Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Although many lung disorders can be diagnosed from the plain chest radiograph, its limitations in providing a definitive diagnosis are widely understood. Complicated chest radiographs, the lack of radiographic abnormalities in symptomatic patients, and the limitations of portable chest studies make evaluation of conventional chest films sometimes difficult.
Imaging evaluation of parenchymal lung disease is essentially confined to chest radiography, high-resolution computed tomography (HRCT), spiral CT, and multidetector CT (MDCT). These techniques serve as important adjuncts to the plain film for the purposes of detection, diagnosis, and characterization of parenchymal lung disease. Compared with conventional radiographic techniques, CT provides better spatial resolution, detects minor differences in radiographic contrast, and expands the ability of radiology to portray boundaries between different tissues.
Imaging of the lung has changed completely during the past decade. With the improvement of CT imaging techniques, shorter examination times, thinner sections, and higher resolution are now possible without loosing image quality. The introduction of fast MDCT scanners further improved the ability to visualize small pulmonary nodules and pulmonary emboli and allowed for three-dimensional (3D) imaging of the lung.
HRCT combines the use of thinnest-beam collimation (1-1.5 to 2 mm in thickness) with reconstruction algorithms designed to produce high spatial resolution. Spatial resolution in the axial plane is a function of pixel size, which is dependent on the image matrix (typically 512 × 512) and field of view. Thin collimation decreases partial volume averaging and improves the ability of CT to demonstrate small pulmonary lesions. The thinnest collimation available on most scanners is 1 to 2 mm. This is closely matched to the size of the anatomic structures of interest within the secondary pulmonary lobule. Use of thin collimation causes increased quantum image noise and decreased contrast sensitivity because fewer photons are available to reach the CT detector system.
The anatomic detail provided by HRCT imaging sections allows a more detailed analysis of pathologic processes affecting the small airways, airspaces or alveolar walls, and interstitium. HRCT has become established as a useful technique for the detection and characterization of diffuse lung disease (DLD). Standard HRCT scanning with single-slice CT allows assessment of the lung at the scanned levels only and cannot exclude abnormalities in the lungs between these sections. HRCT technique is indicated in a variety of clinical settings (e.g., patients with normal chest radiography who have either chronic respiratory symptoms or abnormal pulmonary function tests). HRCT may also play an important role in detecting early morphologic changes in patients with suspected pulmonary disorders such as interstitial lung disease (ILD), emphysema, asbestosis, and bronchiectasis.
Compared with standard CT radiographic technique, the use of higher kilovolt peak (kVp), higher milliamperes (mA), or longer scan duration increases the number of photons incident upon the CT detector system. Although the use of low-dose HRCT has been shown to be slightly less sensitive in detecting some instances of ground-glass opacity and interstitial lines, it has minimal compromise in diagnostic information.
High-spatial-frequency algorithms, by decreasing the amount of smoothing of the image data, improve visualization of both normal and pathologic structures. This technique has been shown to be more sensitive than conventional CT and chest radiography for detection and characterization of diffuse infiltrative lung disease. HRCT can also precisely differentiate emphysema from other cystic lesions. Expiratory CT accurately identifies air trapping. However, assessment of air trapping can be difficult in obese patients or in those who are unable to suspend respiration, especially at end-expiration. Complementary expiratory CT maneuvers can be used to improve the visualization and extent of air trapping.
Prone CT scans can be taken to differentiate focal dependent edema or microatelectasis, commonly seen in the lung bases of normal patients, from early pulmonary fibrosis.
Window settings are important variables in HRCT technique. To view the lung parenchyma, window settings are in the range from 1000 to 2000 HU and level settings from −500 to −750 HU. Images of the mediastinum can be reconstructed using a standard soft tissue algorithm and viewed using mediastinal window settings of 410 HU and level settings of 35 HU. Additional window settings are adjusted manually at the time of imaging to enhance depiction of subtle ground-glass opacities or emphysema. Emphysema may be demonstrated by the use of low window levels of −800 to −900 HU and narrow widths of 500 HU.
