Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The human brain is a fascinating and essentially miraculous structure with layers upon layers of complexity in its anatomic organization, microscopic connectivity, biochemical workings, and functions. No human can grasp all of the complexity that allows the brain to function, but that doesn’t stop us from trying! Learning about the brain’s development is particularly exciting, daunting, and exhausting. Understanding even just a small fraction of the necessary processes that allow the brain to develop from the moment of conception to birth and from birth to adulthood can be rewarding and bring a great sense of wonder.
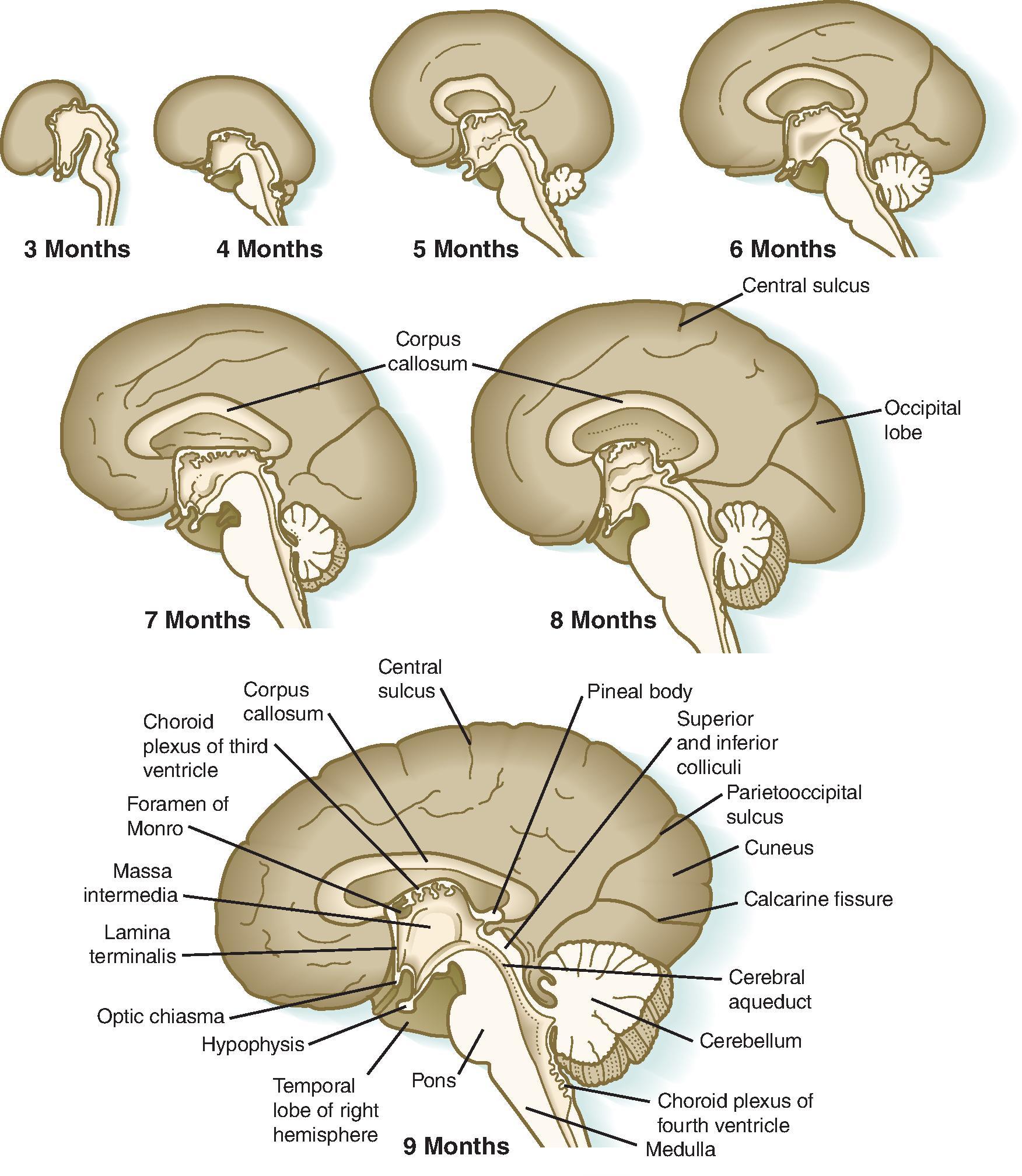
Understanding normal development of the central nervous system begins with a basic understanding of embryology of the brain and spine; however, by the time at which imaging is routinely performed in clinical care of patients, a significant degree of brain and spine development has already occurred. Abnormalities in early brain development account for many of the malformations seen on imaging. As such, this chapter will primarily focus on the imaging appearances seen during normal development and provide a basic overview of embryology.
A general approach for evaluating normal brain development on imaging begins with knowledge of the age of the patient and then determination of normal formation of major structures. A pattern approach is most useful to ensure one does not overlook an important structure. Major intracranial structures that should be evaluated include the size, sulcation, gyration, and myelination of the cerebral hemispheres; size, formation, and proportions of the cerebellum, brainstem, and posterior fossa; presence of the septum pellucidum and formation and size of the corpus callosum; presence of the olfactory bulbs and sulci; formation and size of the basal ganglia and thalami; rotation of the hippocampi; and formation and size of the ventricles. The appearance of these structures will be discussed in this chapter, and useful biometric data will be provided. Importantly, assessment of extracranial structures, including the eyes, ears, and facial bones, should be performed because anomalies in these structures have associated brain anomalies.
In addition to the imaging patterns of normal brain development on conventional imaging, advanced magnetic resonance imaging (MRI) techniques that are not routinely clinically performed, including diffusion tensor imaging, spectroscopy, and functional MRI, will be discussed. These techniques provide additional insight into normal brain development. Last, the formation of the anterior cranial fossa, paranasal sinus development, and spine development will be discussed.
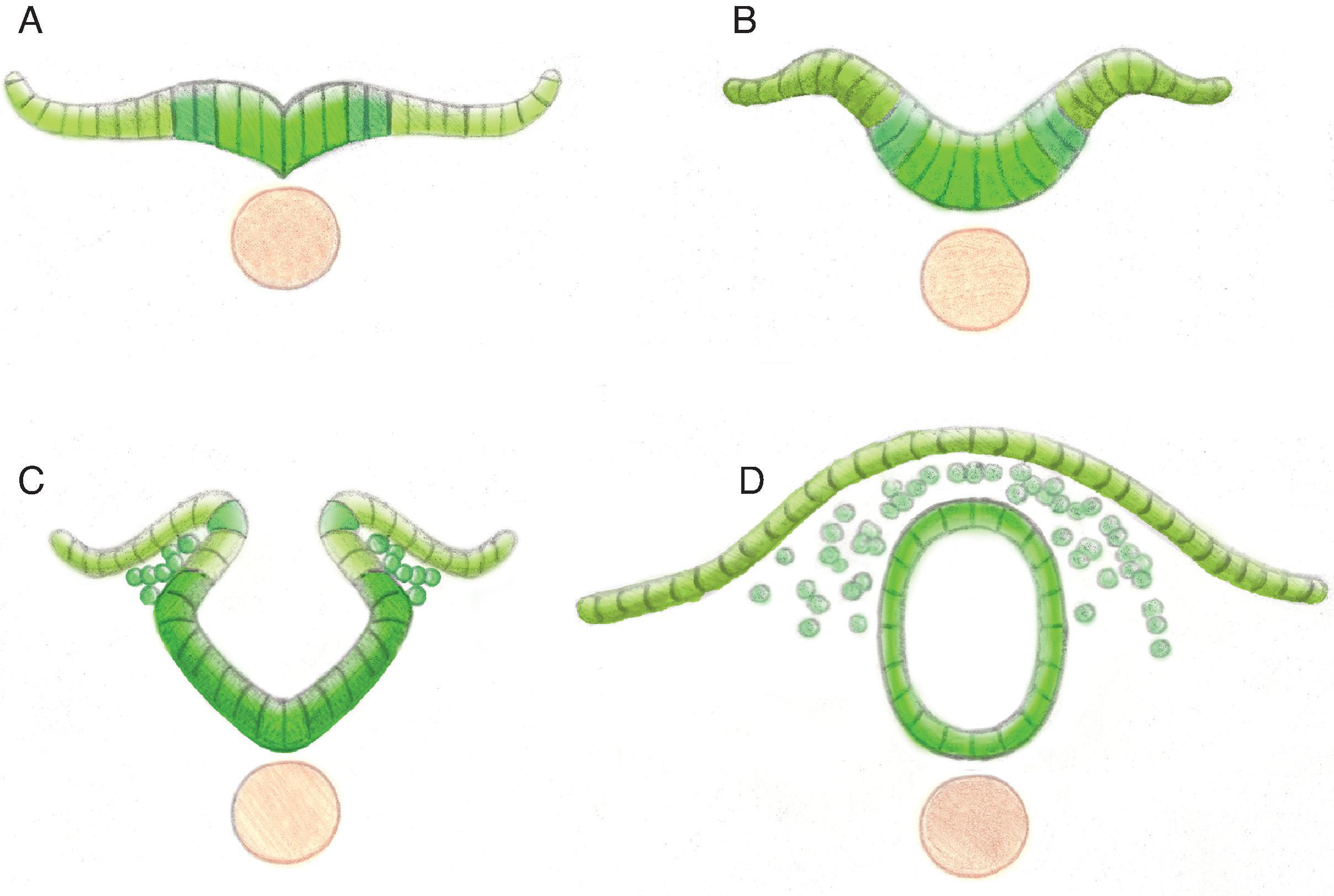
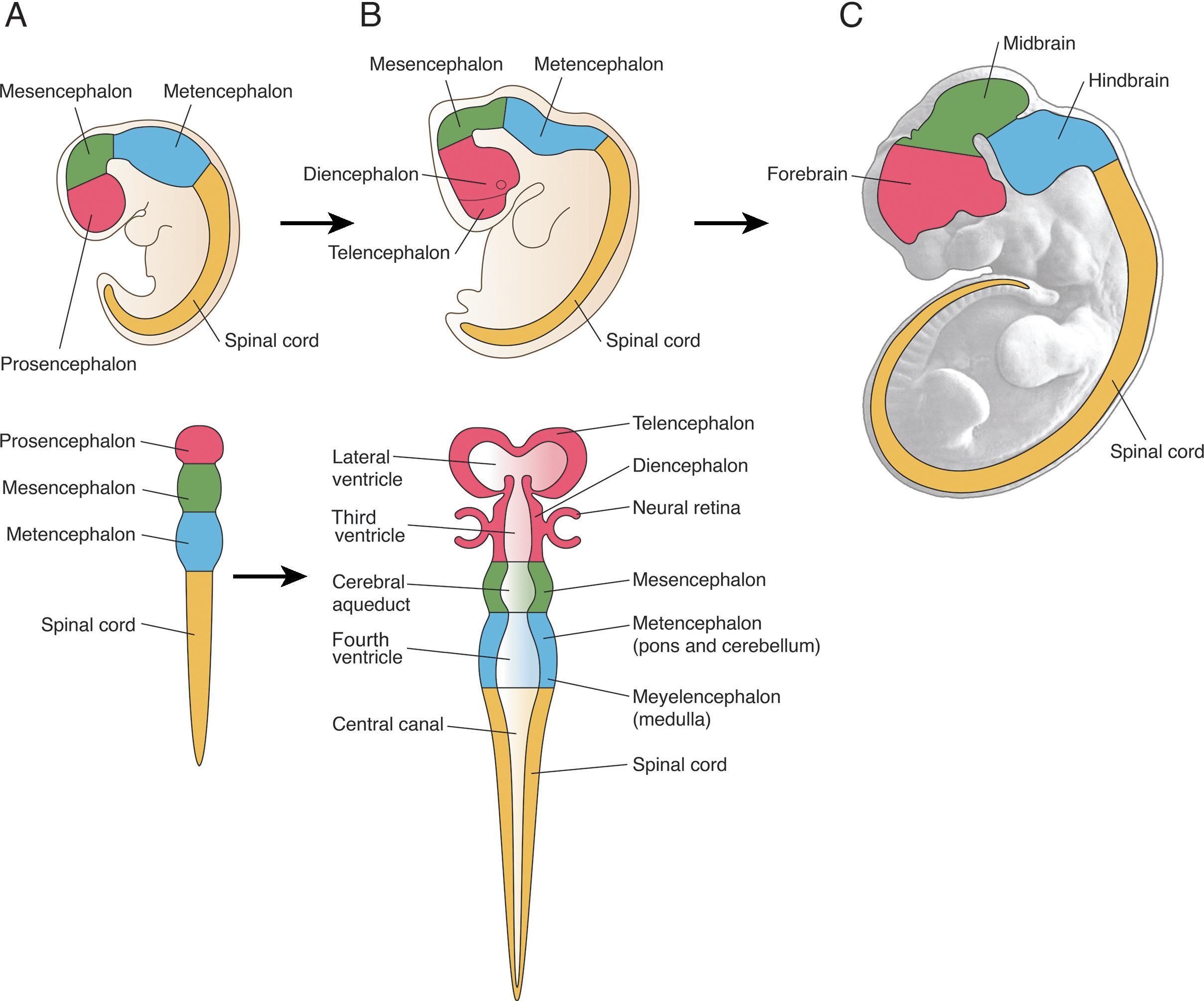
The central nervous system develops through the process of primary neurulation during week 3–4, when notochord induces the ectoderm to become neuroectoderm and develop into the neural plate. The neural plate folds and becomes the neural tube and separates from the ectoderm (disjunction). The cranial end of the neural tube is the anterior/rostral neuropore, and the caudal opening is the posterior/caudal neuropore. Prior to neural tube closure, the anterior neural tube forms three primary brain vesicles or pouches referred to as the prosencephalon (forebrain), mesencephalon (midbrain), and rhombencephalon (hindbrain). Abnormalities in development occurring at these stages lead to malformations, including anencephaly and many of the spine malformations discussed in Chapter 6 .
