Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The author thanks Dr. Susan E. Wert for her writing of this chapter for the fifth edition, which is the foundation for this updated chapter. The author would also like to thank Dr. Jamie Verheyden for assistance with this manuscript.
The respiratory system functions to provide oxygen from the external environment to the organism, while removing excess carbon dioxide from the blood. The respiratory system is divided into two parts, the upper and lower respiratory tracts. The upper respiratory tract is composed of the nasal cavity, sinuses, nasopharynx, and larynx (above the vocal fold), and it warms, moistens, and filters inspired air. The lower respiratory tract includes the larynx (below the vocal fold), trachea, bronchi, and bronchioles, and it distributes air throughout the alveolar region of the lung where exchange of oxygen and carbon dioxide occurs. Integral to respiration are the primary respiratory muscles, which include the intercostal muscles of the thoracic wall and the muscular thoracic diaphragm, which ventilate the lung, moving air in and out of the lung and across the respiratory surface. In addition, the blood vessels of the pulmonary circulation are an integral component of the lung, carrying deoxygenated blood from the heart to the lungs through the pulmonary arteries and returning oxygen-rich blood from the lung to the heart through the pulmonary veins. Appropriate specification of progenitors, patterning and alignment of tissues, differentiation and physiologic maturation of cell types, are all critical for efficient gas exchange and survival starting at birth. This chapter describes the development of the lung and its associated tissues, along with a review of the congenital malformations that arise from defects in pulmonary and vascular morphogenesis. Where known, chromosomal disorders and single-gene mutations associated with these malformations will be discussed.
Human lung development can be divided into five overlapping chronologic stages of organogenesis, which describe the structural and histologic changes that occur during morphogenesis and maturation of the lung. These stages are the embryonic, pseudoglandular, canalicular, saccular, and alveolar stages of lung development, which extend throughout gestation and into the postnatal period ( Table 55.1 ). Lung development begins during the early embryonic period of gestation (at a gestational age [GA] of 3 to 7 weeks) as a small saccular outgrowth, or diverticulum, of the ventral wall of the foregut endoderm. This region is marked by expression of the transcription factor NKX2-1 , which encodes thyroid transcription factor-1 (TTF1), a homeobox transcription factor critical for lung development. During the subsequent pseudoglandular stage of lung development (5 to 17 weeks’ GA), formation of the conducting airways, or tracheobronchial tree, occurs by a process called branching morphogenesis . This process involves rapid growth and repetitive and programmed branching of the epithelial-lined bronchial tubules until all of the branches of the tracheobronchial tree are formed. Outgrowth and branching of the bronchial tubules are dependent on interactions between the epithelium and the surrounding mesenchyme, which are facilitated by a number of pathways that signal across these tissue boundaries, including fibroblast growth factor (FGF), wingless-related integration site (WNT), transforming growth factor beta (TGFβ), bone morphogenetic protein (BMP), sonic hedgehog (SHH), and retinoic acid (RA) pathways. Transcription factors regulating gene expression during this period include NKX2-1, FOXA2, GATA6, and SOX2, among many others ( Fig. 55.1 ). By the end of this stage, the terminal bronchioles have divided into two or more respiratory bronchioles, which will subdivide again into small clusters of short acinar tubules and buds at the periphery of the lung. These peripheral structures will become the adult pulmonary acinus, consisting of the alveolarized respiratory bronchiole, alveolar duct, and alveolus.
