Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acute aortic dissection should be considered a constituent of acute aortic syndrome (AAS) together with intramural hematoma (IMH), penetrating atherosclerotic ulcer (PAU), and aortic rupture. The common denominator of AAS is disruption of the media layer of the aorta, with bleeding within the media layers or separation of the layers of the aorta (dissection). In 90% of cases, an intimal disruption is present that results in tracking of the blood in a dissection plane within the media, potentially rupturing through the adventitia or back through the intima into the aortic lumen ( Figure 1 ). The most common aortic syndrome is aortic dissection, featuring a tear in the aortic intima commonly preceded by medial wall degeneration or cystic media necrosis. Blood passes through the tear, separating the intima from media or adventitia and creating a false lumen. Propagation of dissection can proceed in anterograde or retrograde fashion involving side branches and causing complications such as tamponade, aortic valve insufficiency, or proximal or distal malperfusion syndromes.
Historically, acute aortic syndrome was attributed to syphilis; today, contributing factors are diverse ( Box 1 ). The most common risk condition for aortic dissection or IMH is hypertension (75% of patients have a history of hypertension). Other risk factors include smoking, direct blunt trauma, and use of illicit drugs (such as cocaine or amphetamines). Population-based studies suggest that the incidence of acute dissection ranges from 2 to 3.5 cases per 100,000 person-years, which correlates with 6000 to 10,000 cases annually in the United States. There is weak evidence that aortic dissection is more common in the winter compared to warmer summer months.
Long-standing arterial hypertension
Smoking
Dyslipidemia
Cocaine or crack use
Amphetamine use
Connective tissue disorders
Hereditary disorders
Marfan’s syndrome
Loeys–Dietz syndrome
Ehlers–Danlos syndrome
Turner’s syndrome
Hereditary vascular disease
Bicuspid aortic valve
Coarctation
Vascular inflammation
Autoimmune disorders
Giant cell arteritis
Takayasu’s arteritis
Behçet’s disease
Ormond’s disease
Infection
Syphilis
Tuberculosis
Deceleration trauma
Car accident
Fall from height
Iatrogenic factors
Catheter or instrument intervention
Valvular or aortic surgery
Side or cross clamping or aortotomy
Graft anastomosis
Patch aortoplasty
A review of 464 patients from the International Registry of Acute Aortic Dissection (IRAD) reported a mean age at presentation of 63 years, with significant male predominance (65%). The incidence of dissection appears to be increasing, independent of the aging population, to 16/100,000 men per year. Interestingly, women may be affected less often, but they have worse outcomes as a result of atypical symptoms and a delayed diagnosis. It may in fact be that two to three times as many patients die from aortic dissection than from ruptured abdominal aortic aneurysm, particularly women and younger patients (Marfan’s syndrome, Loeys–Dietz syndrome, Turner’s syndrome, or Ehlers–Danlos syndrome, especially type IV).
The most common mutations appear to lie in either the fibrillin gene (FBN-1) or the transforming growth factor (TGF)-β receptor II gene (TGFBR2) in Marfan and Loeys–Dietz syndromes, respectively. There appears to be little difference in the clinical presentations of these two syndromes; more than half of each group present with aortic symptoms. The commonest nonsyndromic mutation associated with thoracic aneurysms and dissections is in the smooth muscle cell (SMC) actin gene, ACTA2 , found in about one sixth of these patients. The association of mutations in genes encoding the SMC contractile apparatus in both aortic dissections and thoracic aneurysms indicates that SMC tonus and function may be an important phenotype influencing the response of the aorta to wall stress. Two other conditions that affect the proximal aorta and that predispose to acute aortic syndromes are annuloaortic ectasia and bicuspid aortic valve, and both of these might have a genetic basis.
Patients with acute aortic syndromes often come to the hospital with similar symptoms, regardless of the underlying condition. Pain is the most common presenting symptom of acute aortic dissection independent of age, sex, or other associated clinical complaint. Pooled data from more than 1000 patients showed that acute dissection is perceived as abrupt pain in 84% (95% confidence interval [CI], 80%–89%), with initially severe intensity in 90% (95% CI, 88%–92%). Although the pain is classically described as tearing or ripping, patients are more likely to describe the pain of acute dissection as sharp or stabbing, and fluctuating. Pain of aortic origin is often confused with acute coronary syndromes.
Cardiac enzymes, troponin, and electrocardiogram (ECG) changes may be instrumental in the diagnostic workup, but only the absence of both D-dimer elevation and ECG changes is considered specific to rule out acute aortic syndromes. D-dimers elevated above 500 μg/L appear to correlate with extent and severity of acute aortic dissection, but they fail to distinguish AAS from pulmonary embolism. Critically elevated D-dimer should, however, prompt undelayed computed tomography or transesophageal echocardiography for confirmation of either life-threatening entity.