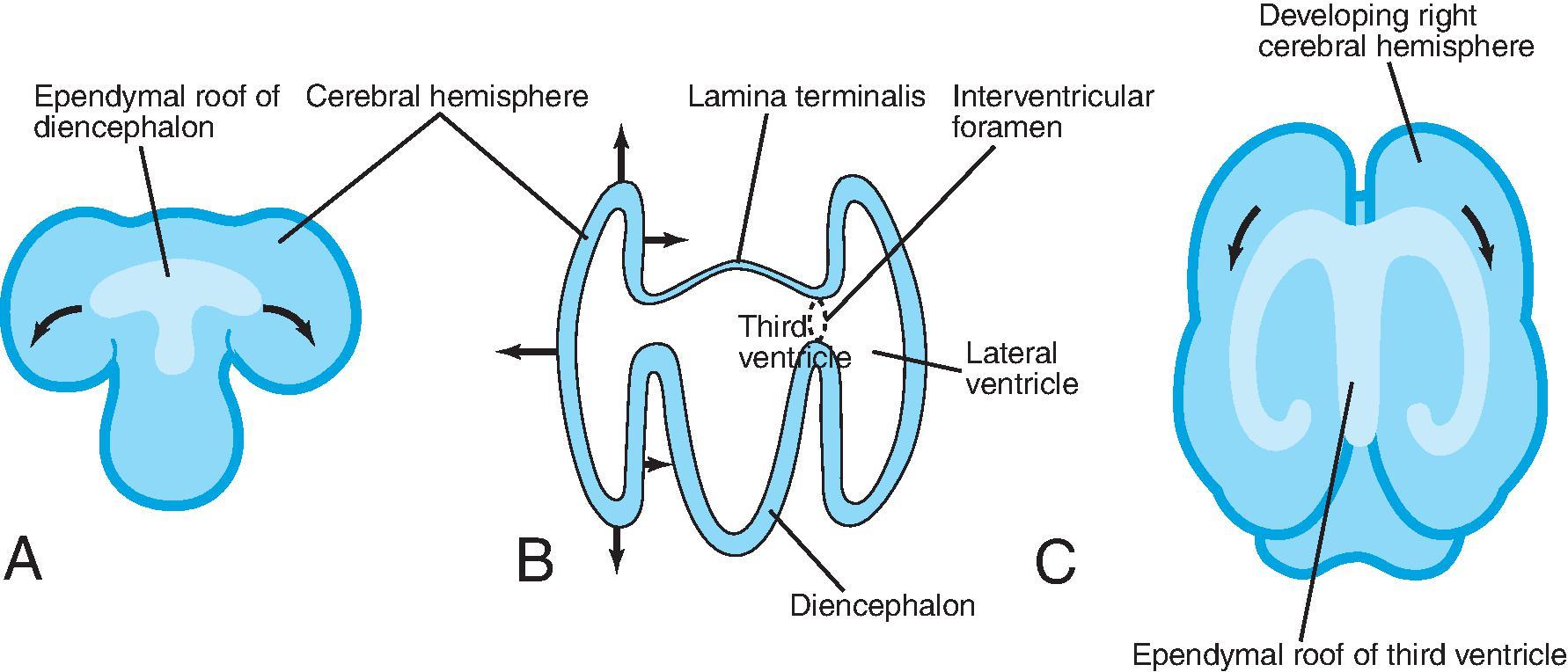
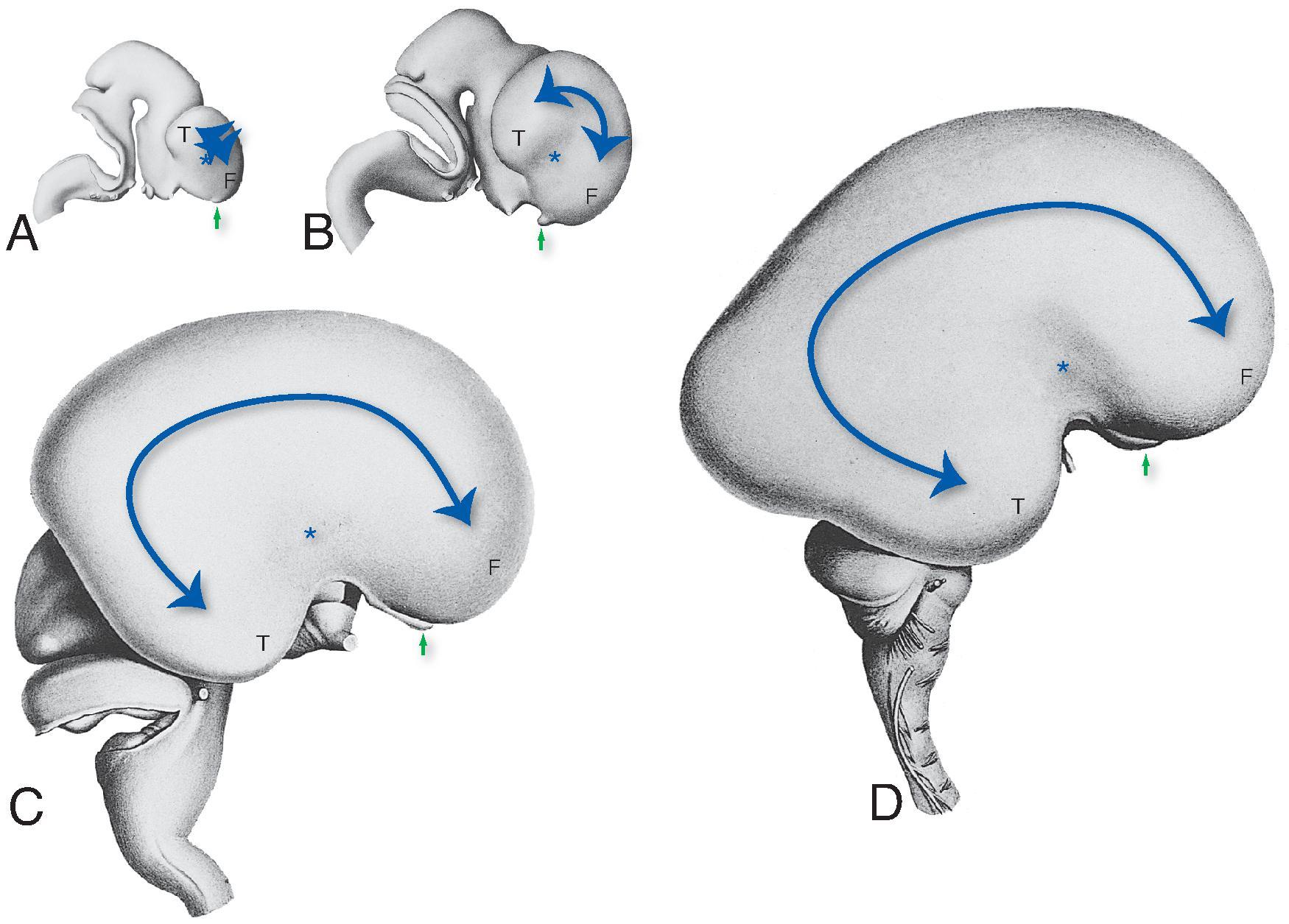
At approximately 35 days of gestation, the prosencephalon divides into the telencephalon and diencephalon. The telencephalon, which forms the cerebral hemispheres (also the caudate and putamen), separates as outpouchings in the region of the foramen of Monro from the diencephalon (which forms the thalami, hypothalamus, and globus pallidi). The thin-walled cerebral hemisphere vesicles expand and cover the diencephalon; cerebral hemispheres will separate in the midline along the roof of the telencephalon. The germinal matrix, which contains the cells that will form the cortex, will develop then within the walls of the telencephalon.
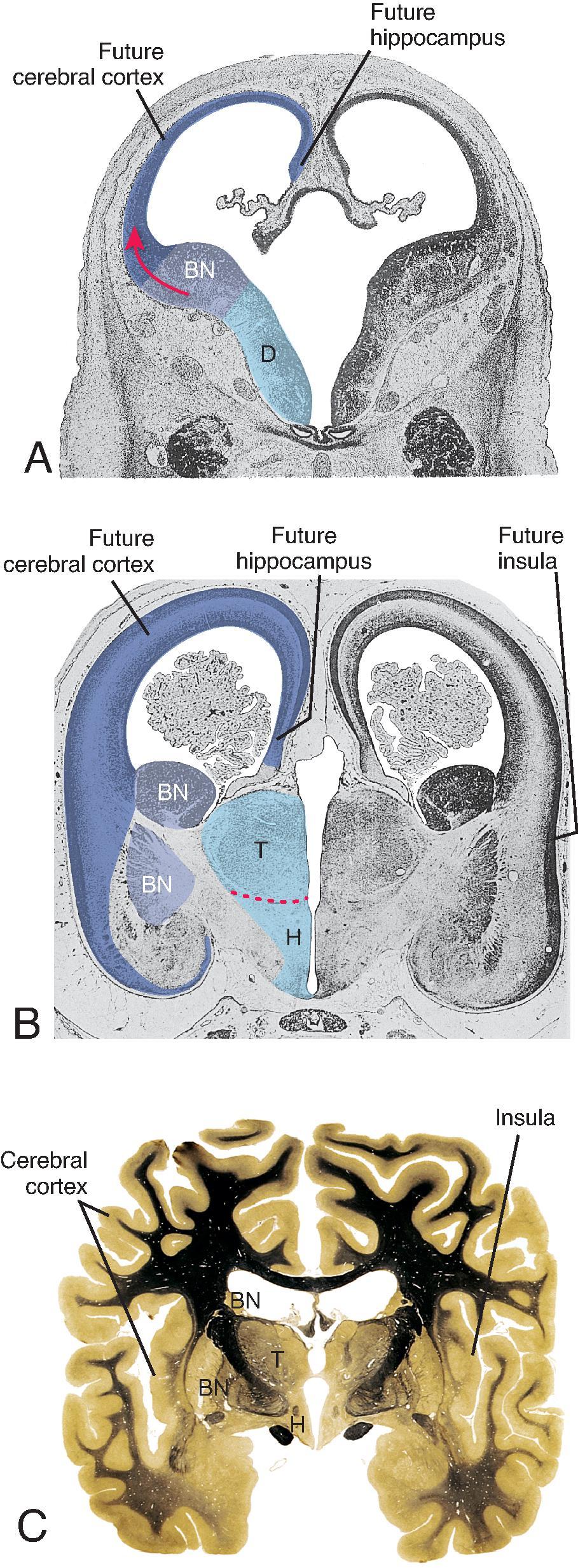
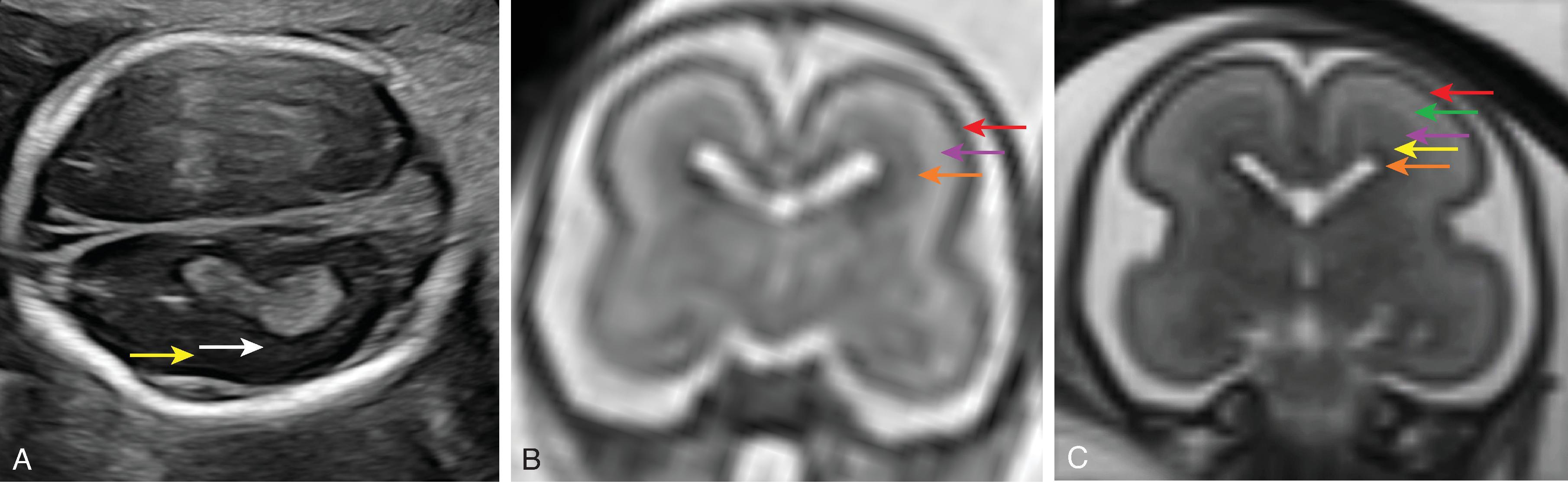
The cerebral cortex, basal ganglia, thalami, and hypothalamic are initially continuous with each but will eventually separate and reach their final positions as the cerebral hemispheres enlarge. The germinal matrix develops in the walls of the telencephalon, and areas known as the ganglionic eminences develop as larger regions of the germinal matrix. As the neurons from the germinal matrix proliferate and migrate to the outer cortex, they also form permanent and transient axonal connections. During this stage of development, zones in the telencephalon are visible. These zones change in appearance during development and peak between 15 and 24 weeks’ gestation ( Fig. 1.1 ).