Spiral CT technique requires a scanner with a continuously rotating x-ray tube capable of generating a series of consecutive scans during a single breath-hold maneuver. Unlike conventional CT, the patient is not scanned section by section but is translated through the scan plane at a uniform table speed during acquisition of raw data. The advantages of spiral CT arise from continuous data acquisition and short total scanning time. The operator chooses three basic parameters for spiral CT: section collimation, table feed per rotation, and reconstruction interval.
The section collimation (0.5 mm, 1 mm, 2 mm, 3 mm, 5 mm, 7 mm, or 10 mm) determines the spatial resolution achieved along the z-axis. The effective slice thickness can be influenced by the table feed speed per tube rotation and the interpolation algorithm used for image reconstruction. Table speed is set to between 1 and 10 mm/second. Pitch is defined as the ratio as the table feed per tube rotation to the collimation. Pitch is usually best kept between 1.0 and 2.0. Whereas with a pitch value of 1.0 (table feed equal to collimation) , longitudinal scan coverage is limited, scanning at a pitch less than 1.0 produces an overlapping scan pattern. Reconstruction of overlapping sections improves the quality of the 3D images. With single-detector-row scanners, routine spiral CT imaging in the thorax is performed with pitch values between 1.0 and 1.5, using collimation in the range of 8 to 10 mm and table movement speed of 8 to 15 mm/sec.
The advent of MDCT represents an enormous technical advance. The development of MDCT allows higher z-axis resolution and faster image acquisition than previously possible, and combined with PACS, greater diagnostic evaluation of the bronchi and vessels and improved visualization of parenchymal processes. It uses a multiple-row detector with narrow detector collimation in conjunction with a relatively rapid table translation to achieve faster scans with thinner slices than single-detector CT. MDCT results in a gapless volumetric acquisition that eliminates problems of slice misregistration caused by variations in the depth of inspiration. The resulting data set is more accurate, more reproducible from one study to the next, and more quickly acquired. The reduced scan time of MDCT reduces motion artifacts, particularly in patients with underlying lung disease.
Knowledge of the capabilities and limitations of MDCT allows the radiologist to use MDCT appropriately and for the best possible patient care. Multislice HRCT may be used to acquire high-resolution images of the lung parenchyma. The image quality of multislice HRCT is equal to that on single-detector HRCT. Although spatial resolution is better on axial HRCT, multislice HRCT is appropriate for evaluating subtle lung abnormalities. Moreover, the image quality of coronal multiplanar reconstructions (MPRs) from isotropic voxel data obtained using 0.5-mm collimation, with or without overlapping reconstruction, is similar to that of direct coronal thin-section CT scans. Careful attention must be paid to technical parameters during scanning, and the dose needs to be tailored to the specific clinical situation. Although low-dose techniques can be used to reduce the radiation dose in patients in whom a volumetric HRCT is indicated, the advantages of accurate diagnosis may outweigh the increased radiation dose.
With the latest generations of 64-slice MDCT and dual-source technology, the performance of CT scanning has dramatically improved. An important advance of MDCT scanners is that from the same data set, thin sections can be reconstructed to minimize partial volume averaging, and thicker sections can be reconstructed to decrease image noise if necessary. MDCT scanners can provide continuous and/or overlapping high-resolution thin sections throughout the lungs in a single breath hold. The provided additional information and its impact on clinical management has justified its use in the area of thoracic imaging.
In combination with advanced image processing techniques, including MPRs, maximum intensity projections (MIPs), minimum intensity projections (MiniPs), external rendering with either 3D shaded-surface displays or volumetric rendering, and internal rendering (or so-called virtual bronchoscopy), CT has become a valuable tool for evaluating diseases affecting the airways ( Fig. 36-1 ).