| Developmental Stage | Major Developmental Events |
|---|---|
| Embryonic | Lung bud arises from ventral foregut endoderm |
| 3–7 wk GA | Branching morphogenesis initiated |
| Primary, secondary, and tertiary bronchi form | |
| Trachea and esophagus separate | |
| Pulmonary arteries bud off sixth pair of aortic arches | |
| Pulmonary veins develop as outgrowths of left atrium | |
| Autonomic innervation extends to trachea and bronchi | |
| Pseudoglandular | Branching morphogenesis continues and completes |
| 5–17 wk GA | Formation of tracheobronchial tree complete by 17 wk |
| Cartilage and mucus glands develop in trachea and bronchi | |
| Airway smooth muscle extends to respiratory bronchioles | |
| Ciliated, mucus, club, neuroendocrine, basal cells differentiate | |
| Respiratory bronchioles, acinar tubules/buds form in distal lung | |
| Pulmonary arterial development parallels airway branching | |
| Pulmonary lymphatics arise from veins/invest bronchi and vessels | |
| Pulmonary veins and lymphatics extend into interlobular septa | |
| Capillary blood vessels form in distal mesenchyme | |
| Autonomic innervation parallels airway branching | |
| Pleuroperitoneal cavity and diaphragm closes | |
| Canalicular | Acinar tubules/buds lengthen, subdivide, and widen |
| 16–26 wk GA | Mesenchyme thins/condenses |
| Primitive alveolar capillary network/blood-air barrier forms | |
| Type I/type II AECs differentiate | |
| Surfactant synthesized in lamellar bodies by type II AECs | |
| Fetal lung fluid production increases | |
| Fetal breathing-like movements initiated | |
| Saccular | Distal airspaces continue to branch and grow |
| 24–38 wk GA | Acinar tubules/buds expand to form fluid-filled saccules |
| Mesenchyme spreads to form alveolar septal walls | |
| Alveolar septa contain double capillary network | |
| Elastin deposited at sites of alveolar septal crest formation | |
| Type I AECs flatten and elongate | |
| Surfactant synthesized and secreted by type II AECs | |
| Ex utero breathing/gas exchange feasible | |
| Alveolar | Alveolar surface area available for gas exchange increases |
| 36 wk GA—8 yr | Secondary alveolar septa subdivide saccules into true alveoli |
| Alveolar septal walls thin with loss of connective tissue | |
| Microvasculature fuses into single capillary network | |
| Subset of interstitial fibroblasts differentiate into myofibroblasts | |
| Collagen, elastin, and fibronectin deposited | |
| Surfactant production increases in type II AEC | |
| Pulmonary vascular resistance decreases at birth |
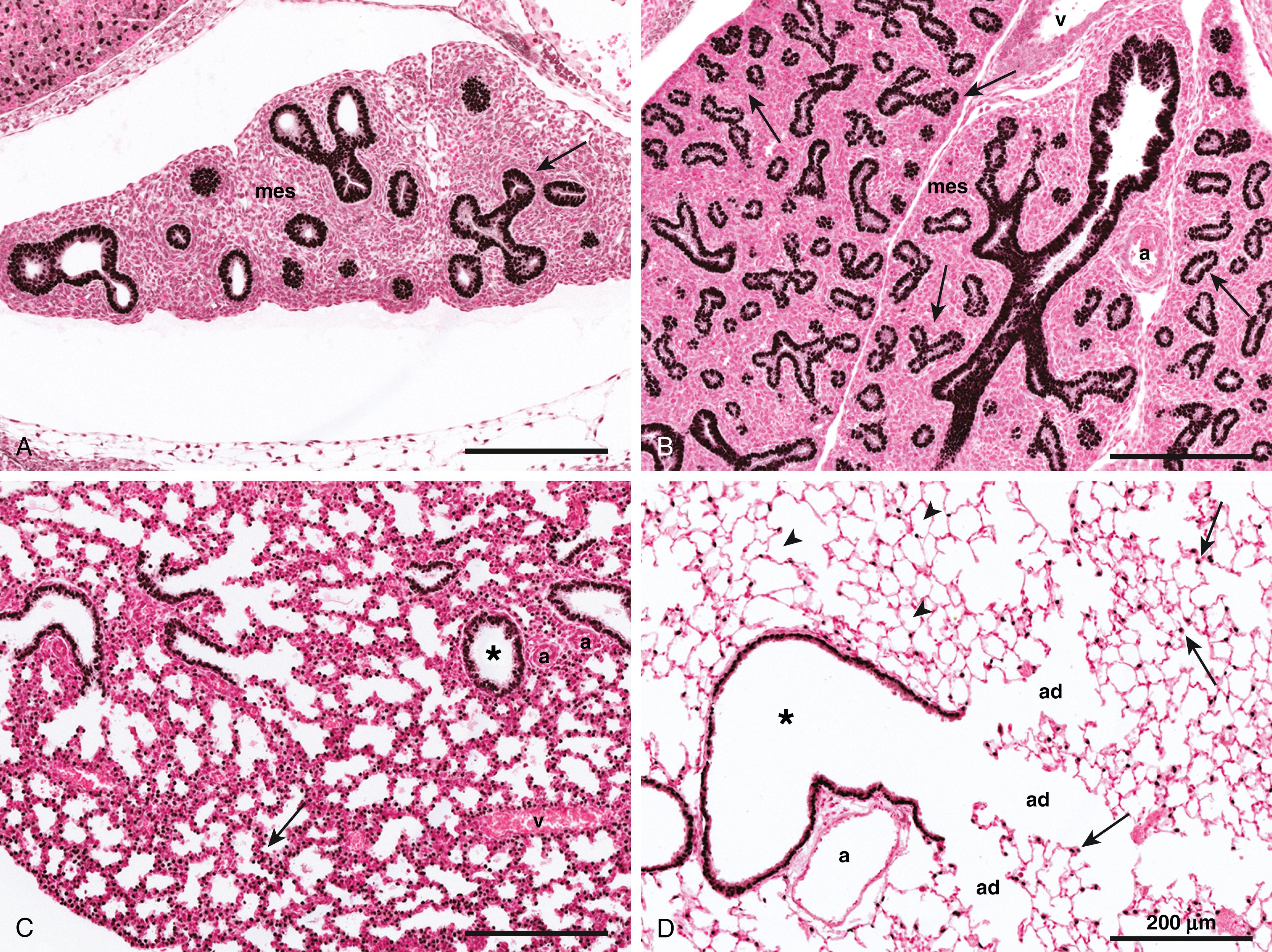
During the canalicular stage of lung development (16 to 26 weeks’ GA), increased vascularization of the surrounding mesenchyme with formation of the peripheral intra-acinar capillary bed gives rise to the blood-air barrier, or alveolar-capillary membrane, of the gas-exchange region of the lung. Molecular pathways important for vascularization during this stage include the vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), TGFα and TGFβ signaling pathways, as well as components of the extracellular matrix (ECM). In addition, differentiation of type 1 and type 2 alveolar epithelial cells (AEC1s and AEC2s) initiated during this period of lung development. NKX2-1, FOXA2, and GATA6 continue to be important transcriptional regulators of target genes involved in AEC differentiation, as well as in surfactant synthesis and fluid and electrolyte transport at this stage (see Fig. 55.1 ).