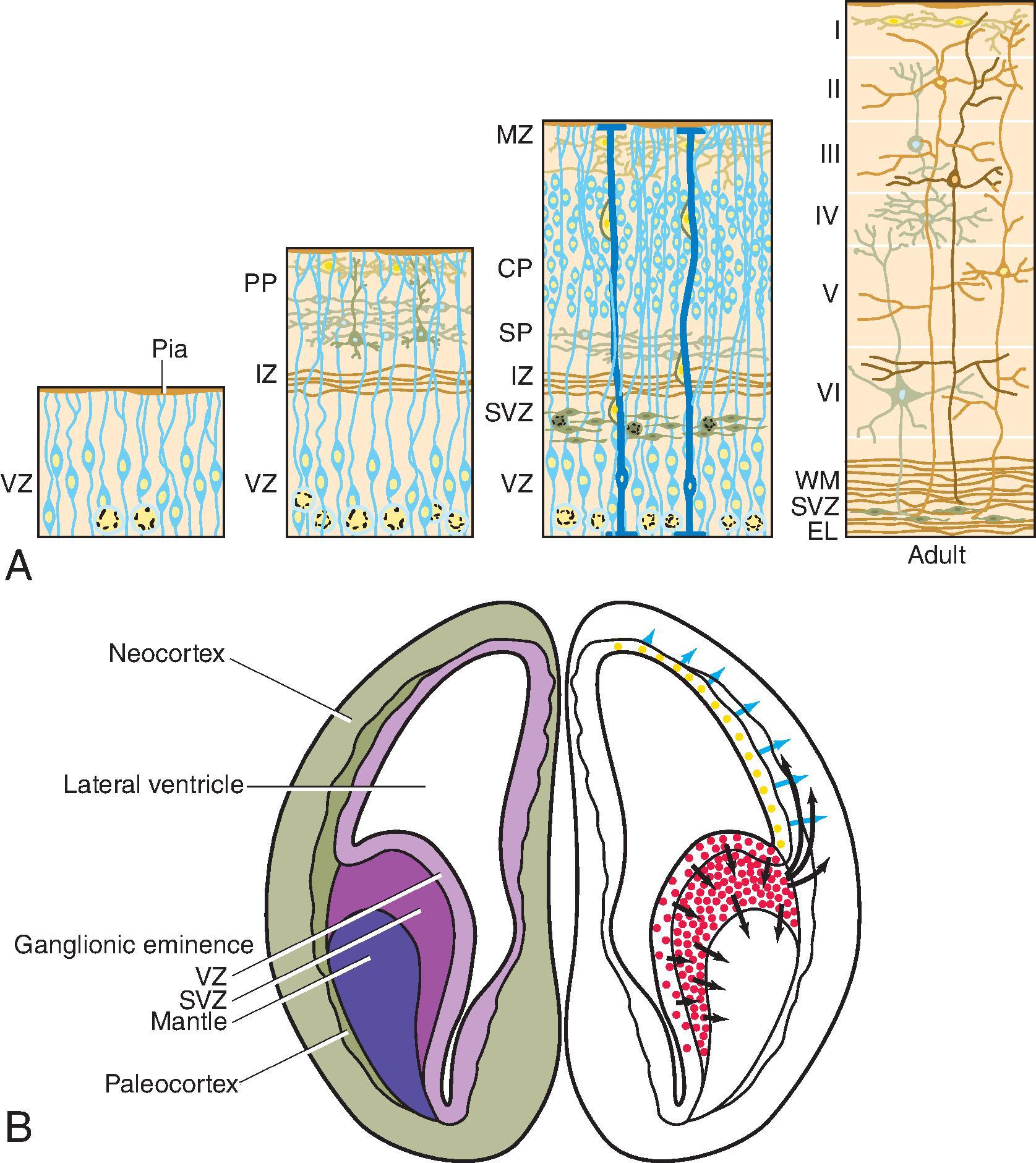
The ventricular zone gives rise to descendants of the neural plate and subventricular zone. The periventricular zone is a cell-sparse layer with axons that give rise to the corpus callosum. The subventricular zone is the source of cortical and striatal neurons, astrocytes, and glial cells. The intermediate zone is a region of axonal connections. The subplate contains maturing neurons and connections important for cortical layer organization. The subplate reaches maximum thickness at 22 weeks’ gestation and disappears at 30 weeks. The cortical plate is the final destination of migrating neurons and will develop into a six-layered neocortex with the innermost layer (layer VI) forming first and, as neurons continue to migrate, will progressively form the more outer layers. Proliferation and migration of glial progenitors continue in the postnatal period into adulthood.
On ultrasound between 17 and 28 weeks, the cortex and subplate are hypoechoic and indistinguishable, and the intermediate zone is mildly hyperechoic.
On MRI between 16 and 20 weeks, the brain has a three-layered pattern on T2W imaging consisting of the:
Germinal matrix (hypointense)
Intermediate zone (hyperintense)
Cortex (hypointense)
On MRI from 20 to 28 weeks, the brain has a five-layered pattern on T2W imaging consisting of the:
Germinal matrix/ventricular zone (hypointense)
Periventricular zone (hyperintense)
Subventricular and intermediate zones (hypointense)
Subplate (hyperintense)
Cortical plate/cortex (hypointense)
Understanding the normal development of the cortex allows one to understand how abnormalities affecting the germinal matrix and neuronal migration can result in heterotopia, polymicrogyria, and cortical dysplasias.
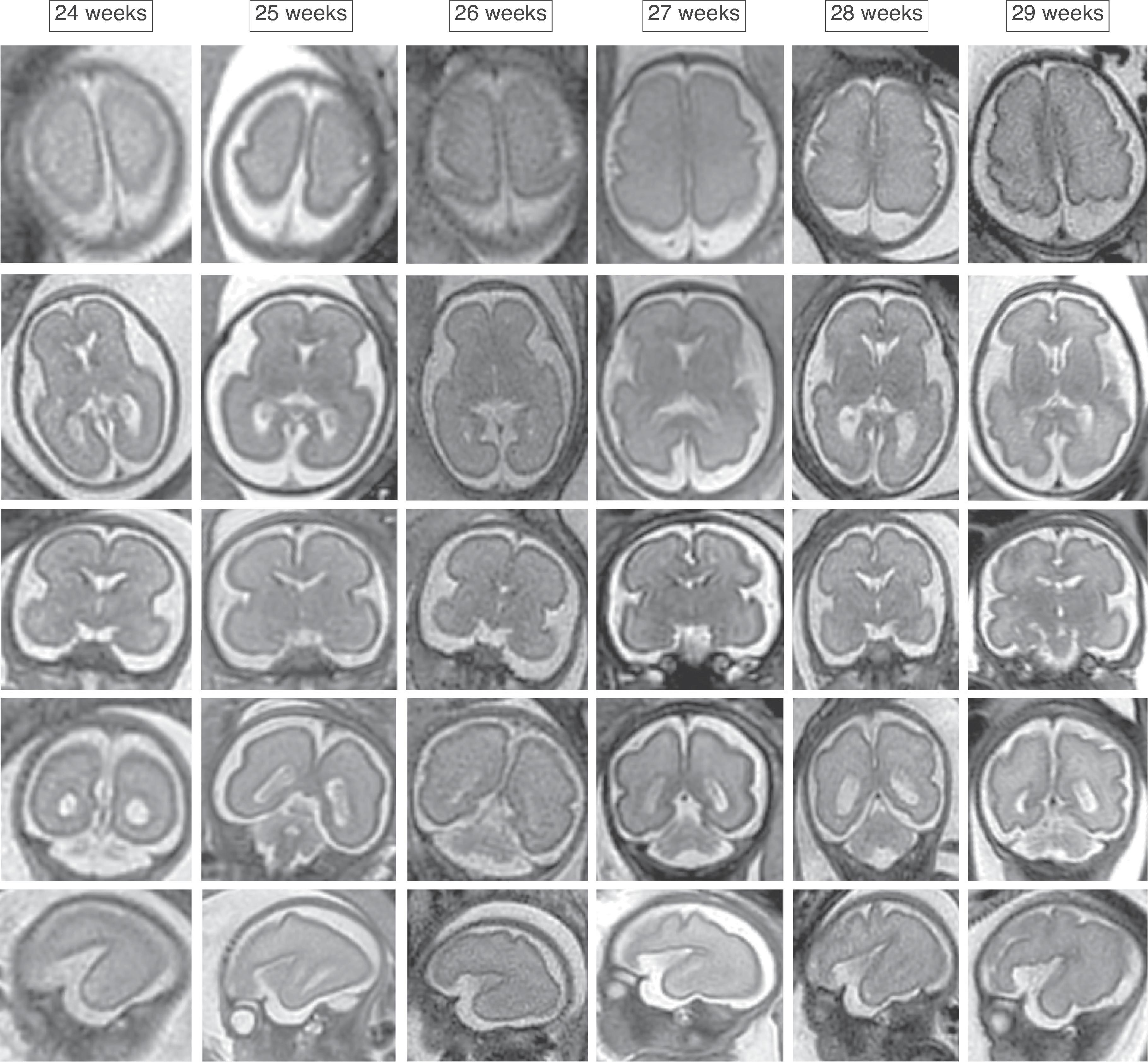
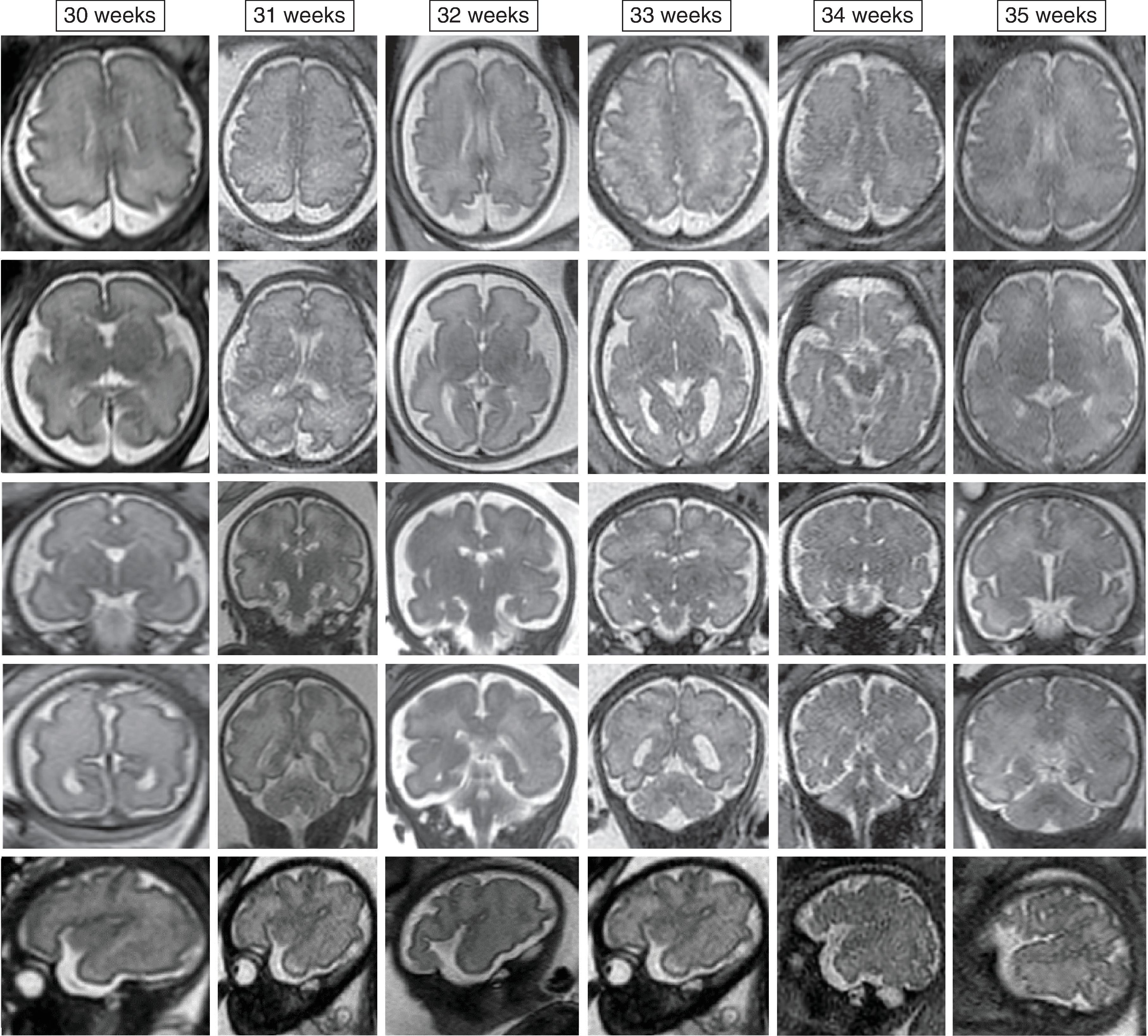
The formation of the brain also requires formation of the gyri and sulci. The fetal brain has a relatively smooth contour until approximately 16 weeks’ gestation, when the sylvian fissure first appears. The primary and secondary sulci will appear in an orderly fashion ( Table 1.1 ). Analysis of normal brain development on fetal MRI requires an understanding of the normal gyration/sulcation pattern of the brain for the gestational age of the fetus. Overgyration and undergyration of the fetal brain may both be signs of a pathologic process. The following pages will illustrate the changing sulcation pattern, major time points for sulci, and reference images of the brain for each gestational week from 24 to 35 weeks. Reference images for sulcation are often useful in routine clinical care when assessing appropriate sulcation with respect to fetal age.
