranes act as emulsifiers that allow fat dispersion in an aqueous phase and limit lipolysis and oxidation, heat treatment or addition of fortifiers and supplements might disrupt this emulsion.
The milk fat content and nutritional value of human milk vary with time, and it does not always provide a complete source of nutrients for infants with very low birth weights. Its composition and energy content may vary in a pumping session and during subsequent changes throughout lactation. The total fat content of human milk at 3 days’ lactation is approximately 2 g/dL; the fat content of mature milk is approximately 4-5 g/dL, with large individual variation possible. The triglyceride content of human milk is its most variable component, changing with gestational and postnatal age, time of day, duration of individual feeds, and maternal diet. Shifts in the dietary practices of a population result in changes in the fatty acid composition of human milk, because the type and amount of fat in the maternal diet affect the composition of milk fat. Maternal diets low in fat and high in carbohydrate lead to de novo synthesis of fatty acids within the mammary gland, which results in high concentrations of fatty acids of less than 16 carbons. Therefore, although the total amount of fat present in the milk remains in the normal range, the fat is more saturated.
Fatty acids represent about 85% of the triglycerides and, therefore, are the principal component of human milk lipids. Fatty acids in human milk are derived from the maternal diet, de novo synthesis by the mammary gland, and mobilization from fat stores. The fatty acid composition of human milk fat reflects the fatty acid composition of the maternal diet. The long-chain polyunsaturated fatty acid (LCPUFA) composition of the milk of women in the United States, Europe, and Africa is quite similar, with the exception of higher amounts of n-3 LCPUFA in the milk of women whose diets contain a large quantity of fish. Medium-chain fatty acids (C8-C10) do not normally account for more than 2% of the fats, even in milk from women who have delivered preterm. Arachidonic acid (C20:4n-6) is the main LCPUFA, and eicosapentaenoic acid (C20:5n-3) is found in small quantities in human milk. Docosahexaenoic acid (C22:6n-3) is the main LCPUFA of the n-3 series.
Fatty acid composition changes with progressing lactation and with gestational age. Most striking is the higher content of C8-C14 fatty acids and of LCPUFAs in preterm milk as compared with term milk; the content of LCPUFA decreases with increasing postnatal age. This may be an advantage for preterm infants because shorter fatty acids are easier to digest, and LCPUFAs are essential for brain and retinal development.
The LCPUFAs play an important role in the development of the infant's brain during the last trimester of pregnancy and also during the first months of life. The precursor C18 fatty acids for the n-6 and n-3 LCPUFA series are linoleic acid (C18:2n-6) and α-linolenic acid (C18:3n-3). These are further elongated and desaturated to form other fatty acids, of which arachidonic acid (AA) and docosahexaenoic acid (DHA) are essential for normal growth and development. Although the capacity for endogenous synthesis of LCPUFA from precursor fatty acids exists in both preterm and term infants, it remains unclear whether DHA and AA can be biosynthesized in quantities sufficient to meet the needs of these infants.
In utero, LCPUFAs are supplied to the fetus across the placenta. After birth and within the first postnatal week, preterm infants developed a deficit of DHA and AA compared to their starting point at birth, while almost tripling their linoleic acid (LA) levels. These acute changes are likely due to the lack of adipose reserves as well as the lack of early nutritional strategies that effectively meet the fatty acid requirements in the preterm infant. Although Smoflipid® contains DHA and AA, the use of Smoflipid® fails to correct these postnatal fatty acid deficits inducing a minimal change in DHA and a further decline in AA, and introduces a substantial increase in eicosapentaenoic acid (EPA) due to its fish oil component. Although the importance of DHA in brain and eye development has been established, accruing evidence suggests that a decline in AA is also of concern with increasing risks of late onset sepsis and retinopathy of prematurity with lower AA levels.
Although naturally contained in human milk, DHA and AA have been added to most infant formulas for term and preterm infants. Multiple randomized controlled trials evaluating the addition of DHA and AA to preterm formulas have been conducted. Added DHA and AA have generally resulted in positive or neutral changes in growth, although there are some reports of a negative effect. Findings of improved visual acuity have been inconsistent. Formula supplemented with DHA and AA has produced positive changes in neurodevelopment measured in infancy in some, but not all, studies. A meta-analysis demonstrated no improvement in visual acuity or neurodevelopment. The current recommendation is that VLBW infants receive 55-60 mg per/day of DHA and 35-45 mg per kg/day of AA.
Cholesterol is a major component of cell membranes and a precursor in the synthesis of bile acids and some hormones. It is present in human milk in concentrations ranging from 10-15 mg/dL, although commercial formulas contain only trace amounts of cholesterol (approximately 1-2 mg/dL). The high cholesterol content of breast milk relative to formula is maintained at this level regardless of maternal diet. The groups that assessed the nutrient requirements for term and preterm infant formulas did not recommend addition of cholesterol to infant formulas, because there was no convincing evidence of a beneficial short- or long-term effect of such an addition. Furthermore, there is no evidence that added cholesterol would be equivalent to the cholesterol in a human milk globule.
Carnitine mediates the transport of long-chain fatty acids into mitochondria for oxidation and the removal of short-chain fatty acids that accumulate in mitochondria. Preterm infants may be at risk for carnitine deficiency, because they are heavily dependent on lipids as an energy source and because the plasma carnitine concentration of preterm infants is low, owing to limited endogenous synthetic ability. In preterm infants not receiving supplemental carnitine, plasma and tissue carnitine levels fall even in the presence of adequate precursor amino acid concentrations. Carnitine is found in human milk and is currently added to standard term and preterm formulas in amounts somewhat higher than in human milk.
The extremely premature neonate is born with glucose stores of only 200 kcal and loses 1% of body protein per day when provided with intravenous glucose alone. Consequently, extreme prematurity should be viewed as a nutritional emergency. A number of observational studies have described the influence of nutritional practices on growth and have found that differences in caloric and protein intake in the first weeks account for the largest difference in growth among premature infants. In addition, there is a growing body of data that shows the association between early nutrient intake and growth and neurodevelopmental outcomes. This section reviews the basis of recommendations for nutritional support of premature infants. Practice decisions related to provision of early nutritional support provided to ELBW infants seem to be related to the perceived severity of illness of the infant by clinicians. The use of standardized protocols for feeding (both parenteral and enteral) extremely premature infants can lessen variation in practice and improve outcomes.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here