Enlargement and expansion of the peripheral acinar tubules during the saccular stage of lung development (24 to 38 weeks’ GA) result in formation of primitive sac-like alveoli with thick inter-alveolar septa. Thinning of these septa and remodeling of the alveolar-capillary membrane occur during the alveolar stage of lung development (36 weeks’ GA to 8 years of age), giving rise to mature alveolar organization of the adult lung. This process of alveologenesis extends into the postnatal period, during which millions of additional alveoli are formed, greatly increasing the surface area of the lung available for gas exchange ( Table 55.2 ). Molecular mechanisms that are important for alveolarization and differentiation of the alveolar epithelium during these last two stages include the PDGF, FGF, Hippo/yes-associated protein (YAP) signaling pathways, as well as steroid hormone pathways such as glucocorticoid and thyroid hormone receptors (see Fig. 55.1 ).
| Term | Adult | Fold Change | |
|---|---|---|---|
| Lung weight (mean) | 50 g | 800 g | ∼16 |
| Lung volume (mean) | 150–200 mL | 4–6 × 10 3 mL | ∼25–30 |
| Alveolar (Alv) surface area (SA) a | 3–5 m 2 | 75–100 m 2 | ∼20–25 |
| SA/kg (mean) | 0.4 m 2 | 1 m 2 | ∼2.5 |
| Alv number (mean) a , b | 150 × 10 6 | 480 × 10 6 | ∼3 |
| Alv number (range) a , b | 110–174 × 10 6 | 274–790 × 10 6 | ∼2.5–4.5 |
| Alv diameter (mean) | 150 μm | 300 μm | ∼2 |
| Alv septal wall thickness (mean) | 5 μm | 2.5 μm | ∼2 |
| Air-blood barrier (mean) | 0.6 μm | 0.6 μm | 0 |
| Tracheal length (mean) | 26 mm | 184 mm | ∼7 |
| Main bronchial length (mean) | 26 mm | 254 mm | ∼10 |
a Hislop AA, Wigglesworth JS, Desai R. Alveolar development in the human fetus and infant. Early Hum Dev . 1986;13:1–11.
b Ochs M, Nyengaard JR, Jung A, et al. The number of alveoli in the human lung. Am J Respir Crit Care Med . 2004;169:120–124.
Although definitive alveoli can be found in the human lung by 36 weeks’ GA, greater than 85% to 90% of all alveoli are formed within the first 6 months of life. After 6 months, alveolar formation continues at a slower pace until at least 8 years of age. A more recent study presented helium imaging data demonstrating that new alveoli may be added into the early 20-years-of-age period. Overall, the number of alveoli increases from an average of 150 million alveoli (ranging from 110 to 174 million) in the term lung to 480 million alveoli (ranging from 274 to 790 million) in the adult human lung. , , , Likewise, the conducting airways increase in length and diameter (see Table 55.2 ), whereas the diffusion capacity and surface area of the alveolar parenchyma increase linearly with body weight up to about 18 years of age.
The lung is a derivative of the definitive foregut endoderm and the adjacent mesoderm. , The respiratory primordium of the lung first appears at day 22 GA as an enlargement of the caudal end of the laryngotracheal sulcus, which is located in the medial pharyngeal groove, an outgrowth of the ventral wall of the definitive foregut endoderm. The primitive respiratory diverticulum, or lung bud, appears at day 26 GA, when the embryo is only about 3 mm long, and grows ventrocaudally into the mesoderm surrounding the foregut in a position that is anterior and parallel to the primitive esophagus. Epithelial cells of the primitive respiratory diverticulum invade the surrounding mesoderm, or splanchnic mesenchyme. At day 28 GA, the respiratory diverticulum bifurcates into right, and left primary bronchial buds, which will become the main stem, or primary, right and left bronchi. The region proximal (or superior) to the first bifurcation becomes the trachea and larynx. Shortly thereafter, the trachea and the esophagus begin to separate into two distinct structures.