| Gestational Age (wk) | |||
|---|---|---|---|
| Neuropathologic Appearance a | Detectable in 25% to 75% of Brains in Present MR Study | Present in More Than 75% of Brains in Present MR Study | |
| Sulci of the Medial Cerebral Surface | |||
| Interhemispheric fissure | 10 | 22–23 | |
| Callosal sulcus | 14 | 22–23 | |
| Parietooccipital fissure | 16 | 22–23 | |
| Cingular sulcus | 18 | 22–23 | 24–25 |
| Secondary cingular sulci | 32 | 31 | 33 |
| Marginal sulcus | 22–23 | 27 | |
| Calcarine fissure | 16 | 22–23 | 24–25 |
| Secondary occipital sulci | 34 | 32 | 34 |
| Sulci of the Ventral Cerebral Surface | |||
| Hippocampic fissure | 22–23 | ||
| Collateral sulcus | 23 | 24–25 | 27 |
| Occipitotemporal sulcus | 30 | 29 | 33 |
| Sulci of the Lateral Cerebral Surface | |||
| Superior frontal sulcus | 25 | 24–25 | 29 |
| Inferior frontal sulcus | 28 | 26 | 29 |
| Superior temporal sulcus (posterior part) | 23 | 26 | 27 |
| Superior temporal sulcus (anterior part) | 30 | 32 | |
| Inferior temporal sulcus | 30 | 30 | 33 |