Acute aortic dissections can be classified according to either the origin of the intimal tear or whether the dissection involves the ascending aorta, regardless of the site of origin. Accurate classification is important because it drives decisions regarding surgical versus nonsurgical management. The two most commonly used classification schemes are the DeBakey and the Stanford systems ( Table 1 ). For purposes of classification, the ascending aorta refers to the aorta proximal to the brachiocephalic artery, and the descending aorta refers to the aorta distal to the left subclavian artery. The DeBakey classification system categorizes dissections based on the origin of the intimal tear and the extent of the dissection. The Stanford classification system divides dissections into two categories, those that involve the ascending aorta and those that do not.
| Type | Description |
|---|---|
| Stanford Classification | |
| A | All dissections involving the ascending aorta regardless of the site of origin (surgery is usually recommended) |
| B | All dissections that do not involve the ascending aorta (nonsurgical treatment is usually recommended) Note that involvement of the aortic arch without involvement of the ascending aorta in the Stanford classification is labeled type B |
| DeBakey Classification | |
| I | Dissection originates in the ascending aorta and propagates distally to include at least the aortic arch and typically the descending aorta (surgery is usually recommended) |
| II | Dissection originates in and is confined to the ascending aorta (surgery is usually recommended) |
| III | Dissection originates in the descending aorta and propagates most often distally (nonsurgical treatment is usually recommended) |
| IIIa | Dissection is limited to the descending thoracic aorta |
| IIIb | Dissection extends below the diaphragm |
The risk of death is increased in patients who come to the hospital with or develop complications of pericardial tamponade, involvement of coronary arteries causing acute myocardial ischemia or infarction, or malperfusion of the brain. Other predictors of increased in-hospital death include age 70 years or older, hypotension, kidney failure, and pulse deficits. Less appreciated predisposing factors for type A dissection include prior cardiac and valvular surgery (15%) and iatrogenic dissection with surgery or catheterization (5%). In the absence of immediate surgical repair, medical management of proximal dissection is associated with a mortality of nearly 20% at 24 hours after presentation, 30% by 48 hours, 40% by day 7, and 50% by 1 month. Even with surgical repair, mortality rates are 10% by 24 hours, 13% by 7 days, and nearly 20% by 30 days ( Figure 2 ). The most common causes of death include aortic rupture, stroke, visceral ischemia, cardiac tamponade, and circulatory failure.
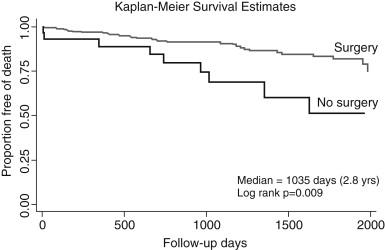
Patients with uncomplicated type B dissection have a 30-day mortality of 10% and may be candidates for long-term medical management based on antiimpulsing medication. However, with the evolution of ischemic complications such as kidney failure, visceral ischemia, or contained rupture, mortality increases to 20% by day 2 and 25% by day 30. Similar to type A dissection, advanced age, rupture, shock, and malperfusion are important independent predictors of early mortality. Nonetheless, all types of acute dissection require initial medical management until they can have definitive treatment.
Acute aortic syndrome (dissection or IMH) involving the ascending aorta is a surgical emergency. In selected cases, hybrid approaches of an endovascular and open combination may be considered. Conversely, acute aortic pathology confined to the descending aorta is subject to medical treatment unless it is complicated by organ or limb malperfusion, progressive dissection, extra-aortic blood collection (impending rupture), intractable pain, or uncontrolled hypertension.
Initial management of an acute aortic syndrome, in general and particularly in dissection of both the proximal (A) and distal (B) aorta, is directed at limiting propagation of dissected wall components by control of blood pressure and reduction of d P /d t as an antiimpulse strategy. Reduction of pulse pressure to just maintain sufficient end-organ perfusion is a priority with the use of intravenous β-blockade as first-line therapy before swift surgical repair in type A lesions ( Box 2 ). Labetalol, with both α- and β-blocking characteristics, is useful for lowering both blood pressure and d P /d t , with target systolic pressure of 100 to 200 mm Hg and heart rate of 60 to 80 beats/min. Often multiple agents are required, with patients ideally managed in an intensive care setting. Opiate analgesia should be prescribed to attenuate the sympathetic release of catecholamines triggered by pain, with resultant tachycardia and hypertension ( Table 2 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here