A second round of branching occurs during the fifth week of gestation (days 33 to 41 GA) to yield three secondary (or lobar) bronchial buds on the right and two on the left, which will become the primary lobes of the right and left lung. During the sixth week of gestation (days 41 to 44 GA), a third round of branching yields 10 tertiary (or segmental) bronchi on the right and 8 to 9 on the left. These will become the bronchopulmonary segments of the mature lung. During this period, there is extensive proliferation of both epithelial and mesenchymal cells, with focal apoptosis in the mesenchyme around bronchial branch points and in regions of new bronchial bud formation.
At this stage of development, the mesenchyme is composed of a loose arrangement of primitive cells that will at a later stage give rise to the pulmonary vascular plexus, airway and vascular smooth muscle, cartilage, and other fibroblast-like cells. The ECM is composed primarily of hyaluronic acid, whereas the basement membrane underlying the epithelium contains type IV collagen, laminin, and fibronectin. The trachea and bronchial tubules are lined by pseudostratified columnar epithelium, composed of relatively primitive and undifferentiated epithelial cells. At the end of this stage, the lung resembles a small tubuloacinar gland, and separation of the trachea and esophagus is complete.
Autonomic innervation of the lung is derived from the ectoderm, neural plate, and associated neural crest cells, which migrate through the mesoderm to take up positions in the walls of the trachea and lung buds before separation from the esophagus. Ganglion cells appear in the mesenchyme around the trachea by 7 weeks of gestation. As growth proceeds, these cells form segmental ganglia and nerve fibers that develop into primitive neural plexuses, encircling the trachea and extending as far as the main stem and lobar bronchi.
Vascular connections with the right and left heart are established at the end of the embryonic stage (5 to 8 weeks’ GA), creating the primitive pulmonary vascular circulation. , The pulmonary arteries arise from the sixth pair of aortic arches and grow into the surrounding mesoderm, where they accompany the developing airways, segmenting with each bronchial subdivision, and then anastomose with the vascular plexus developing in the pulmonary mesenchyme around the bronchial buds. During fetal life, the pulmonary artery is connected to the aortic arch by the ductus arteriosus, which enables the right ventricular output from the heart to bypass the pulmonary vascular bed. The pulmonary veins originate from the left atrium and grow into the surrounding mesoderm, dividing several times before connecting to the pulmonary vascular bed.
Developmental abnormalities that arise during the embryonic stage of lung development are related to lung bud formation, separation of the trachea and esophagus, formation of the proximal conducting airways, and initial lobe formation. These abnormalities include laryngeal, esophageal, tracheal, and bronchial atresia; tracheal and bronchial stenosis; tracheo- and bronchoesophageal fistulas; pulmonary agenesis; bronchogenic cysts; ectopic lobes; and extralobar pulmonary sequestration.
The pseudoglandular stage of fetal lung development is marked by the formation of the bronchial portion of the lung. This occurs through a process during which the segmental tubules of the developing lung undergo a set program of branching morphogenesis to form the primitive bronchial tree. Expansion of the bronchial tubules is due to rapid proliferation of their epithelial cells, particularly at the ends of the tubes, or bronchial buds. By the end of this stage, formation of the conducting airways, including the terminal bronchioles, is complete (17 weeks), with 12 to 17 generations of bronchial tubules in the upper lobes, 18 to 23 in the middle lobes, and 14 to 23 in the lower lobes. , Numerous acinar tubules and buds, which give rise to the adult pulmonary acinus, are formed in the periphery of the lung, arising as distal branches of the respiratory bronchioles. , By the end of this stage, the pseudoglandular lung is composed of millions of epithelial tubules surrounded by relatively extensive regions of mesenchyme, which gives it a distinct glandular appearance.
The bronchial tubules are lined initially by a pseudostratified columnar epithelium. These cells are morphologically undifferentiated and contain large pools of intracellular glycogen, deriving most of their energy needs from anaerobic glycolysis. A prominent basement membrane, rich in laminin and collagen type IV, underlies the epithelium. Mesenchymal cells adjacent to these tubules differentiate into smooth muscle cells starting at 7 weeks’ GA, aligning themselves in a circumferential orientation perpendicular to the long axis of the tubules. The ECM is composed of various types of glycosaminoglycans and proteoglycans, such as chondroitin, dermatan, and heparan sulfate, as well as laminin, fibronectin, tenascin, entactin, and type I and type III collagen. , These macromolecules are important for cell proliferation, migration, adhesion, and differentiation during lung development.