| Intraparietal sulcus | 26 | 27 | 28 |
| Insular sulci | 34–35 | 33 | 34 |
| Sulci of the vertex | |||
| Central sulcus | 20 | 24–25 | 27 |
| Precentral sulcus | 24 | 26 | 27 |
| Postcentral sulcus | 25 | 27 | 28 |
* According to Chi et al., sulci observed in 25% to 50% of the brains. (Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol . 1977;1:86–93.) From Garel C, Chantrel E, Brisse H, et al. Fetal cerebral cortex: normal gestational landmarks identified using prenatal MR imaging. AJNR Am J Neuroradiol . 2001 Jan;22(1):184–189. PMID: 11158907.
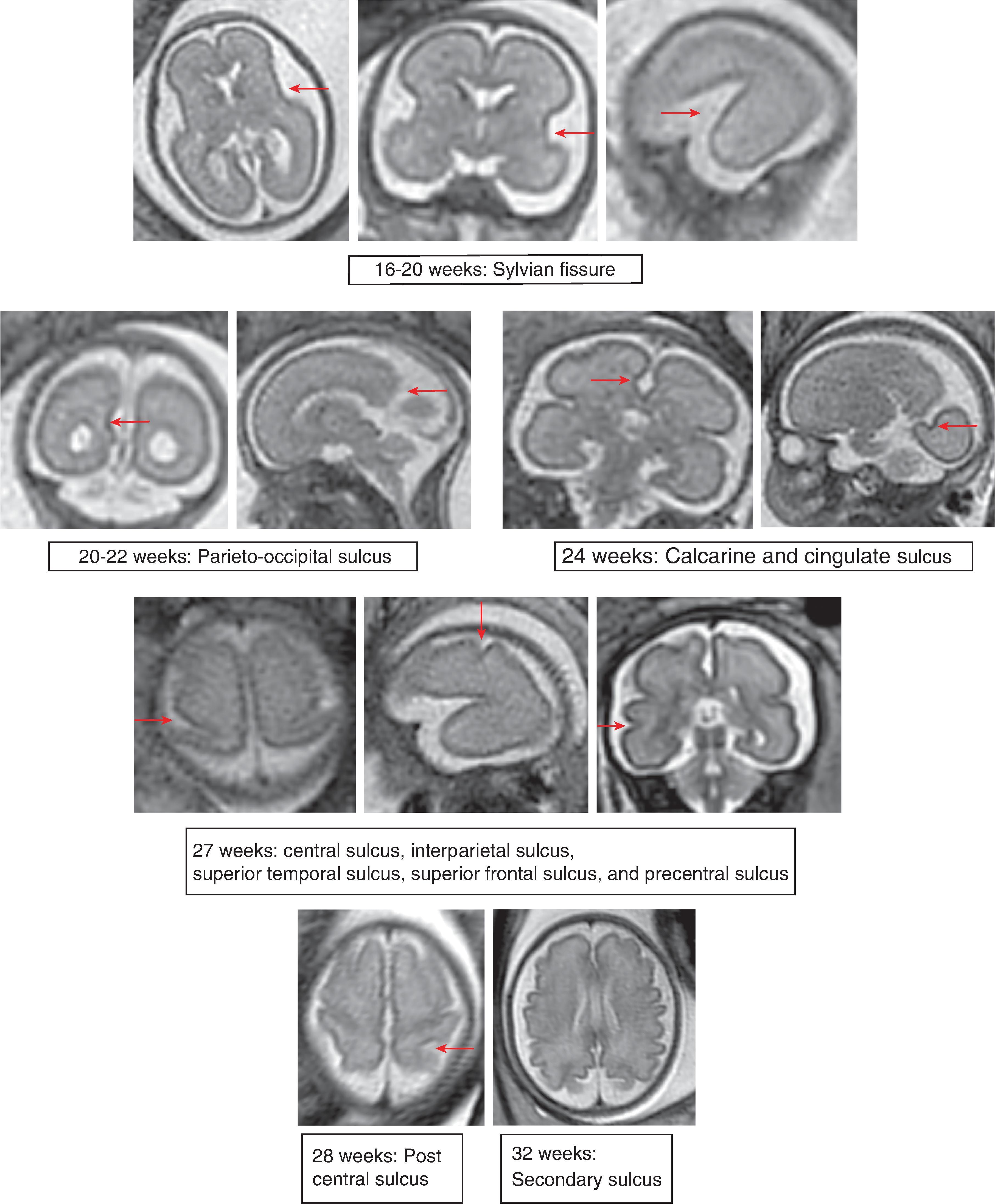
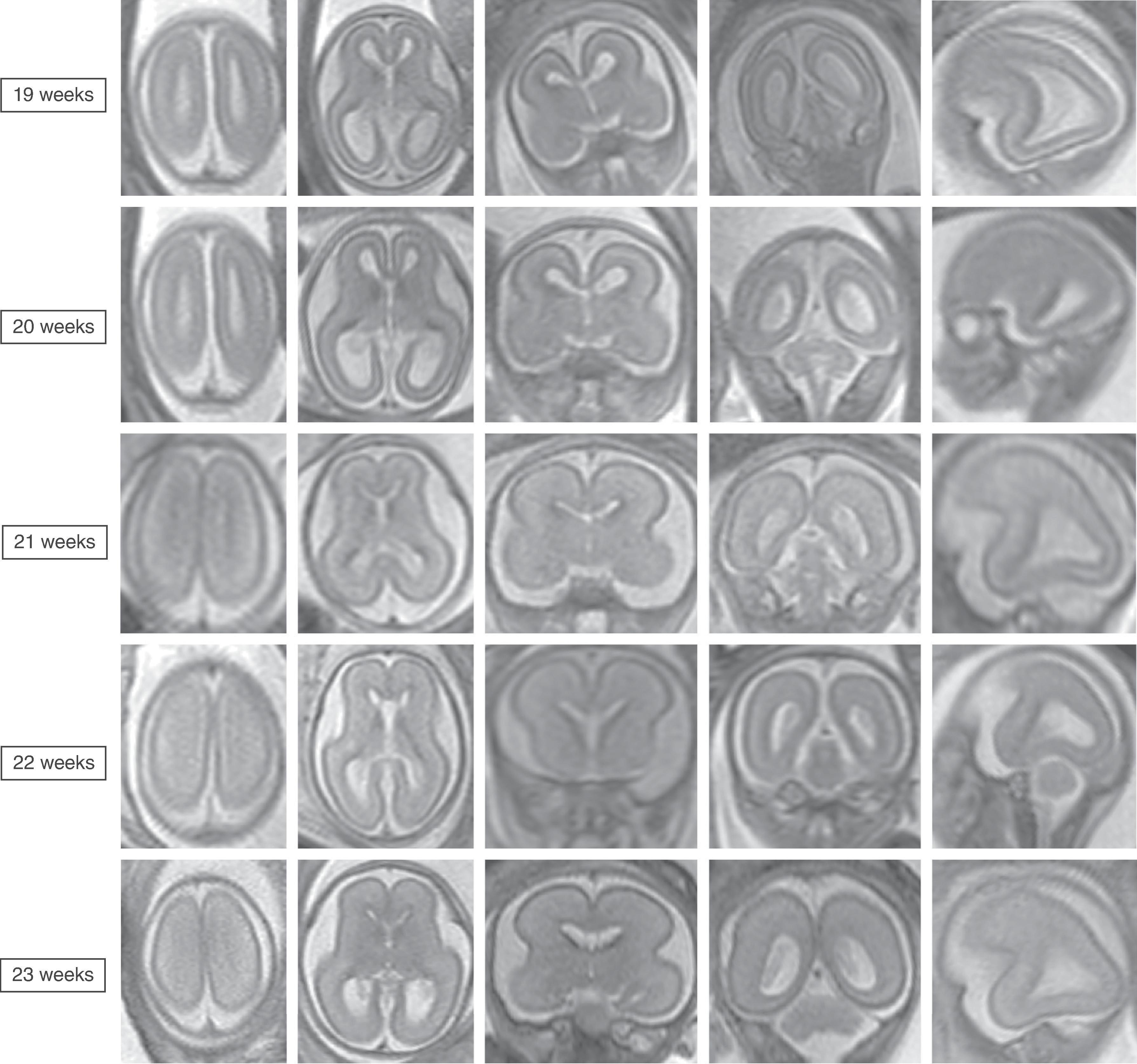
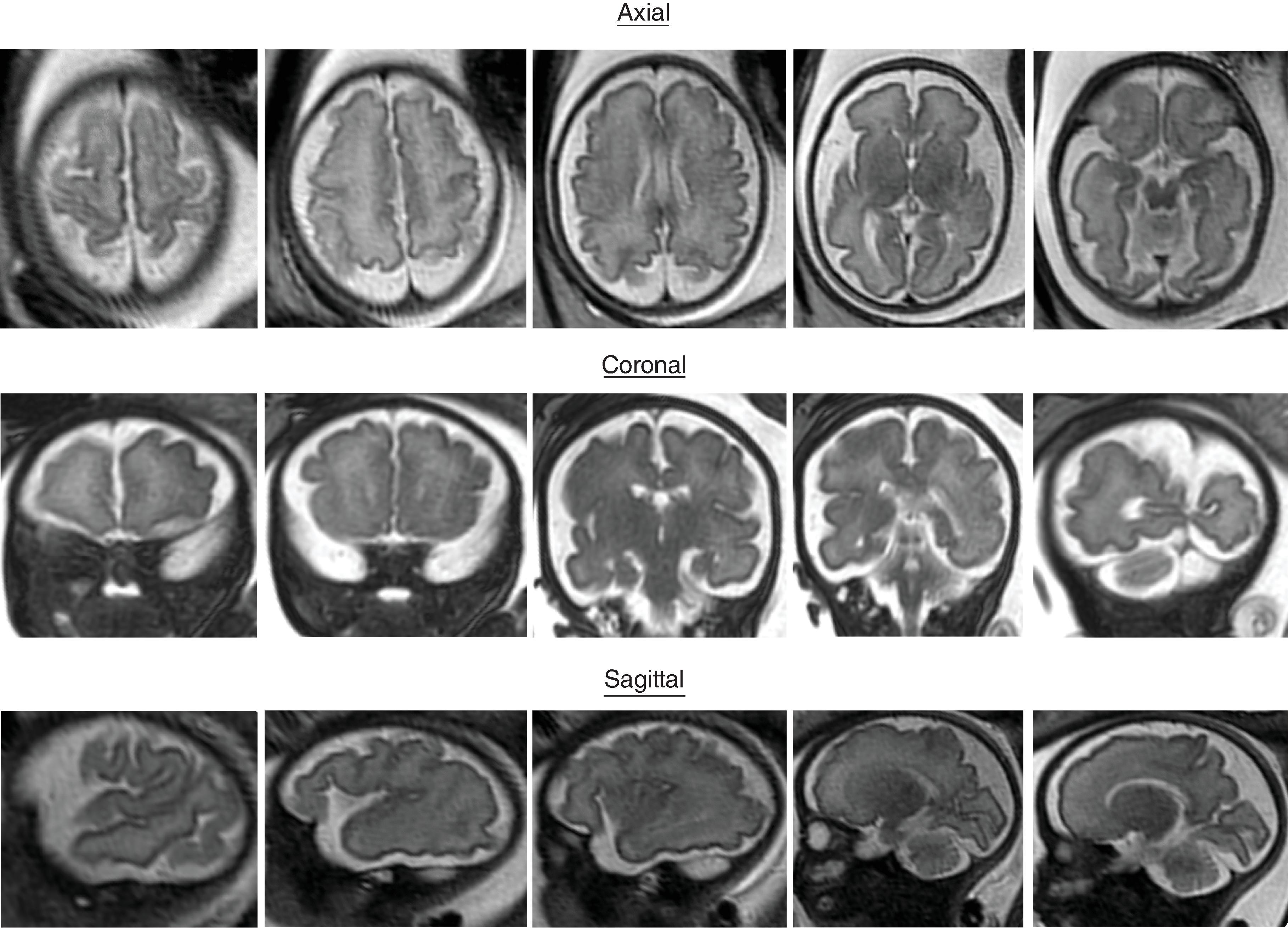
Sulci present: Sylvian fissures. Minimal differences in the sulcation between 19 and 23 weeks
Sulci developing: Parietooccipital sulcus
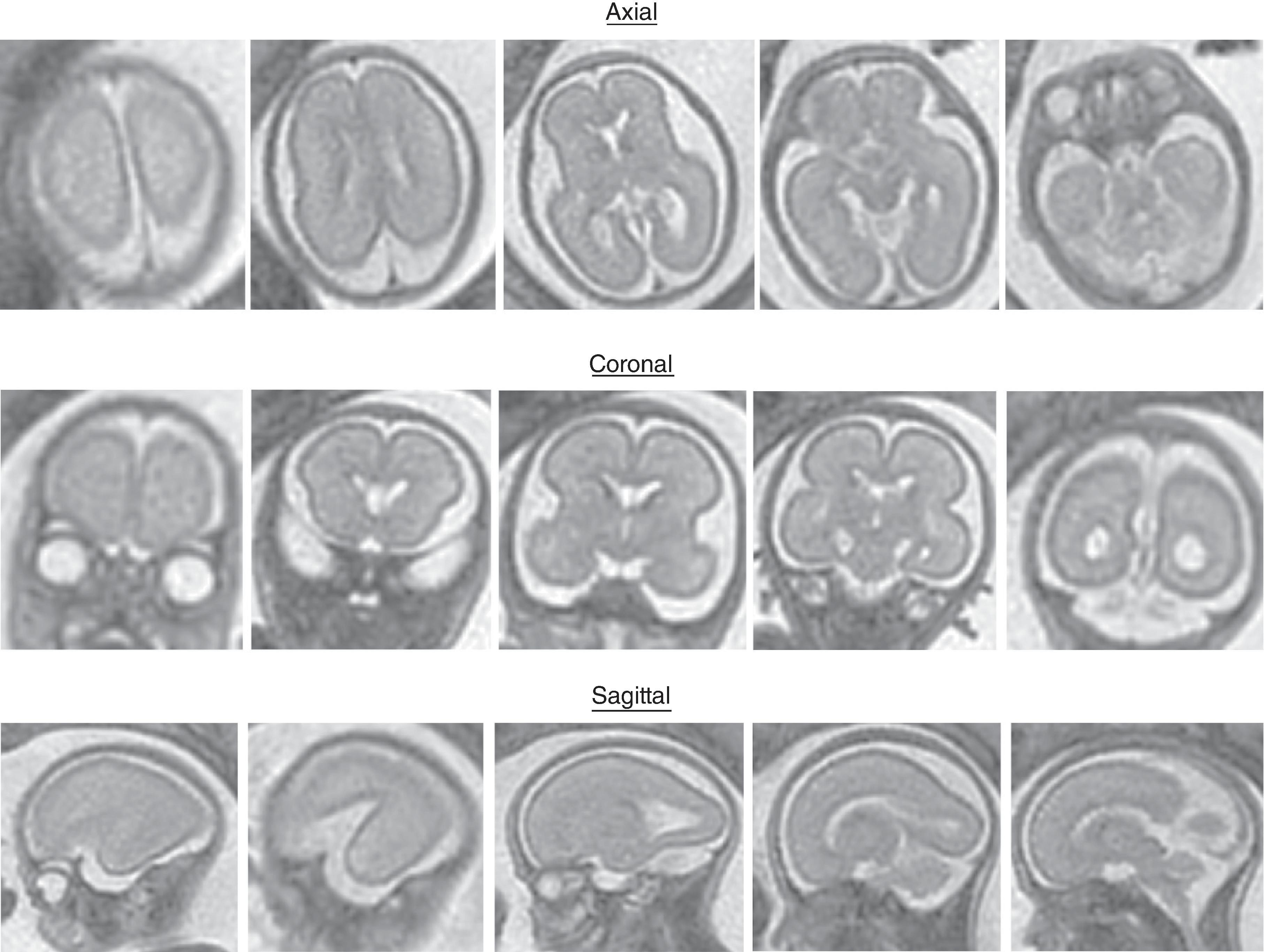
Sulci present: Sylvian, parietooccipital
Sulci developing: Calcarine, cingulate
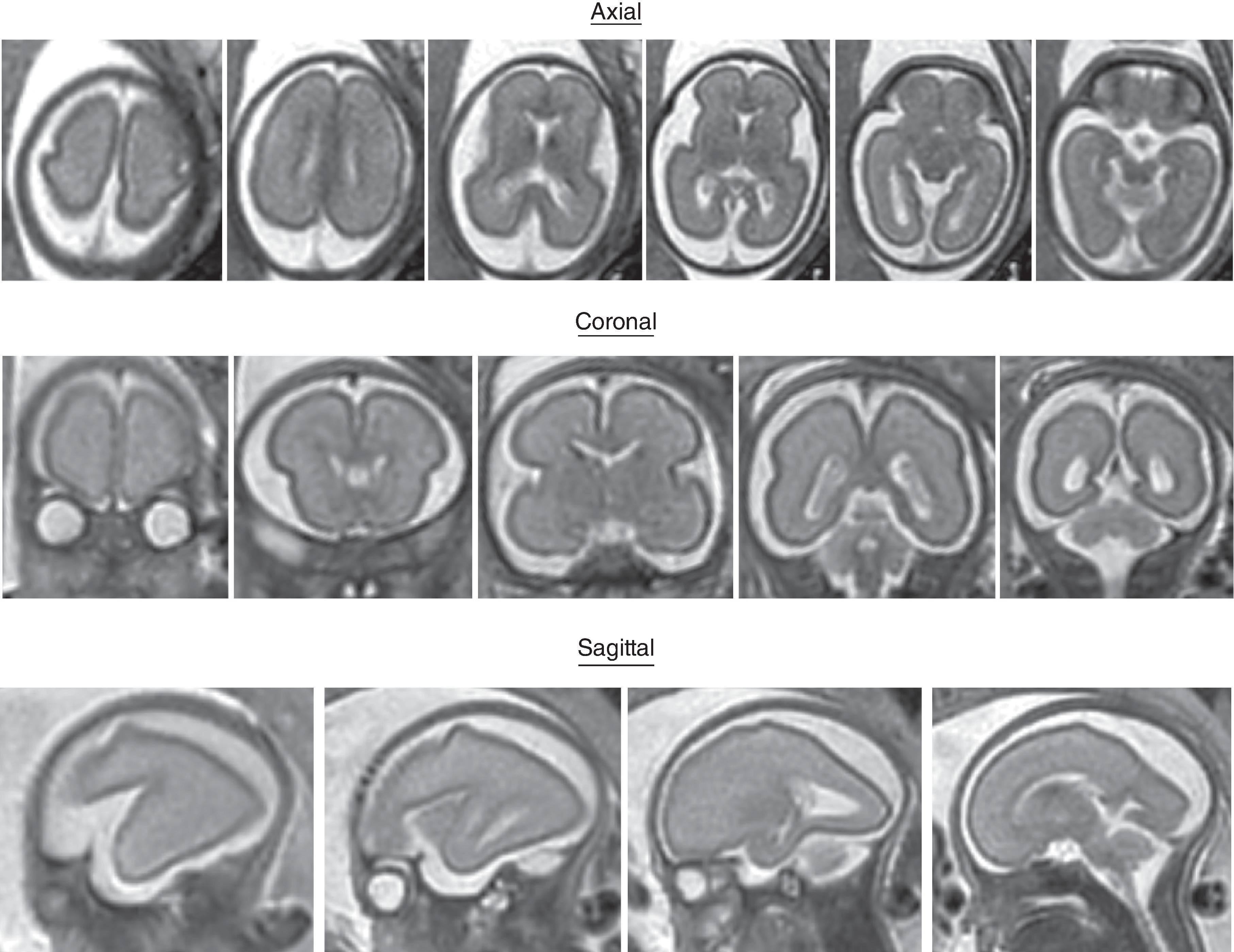
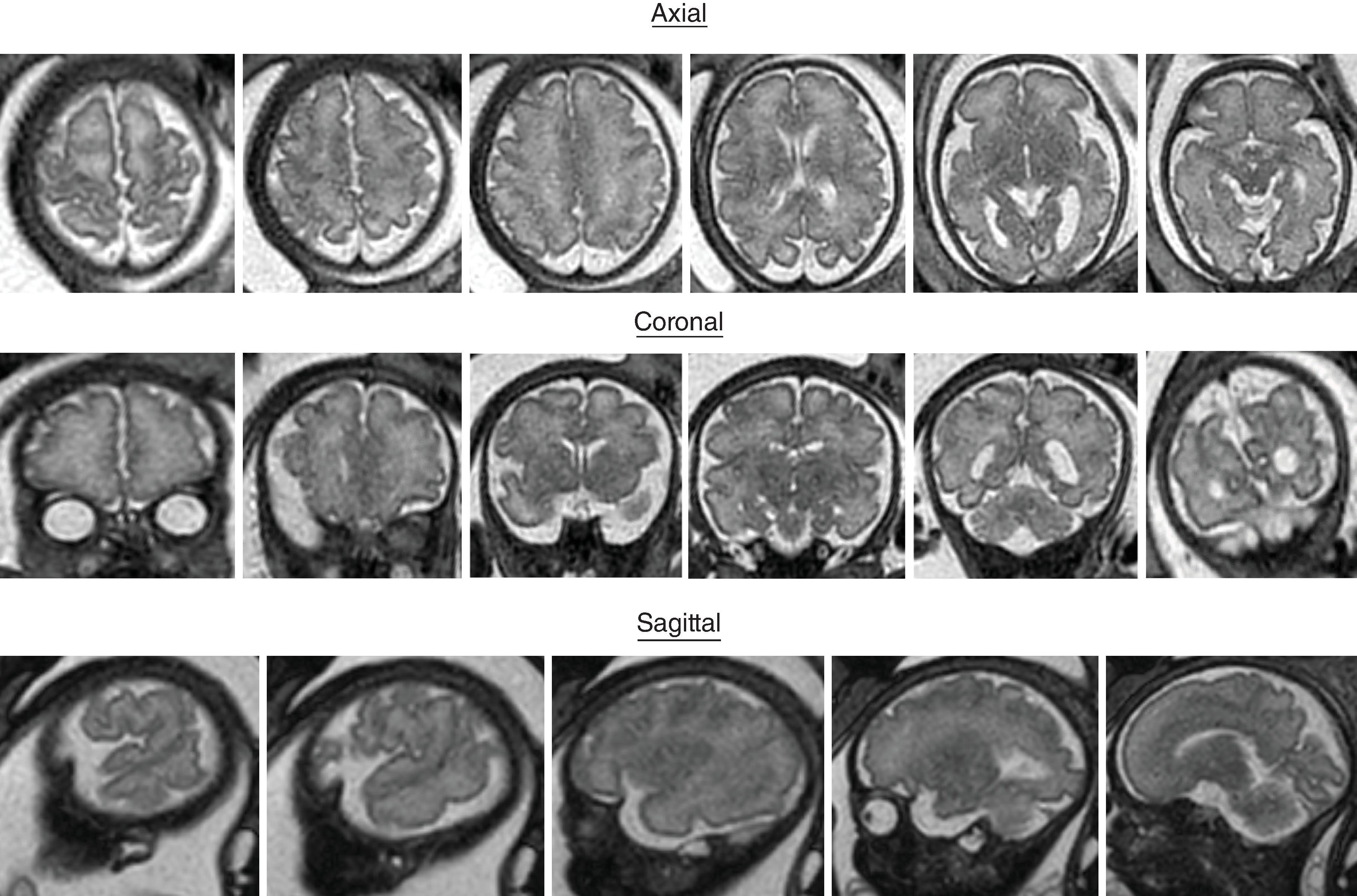
Sulci present: Sylvian, parietooccipital
Sulci developing: Calcarine, cingulate
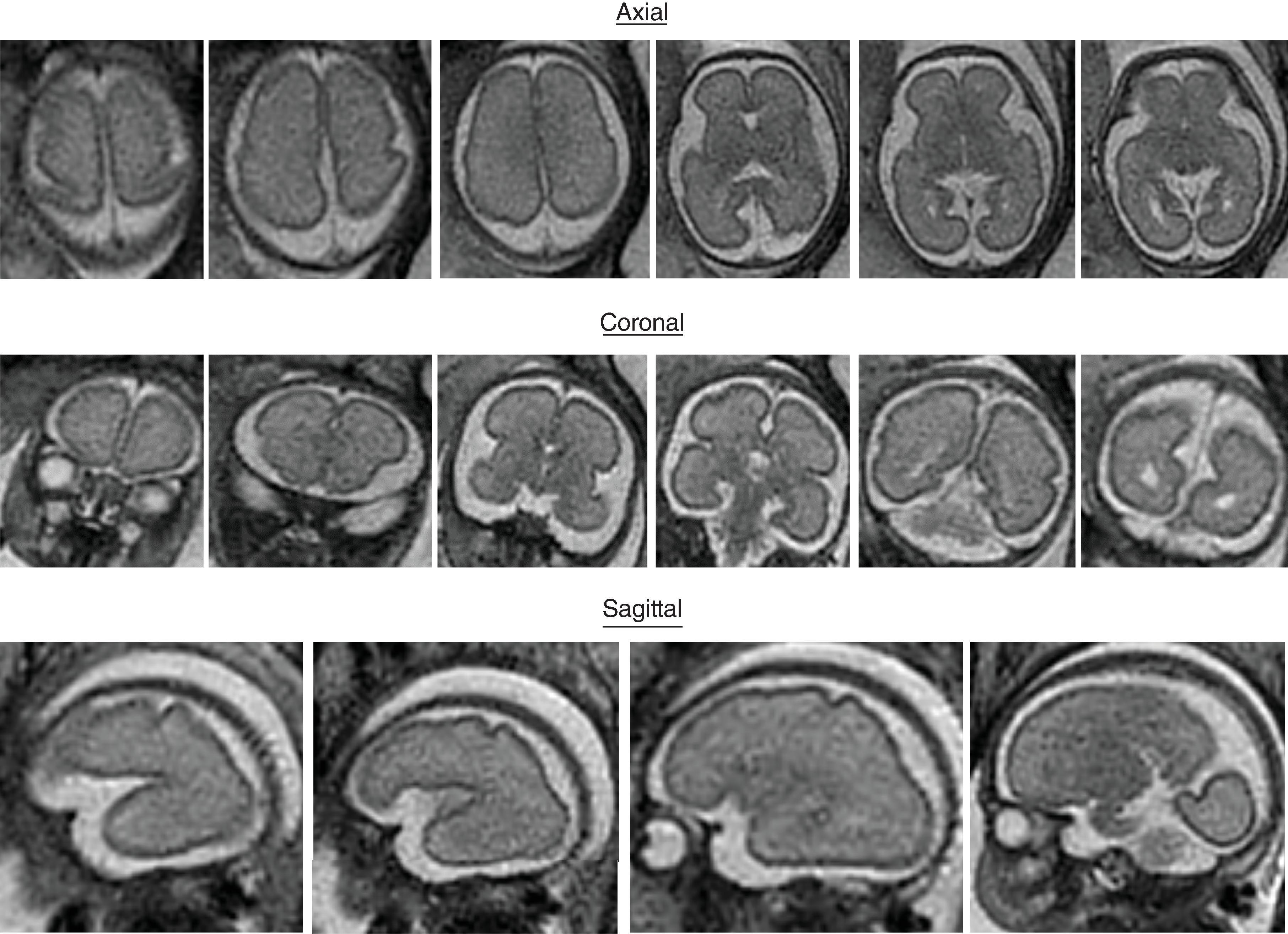
Sulci present: S ylvian, parietooccipital
Sulci developing: Calcarine, cingulate
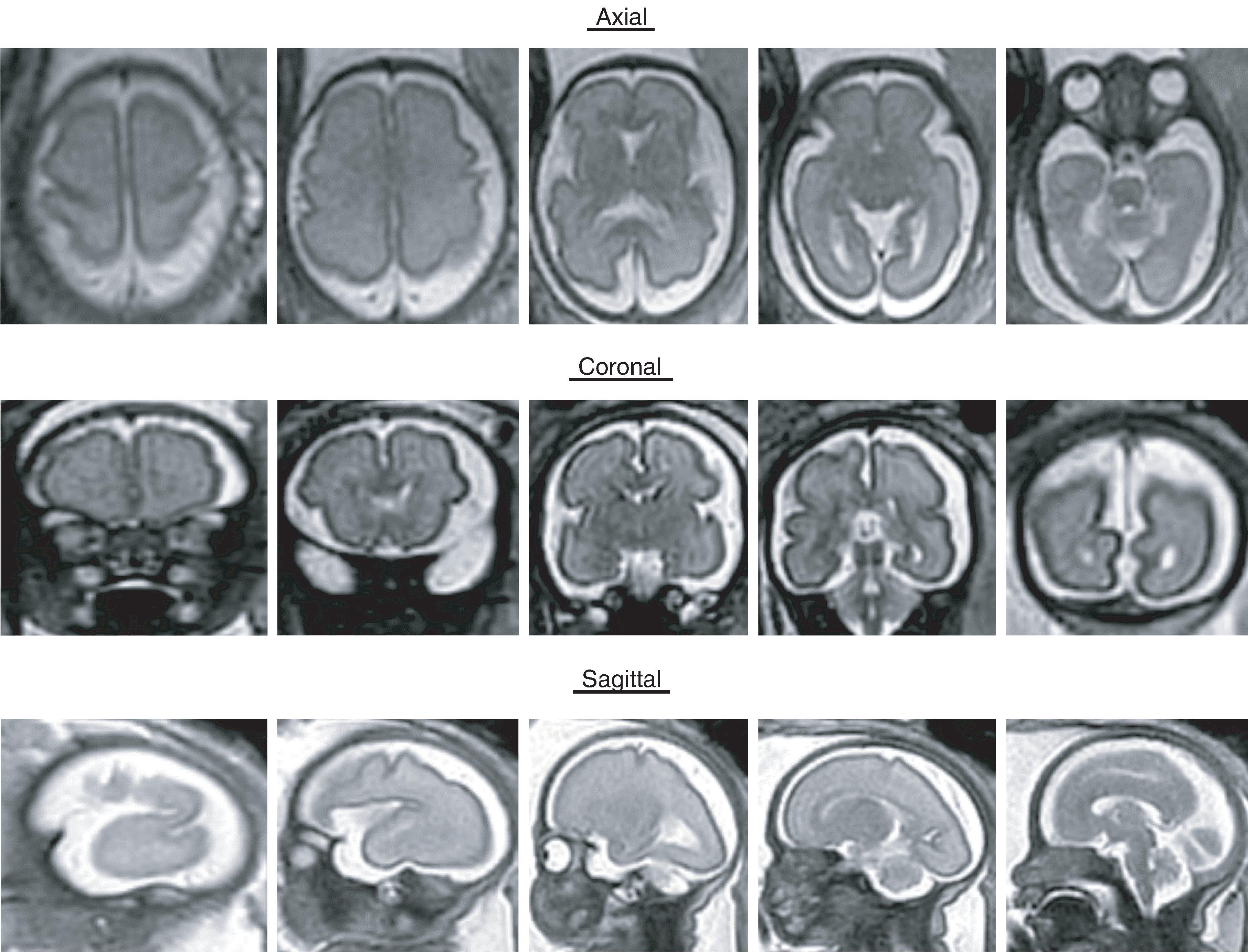
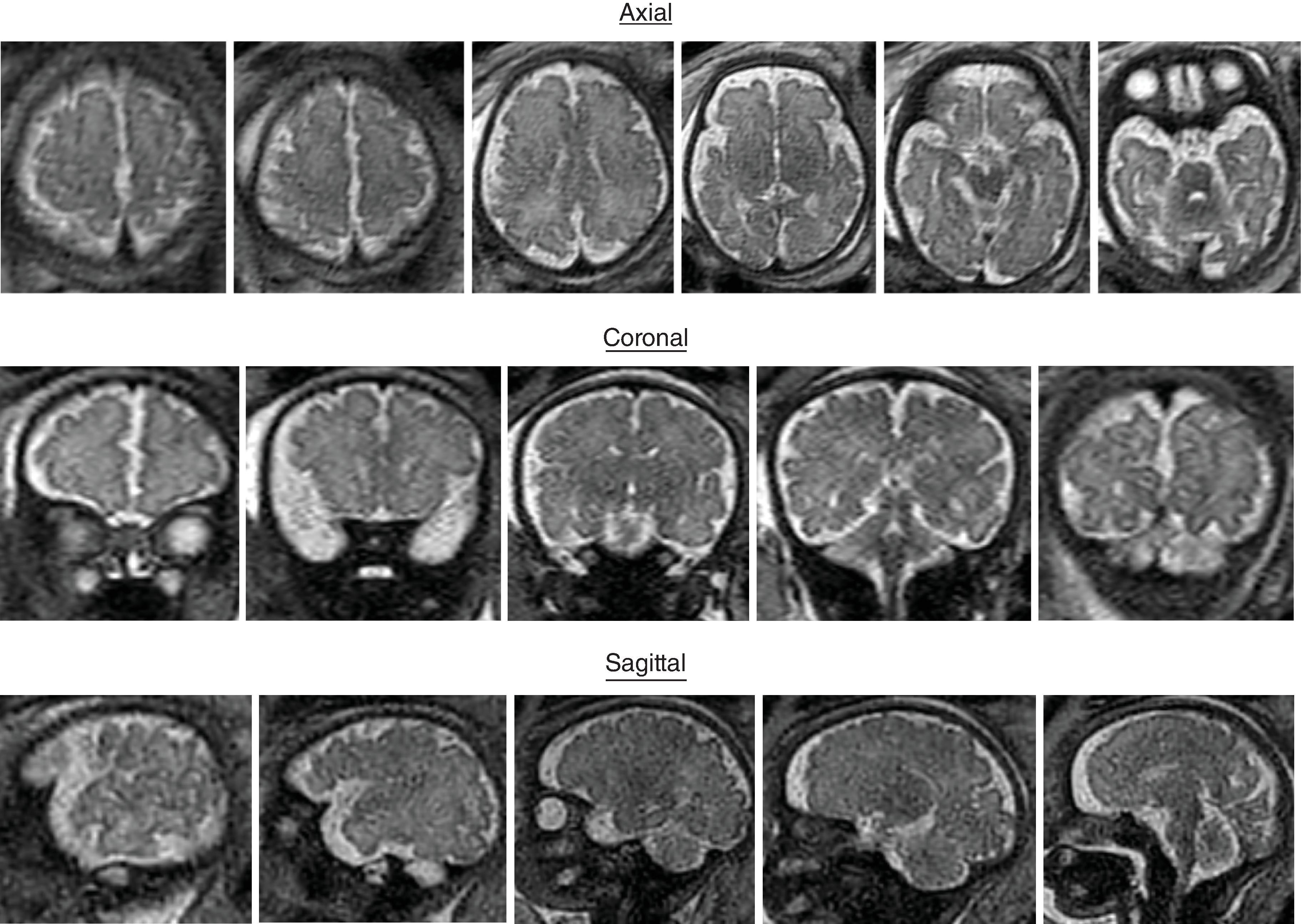
Sulci present: Sylvian, parietooccipital, calcarine, cingulate
Sulci developing: Central, interparietal, superior temporal, superior frontal, precentral
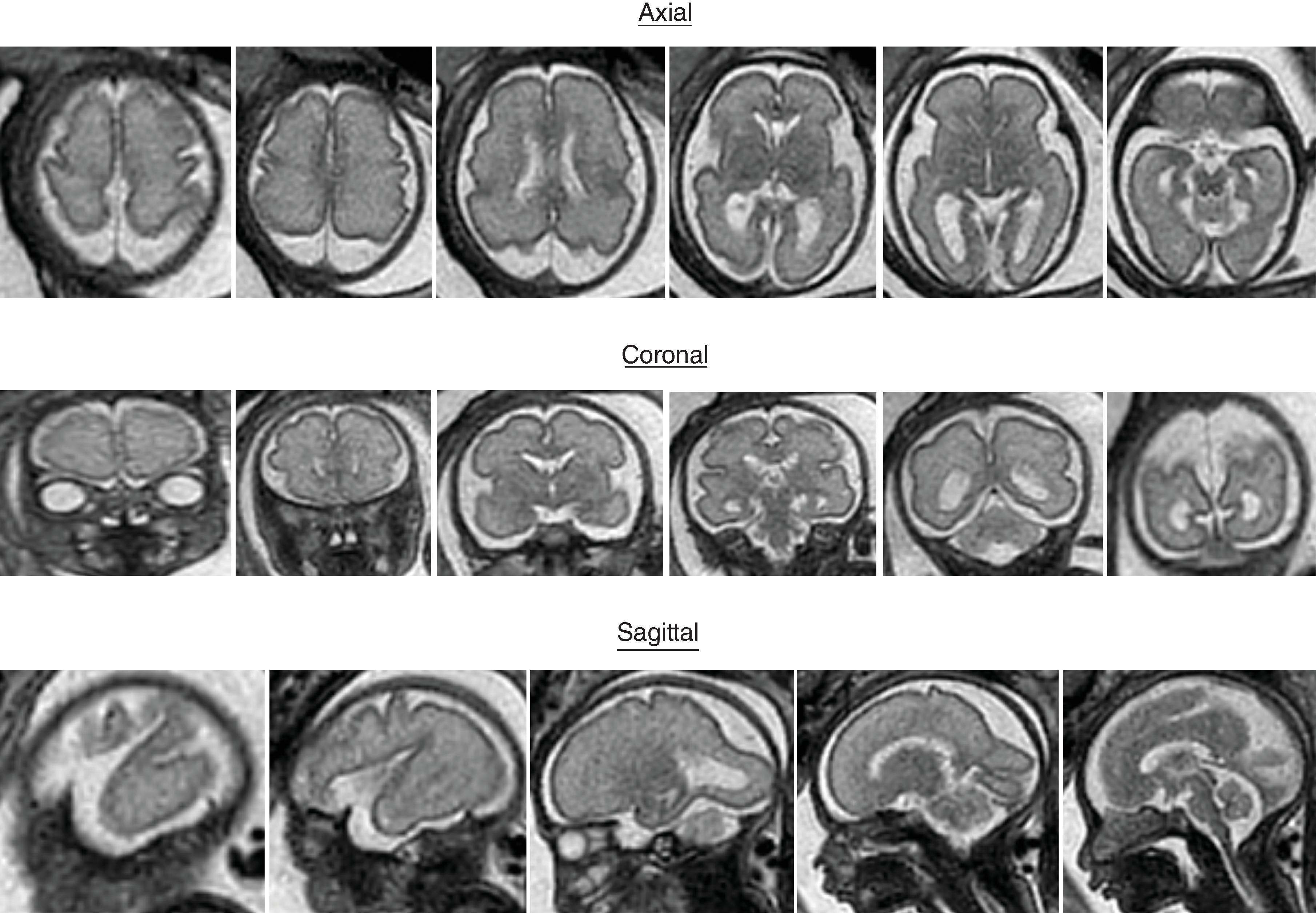
Sulci present: Sylvian, parietooccipital, calcarine, cingulate, central, interparietal, superior temporal, superior frontal, precentral
Sulci developing: Postcentral sulci
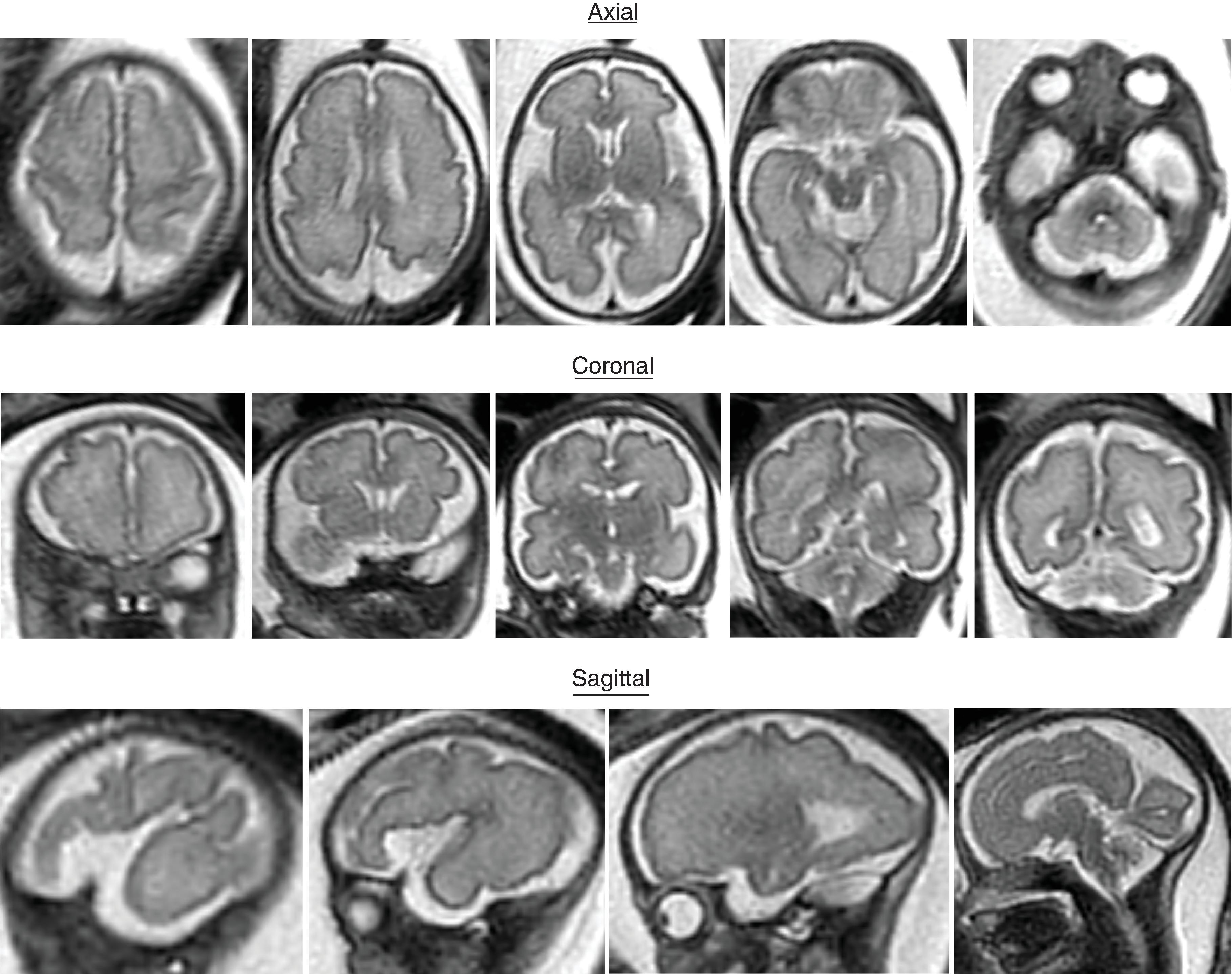
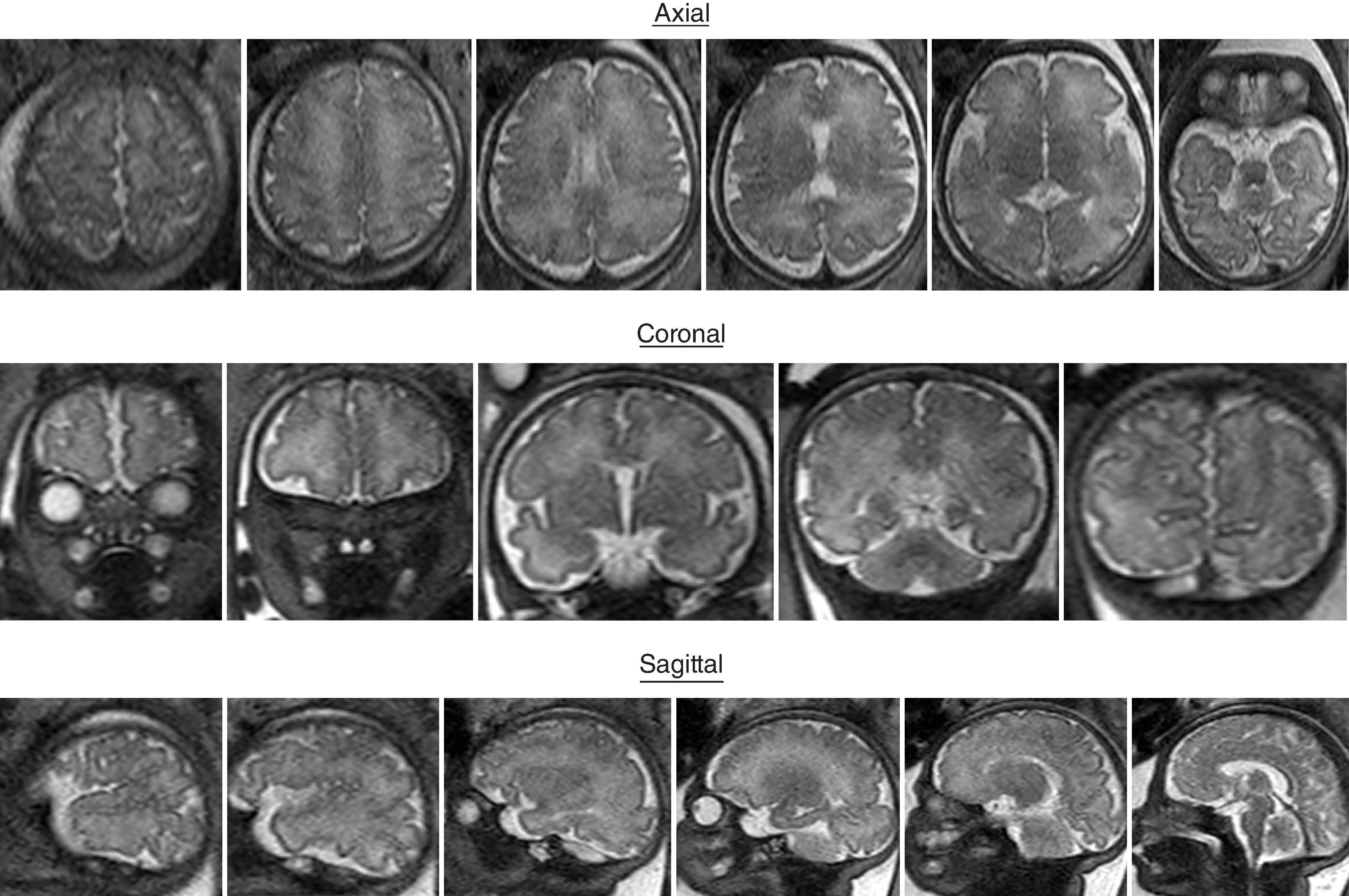
Sulci present: Sylvian, parietooccipital, calcarine, cingulate, central, interparietal, superior temporal, superior frontal, precentral
Sulci developing: Postcentral sulci
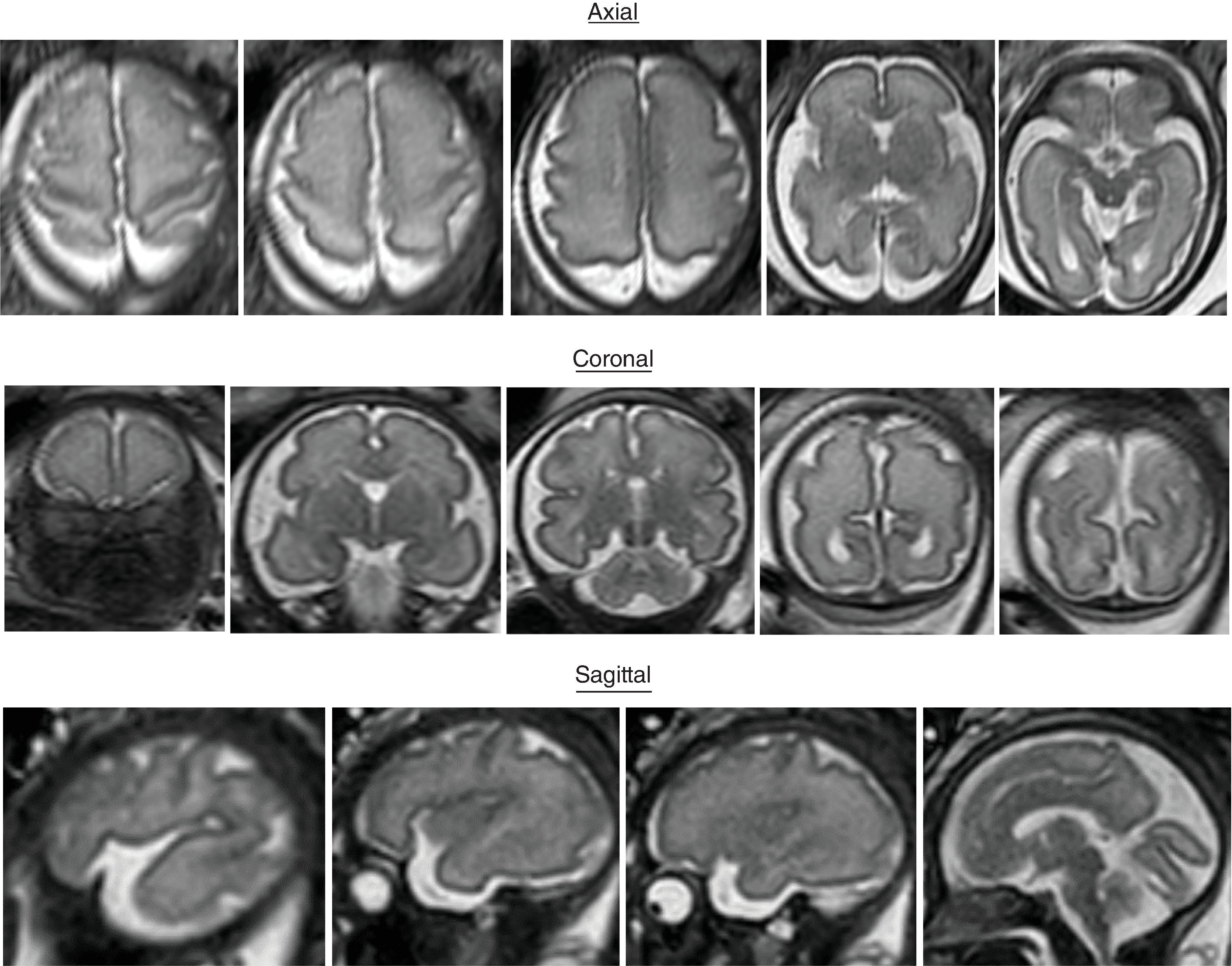
Sulci present: Primary sulci
Sulci developing: Secondary sulci

Sulci present: Primary sulci
Sulci developing: Secondary sulci
Sulci present: Primary sulci
Sulci developing: Secondary sulci
Sulci present: Primary sulci
Sulci developing: Secondary sulci
Sulci present: Primary sulci
Sulci developing: Secondary sulci
Sulci present: Primary sulci
Sulci developing: Secondary sulci

Myelination increases the transmission of an action potential 10–100× that of an unmyelinated axon. Myelination is important for normal brain development as it allows efficient transmission of nearly an infinite number of transmissions between neurons. Myelination also aids in axonal integrity and regulation of ion composition and fluid around the axon. Myelin consists of a lipid bilayer formed by an extension of the oligodendrocyte cell process, and multiple sheaths wrap around an axon. Myelin is composed of ∼70% lipid and 30% protein. Lipids in myelin include cholesterol, phospholipids, and glycosphingolipids. The main proteins in myelin include proteolipid protein (50%), myelin basic protein (30%), and phosphodiesterase (4%).