As branching progresses, the pseudostratified columnar epithelium is reduced to a tall columnar epithelium in the proximal airways and to a cuboidal epithelium in the distal acinar tubules and buds. Differentiation of the conducting airway epithelium occurs with ciliated, club (serous), goblet (mucus), neuroendocrine, and basal cells appearing first in the more proximal airways. , , Isolated neuroendocrine cells, the first bronchial epithelial cells to differentiate, can be detected in the proximal airways by 8 to 9 weeks’ GA. Clusters of neuroendocrine cells, called neuroepithelial bodies , are detected by 9 to 10 weeks’ GA. These are located at branch points along the bronchial tree and are innervated by parasympathetic, sympathetic, and sensory nerve fibers. Ciliated cells appear in the epithelium of the trachea by 10 weeks’ GA, in the main stem bronchi by 12 weeks’ GA, and in the segmental bronchi by 13 weeks’ GA. Whereas mucus cells are present in the epithelium by 13 weeks’ GA, submucosal glands appear in the trachea by 11 to 12 weeks’ GA and in the bronchi by 13 weeks’ GA, with active mucus production by 14 weeks’ GA. , Epithelial cell differentiation is initiated in the distal acinar structures with the onset of surfactant protein B (SFTPB) and C (SFTPC) expression. These two hydrophobic lung-specific surfactant components are expressed selectively in the distal respiratory epithelium by 12 to 14 weeks of GA.
Cartilage appears in the trachea and bronchi by 10 weeks’ GA and in the segmental bronchi by 16 weeks. By the end of this developmental stage, cartilaginous structures extend as far as the segmental bronchi, and airway smooth muscle extends as far as the respiratory bronchioles. Spontaneous and peristaltic contraction of fetal airway smooth muscle can be observed in cultured human fetal lung explants at this stage of development. The smooth muscle layer enveloping the conducting airways is invested by an extensive network of neural ganglia and nerve bundles, which is detected by 7.5 to 8 weeks’ GA. Well-defined neural plexuses can be found between the cartilage and the tracheobronchial epithelium, with nerve fibers extending to the submucosal glands and trachealis muscle; ganglia are found at bronchial bifurcations and in the adventitia of smaller bronchi. Elastic fibers are detected in the walls of the trachea and the main stem bronchi, pleura, and pulmonary artery.
During this period, the pulmonary arterial system develops along with the bronchial and bronchiolar tubules, branching in parallel with these airways by angiogenesis . , , The pulmonary veins and lymphatic vessels take a different pathway through the lung, coursing through the interlobular connective tissue septa that surround each pulmonary lobule. Between the arteries and veins the preacinar, or capillary, blood vessels are formed. The pulmonary arteries gradually become invested with smooth muscle cells, so that smooth muscle actin and myosin can be detected in all of the vasculature by the end of this developmental stage. , Intrapulmonary bronchial arteries, which are supplied by the descending aorta, extend along the airways in parallel with cartilage formation. ,
Little is known about the ontogeny of the pulmonary lymphatic vessels, but these vessels appear to originate by budding, or sprouting, from the cardinal veins. Progenitor cells in these sprouts then proliferate and migrate into the lung mesenchyme to form primitive lymphatic sacs adjacent to the hilar bronchi. During the pseudoglandular period, the lymphatic system expands, forming an extensive network around the bronchi and pulmonary vessels, and extends to the pleura.
A variety of congenital defects in branching morphogenesis may arise during the pseudoglandular stage of lung development, including tracheo- and bronchomalacia, intralobar bronchopulmonary sequestration, congenital pulmonary airway malformations, acinar aplasia or dysplasia, pulmonary hypoplasia, pulmonary lymphangiectasis, and other pulmonary vascular malformations. The pleuroperitoneal cavity also closes during the pseudoglandular period (6 weeks’ GA), forming the diaphragm. Failure to close the pleural, or thoracic, cavity is often accompanied by herniation of the abdominal contents into the thorax (congenital diaphragmatic hernia), which limits the space available for further growth of the lung, causing pulmonary hypoplasia.
The canalicular stage is so named because of the appearance of vascular canals, or capillaries, that multiply in the interstitial compartment to form the blood-air barrier, or alveolar-capillary membrane, the future gas-exchange surface of the lung. Development of the alveolar-capillary membrane, along with the synthesis and secretion of pulmonary surfactant, is critical for extrauterine survival of the immature fetus, if delivered prematurely near the end of this stage. Gas exchange cannot occur in the premature infant unless these capillaries are close enough to the adjacent alveolar epithelium for optimal gas diffusion to take place across their surfaces. Rapid expansion of the capillary bed, with condensation and thinning of the mesenchyme, is the first critical step in the formation of the gas-exchange regions of the lung. During this stage of lung development, the total surface area of the alveolar-capillary membrane increases exponentially with a concomitant decrease in the mean mesenchymal wall thickness, thereby increasing the potential for gas exchange in the immature lung. Disturbances in this stage of lung development result in severe hypoxemia and are not compatible with life after birth.