Myelination generally proceeds from inferior → superior, posterior → anterior, and central → peripheral and is best assessed using T1W and T2W imaging. Myelination can be seen on fetal MRI; however, because obtaining high-quality fetal MRI T1W imaging can be difficult, most of the changes are seen on T2W. In the postnatal brain, high-quality T1W and T2W brain imaging can be obtained. The T1W hyperintensity progresses faster than T2W hypointensity such that after the first year of life the brain will appear completely myelinated on T1W images but will only appear complete on T2W images between 3 and 4 years of age. This delay in T2 signal is due to the immature myelin containing greater water content than mature myelin. Histologically, the brain continues to myelinate up to approximately age 30.
Understanding the normal myelination pattern for age is necessary for detecting abnormalities that may manifest with abnormal development of myelination. For example, hypomyelination may indicate a metabolic disorder. Some disorders cause accelerated myelination, such as focal cortical dysplasia, hemimegalencephaly, and Sturge-Weber syndrome. Acute and subacute ischemic injuries can result in loss of normal myelin signal in areas in newborns such as in the posterior limb of the internal capsule. Last, age adjustment for weeks of prematurity should be performed when determining appropriate myelination in a premature infant.
The following section will illustrate the imaging appearance of the brain as myelination progresses to completion.
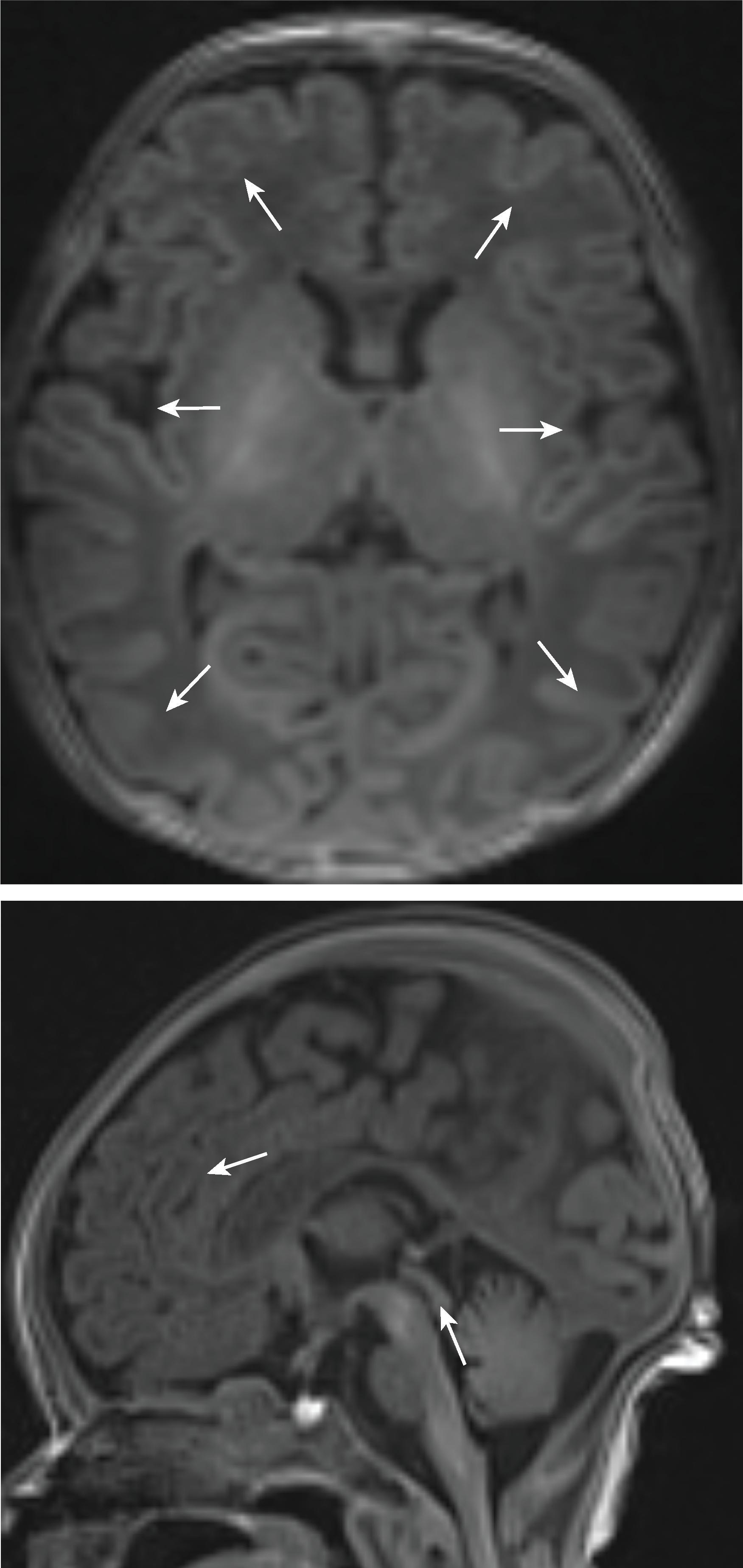
Myelination can be detected on fetal MRI. Myelination first occurs in the medulla at 18 weeks followed by the dorsal pons between 20 and 30 weeks and the midbrain at 32 weeks. After 32 weeks, myelination can be seen in the putamen, ventral lateral thalami, and posterior limb of the internal capsule .
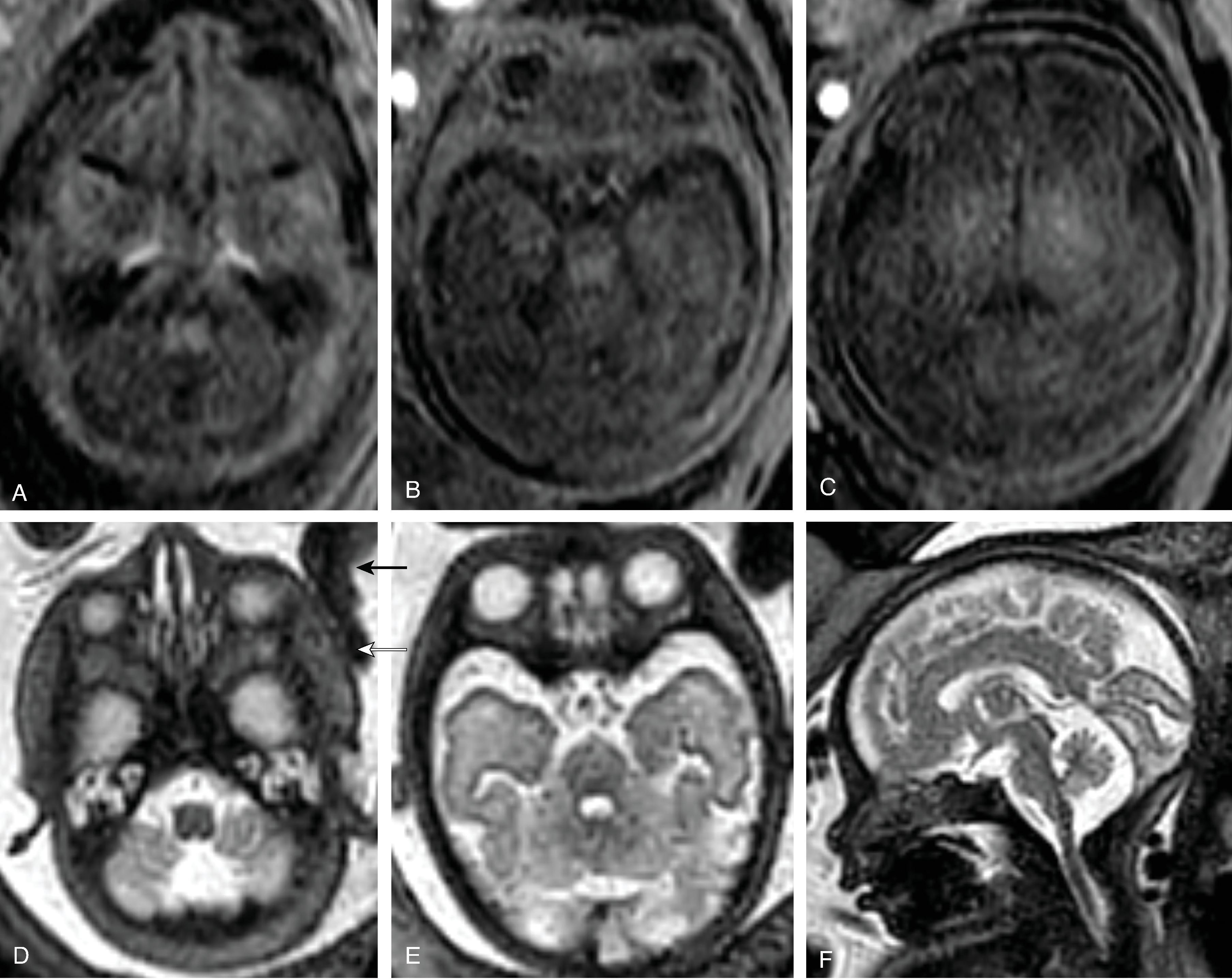
Between 15 and 28 weeks’ gestation, the fetal cerebral hemisphere have predominantly a tangential organization and prominent crossroads of projection, association, and commissural fibers.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here