At the beginning of the canalicular stage, branching morphogenesis and formation of the bronchial tree is complete, and the terminal bronchioles have divided into two or more respiratory bronchioles that have subdivided further into small clusters of short acinar tubules and buds lined by cuboidal epithelium. These structures undergo further differentiation to become the adult respiratory unit, or pulmonary acinus, consisting of two to four alveolarized respiratory bronchioles, each ending in six to seven generations of branched alveolar ducts and alveoli. Clusters of acinar tubules and buds grow by lengthening, subdividing, and widening at the expense of the surrounding mesenchyme. The proportion of epithelial and endothelial cells increases, and the proportion of interstitial fibroblasts decreases. Apoptosis of cells in the interstitial tissue contributes to mesenchymal involution and thinning of the alveolar septa at this stage. Epithelial growth drops in the larger airways and cell proliferation occurs predominantly in the peripheral acinar tubules and buds. This peripheral growth is accompanied by the growth and development of inter-acinar capillaries, which align themselves around the air spaces, establishing contact with the adjacent epithelium to form the primitive alveolar-capillary membrane.
Epithelial cell differentiation becomes increasingly complex and is especially apparent in the distal regions of the lung parenchyma, where type I and type II AECs can be detected. Bronchiolar cells begin to express differentiated features and to synthesize cell-specific secretory proteins, such as secretoglobin 1A1. Cuboidal type II AECs lining the distal acinar tubules express increasing amounts of surfactant proteins and phospholipids. , Nascent lamellar bodies, the storage form of pulmonary surfactant, are seen in association with rich glycogen stores in cuboidal pre–type II AECs lining the acinar tubules and buds. Cells of the proximal acinar tubules become flattened and attenuated, acquiring features of typical squamous type I AECs. Type I AEC differentiation occurs in conjunction with formation of the alveolar-capillary membrane—that is, wherever endothelial cells of the developing capillary system come into contact with adjacent acinar epithelial cells. Where this occurs, the intercellular junctional complexes, originally localized around the epithelial cell apex, shift to the basolateral aspect of the intercellular cleft. The cells develop thin cytoplasmic attenuations, differentiating into squamous type I AECs and losing features previously associated with pre–type II AECs.
By the end of the canalicular stage of lung development, fully differentiated mucus and ciliated cells are found in the conducting airways, and cartilage, submucosal glands, and smooth muscle extend as far down the airway as they do in the adult lung. The epithelial cells are capable of producing fetal lung fluid, and the primitive alveolar-capillary membrane is thin enough to support gas exchange, although pulmonary surfactant production is low. Disturbances in this stage of lung development often result in severe hypoxemia and are incompatible with life after birth. Abnormalities of lung development associated with the canalicular stage include diverse causes of congenital alveolar dysplasia and pulmonary hypoplasia, which usually lead to severe respiratory insufficiency shortly after birth. In addition to genetic causes, acquired forms of pulmonary hypoplasia include (1) congenital diaphragmatic hernia, (2) compression from thoracic or abdominal masses, (3) decreased fetal breathing movements, (4) renal agenesis (such as with Potter syndrome) or obstruction of the urinary tract, in which amniotic fluid production is impaired, and (5) prolonged rupture of membranes in which amniotic fluid is lost.
Although postnatal gas exchange can be supported late in the canalicular stage, premature infants born during this period generally suffer severe complications related to low levels of pulmonary surfactant (surfactant deficiency of prematurity) with injury to the alveolar epithelium, which causes hyaline membrane disease (HMD) and respiratory distress syndrome (RDS). The administration of exogenous surfactants improves survival in these infants, but bronchopulmonary dysplasia (BPD), a complication secondary to antenatal or postnatal injuries that can include hyperoxia and ventilatory therapy for RDS, frequently develops as a consequence. Surfactant synthesis and mesenchymal thinning can be accelerated by glucocorticoids, , which are administered to mothers to prevent RDS and HMD when a premature birth is anticipated. ,
During the saccular stage of lung development, the terminal clusters of acinar tubules and buds dilate and expand into thin smooth-walled transitory alveolar ducts and saccules, and there is a marked reduction, or condensation, of the surrounding mesenchymal tissue. The lung continues to grow peripherally by branching and growth of the transitory ducts, so that by the end of this period, three additional generations of transitory alveolar ducts ending in terminal, or primary, saccules have formed. The peripheral regions of the lung also increase in size as a result of lengthening and widening of all segments distal to the terminal bronchioles (respiratory bronchioles, transitory alveolar ducts, and terminal saccules). Intersaccular and interductal septa develop, which include myofibroblasts at the tip and contain delicate collagen fibers and a double capillary network. Overall, cell proliferation slows as a result of a sharp drop in division of the epithelial cell population.
With the reduction in epithelial cell proliferation comes ultrastructural evidence of cell differentiation. Maturation of type II AECs continues and is associated with increased synthesis of surfactant phospholipids, , the surfactant-associated proteins A, B, C, and D, , , , , and ABCA3, a phospholipid transporter important for lamellar body formation. Glycogen content is reduced, and mitochondrial enzyme activity increases, indicating a shift to aerobic oxidative pathways. Lamellar bodies increase in number and size, and increasing amounts of tubular myelin, the secretory form of pulmonary surfactant, are seen in the terminal air spaces. The concentration of pulmonary surfactant is still low, however, and its phospholipid composition differs significantly from that at term.
Squamous type I AECs continue to differentiate from type II AECs and line an increasing proportion of the surface area of the distal lung. Enlargement of the potential gas exchange surface is dependent on the development of type I AECs with their flattened and squamous cell shape. Capillaries become more closely associated with squamous type I AECs, decreasing the diffusion distance between the air spaces and the capillary bed. The basal lamina of the epithelium and endothelium fuse to form the thin-walled alveolar-capillary membrane. In the newborn and adult lung, the mean thickness of the alveolar-capillary membrane is 0.6 µm, which permits passive diffusion of oxygen and carbon dioxide between the alveolar lumen and the capillary bed. Near the end of this stage, the interstitial tissue, or stroma, contains increasing amounts of ECM, and elastin is deposited in areas where future interalveolar septa will form, subdividing the terminal alveolar saccules into true alveoli. At this time, the immature lung contains relatively few elastin and collagen fibers, has little elastic recoil, and can be easily ruptured by mechanical ventilation. Abnormalities associated with the saccular stage of lung development are similar to those associated with the canalicular stage of lung development, including pulmonary hypoplasia, alveolar capillary dysplasia with or without misaligned pulmonary veins, and respiratory insufficiency, resulting in RDS, HMD, and BPD in the premature infant ( Table 55.3 ).
| Developmental Stage | Malformations/Disorders |
|---|---|
| Embryonic | Laryngeal, esophageal, tracheal agenesis/atresia |
| 3–7 wk GA | Tracheo- and bronchoesophageal fistula |
| Tracheal and bronchial stenosis | |
| Bronchogenic cysts | |
| Extralobar bronchopulmonary sequestration | |
| Pulmonary agenesis/aplasia | |
| Pulmonary vascular malformations/cardiac defects | |
| Pseudoglandular | Tracheo- and bronchomalacia |
| 5–17 wk GA | Intralobar bronchopulmonary sequestration |
| Cystic pulmonary airway malformation | |
| Congenital acinar aplasia/dysplasia | |
| Pulmonary hypoplasia/renal agenesis with oligohydramnios | |
| Pulmonary hypoplasia/congenital diaphragmatic hernia | |
| Congenital pulmonary lymphangiectasis | |
| Pulmonary vascular malformations/cardiovascular defects | |
| Canalicular | Pulmonary hypoplasia/oligohydramnios |
| 16–26 wk GA | Congenital alveolar dysplasia |
| Pulmonary arteriovenous malformations | |
| Pulmonary immaturity/surfactant deficiency | |
| Respiratory insufficiency/RDS | |
| Saccular | Pulmonary hypoplasia/oligohydramnios |
| 24–38 wk GA | Alveolar capillary dysplasia with misaligned pulmonary veins |
| Pulmonary immaturity/surfactant deficiency | |
| Respiratory insufficiency/RDS | |
| Hyaline membrane disease | |
| Bronchopulmonary dysplasia | |
| Alveolar | Congenital lobar overinflation |
| 36 wk GA—2 yr | Pulmonary hypoplasia/oligohydramnios |
| Alveolar capillary dysplasia with misaligned pulmonary veins | |
| Persistent fetal circulation/pulmonary hypertension | |
| Genetic surfactant deficiency/dysfunction | |
| Alveolar simplification/lung growth disorders |
The alveolar stage is the last stage of lung development and is marked by the formation of secondary alveolar septa, which partition the transitory ducts and terminal saccules into true alveolar ducts and alveoli, as well as by the maturation of the alveolar-capillary membrane. This process, known as alveologenesis , greatly increases the surface area of the lung available for gas exchange. A key cell type that drives the formation of new secondary septa is the myofibroblast; these cells differentiate from progenitors in the lung mesenchyme. At the beginning of this stage, the alveolar septa are relatively thick and contain a capillary network on each side of a central core of connective tissue, often referred to as a double capillary network . With the appearance of myofibroblasts at organized positions, their contractile property leads to lengthening and thinning of the secondary septa. , This is accompanied by the reduction of septal interstitial tissue, and remodeling of the capillary bed by fusion of the two septal capillary networks into one. Pulmonary vascular resistance decreases just before birth, allowing increased blood flow and commencement of gas exchange.
This stage is accompanied by a phase of rapid cellular proliferation in both the epithelial and mesenchymal cell populations. Interstitial fibroblasts actively proliferate early in this stage, but then slow down as increased synthesis and deposition of collagen, elastin, and fibronectin occur. Endothelial growth is brisk throughout this stage, and dividing endothelial cells are located primarily in the developing secondary alveolar septal crests. Both type II and type I AECs increase in number during this stage, but only type II AECs are proliferating actively, while type I AECs are derived from type II AECs. Type I AECs are thought to be terminally differentiated and to lack the capacity for mitosis. Although type II AECs account for two thirds of the total number of AECs in the adult human lung, the larger squamous type I AECs actually occupy 93% of the total alveolar surface. Although type I AECs form a tight epithelial barrier that is impervious to extracellular fluid and ions, they are easily injured by oxidants, barotrauma, and infection, readily detaching from the alveolar wall when injured. Injury to the lung during this stage of development can result in abnormal remodeling of the lung with a reduction in the number of alveoli and the development of chronic interstitial lung disease. Additional factors that may cause disturbances in alveolarization include administration of glucocorticoids, which inhibit cellular proliferation and reduce septation and formation of alveoli. Conversely, glucocorticoid administration enhances thinning of the alveolar septa, increases maturation of type II AECs, and enhances the production of surfactant in the premature lung.
Disorders associated with disturbances in the alveolar stage of lung development, which present at birth, include persistent fetal circulation and pulmonary hypertension of the newborn, BPD, congenital lobar emphysema, alveolar capillary dysplasia with or without misaligned pulmonary veins (often due to a FOXF-1 gene mutation), pulmonary hypoplasia, and RDS caused by surfactant deficiency due to mutations in the SFTPB, SFTPC , and ABCA3 genes.
Formation of the pulmonary circulation is linked directly to cardiovascular development, which begins on day 19 GA with formation of the lateral endocardial tubes. Subsequent embryonic folding on day 20 GA brings the endocardial tubes together in the midline, where they fuse to form a single primitive heart tube, or truncus arteriosus. Simultaneously, both the outflow (dorsal aortae) and inflow (venous) tracts of the heart form in the dorsal mesenchyme and connect with the endocardial tubes before they fuse. During additional embryonic folding (days 23 to 28 GA), the cranial ends of the dorsal aortae are pulled ventrally until they form a dorsoventral loop, or the first aortic arch, connecting the upper end of the truncus arteriosus to the paired dorsal aortae. Four additional pairs of aortic arches develop over the next week from the aortic sac, an expansion of the cranial end of the truncus arteriosus, and connect to the dorsal aortae. These arches will form the major arteries of the head, neck, upper thorax, upper extremities, lungs, and dorsal aorta. Along with remodeling of the atrial and ventricular chambers (5 to 8 weeks’ GA), the truncus arteriosus eventually splits vertically to form the ascending aorta and the main pulmonary artery, which is complete by week 8 of GA. During this period, the right and left pulmonary arteries arise from the paired sixth aortic arches and grow towards the lung, where their distal ends anastomose with the vascular plexus developing in the mesenchyme surrounding the bronchial buds. Subsequent growth and development of the pulmonary arteries follow branching of the bronchial tree (5 to 17 weeks’ GA). The bronchial arteries, which develop later (between 20 and 32 weeks’ GA), arise from the descending aorta and also supply blood to the lung. Along with the esophageal arteries, the bronchial arteries are remnants of the original, segmental, arterial supply from the aorta to the foregut vascular plexus, which include vessels to the esophagus, trachea, and lung buds.
As can be seen from Table 55.3 , most pulmonary malformations arise during the embryonic and pseudoglandular stages of lung development. These malformations represent a spectrum of closely related abnormalities associated with lung bud formation, branching morphogenesis, separation of the trachea from the esophagus, and failure of the pleuroperitoneal cavity to close properly. Abnormalities in other organ systems, such as renal agenesis, dysplastic growth of the kidney, or congenital diaphragmatic hernia, may also affect branching morphogenesis of the lung during these early developmental stages. Later during the canalicular and saccular stages of lung development, abnormalities related to growth and maturation of the respiratory parenchyma and its vasculature predominate, leading to abnormalities in acinar development, alveolar capillary dysplasia, pulmonary hypoplasia, and respiratory insufficiency. Infants born prematurely during the saccular and early alveolar stages of development are also subject to acute lung injury following ventilation and supplemental oxygen treatment in the neonatal period, resulting in BPD or chronic interstitial lung disease. Postnatally, mutations in the surfactant-related genes SFTPB , SFTPC, and ABCA3, have been associated with pulmonary surfactant deficiency and/or dysfunction, causing respiratory distress and failure in term infants, , as well as chronic interstitial lung disease in older infants, children, adolescents, and adults. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here