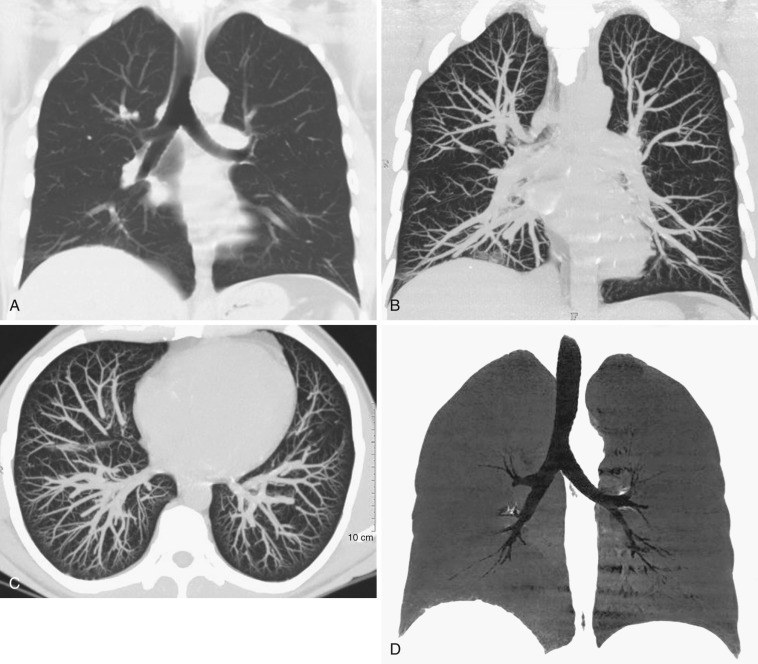
Given the shorter acquisition times, correct scan timing after intravenous (IV) contrast medium administration is mandatory to achieve adequate enhancement of the organ or vessels within the area of interest. Because of different MDCT scanners, the protocols need to be adjusted to match the suggested iodine doses and administration rates. A small to moderate amount of contrast medium (60-120 mL of 300 mg iodine[I]/mL) delivered at a slow injection rate (1.5-3 mL/sec), scan delay time of 25 seconds, and long injection duration (>30 seconds) results in sufficient opacification of thoracic vessels. Saline flushing is favorable to reduce perivenous artifacts. Moderate to large amounts of iodine (3-4 mL/sec) are required to study diverse abnormalities of pulmonary arteries such as acute pulmonary embolism (PE), chronic thromboembolic disease, pulmonary arteriovenous malformations (AVMs), pulmonary artery aneurysms, and arteriovenous fistulas. A slower injection is recommended in those patients with diminished cardiac output. For fast acquisitions (16- and 64-channel MDCT), thoracic and coronary CT angiography require moderate amounts of iodine at high iodine administration rates.
The value of MDCT for evaluating patients with chest pain presenting to the emergency department has also been recently discussed. In addition to evaluation of the coronary arteries, CT angiography has long been used to evaluate patients for other dangerous causes of chest pain such as aortic dissection and pulmonary embolus (“triple rule-out”).
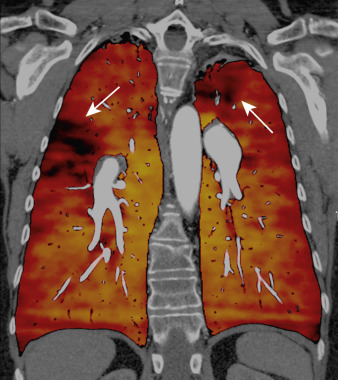
Dual-energy computed tomography (DECT) is very helpful in the detection and diagnosis of thoracic abnormalities. Selective iodine mapping is made possible by the x-ray absorption characteristics of iodine, which has a high atomic number compared with elements that constitute air or soft tissue.
A major advantage of dual-energy imaging over conventional radiography is its superior sensitivity for the detection of calcification within a pulmonary nodule. Other advantages of dual-energy imaging are related to identification of bone and pleural abnormalities, recognition of hilar and mediastinal masses, detection of tracheal narrowing and airway disease, and localization of stents, catheters, and other indwelling devices. DECT can also be used for visualization of pulmonary microvascular contrast material distribution (DECT angiography) and detection of PE ( Fig. 36-2 ).
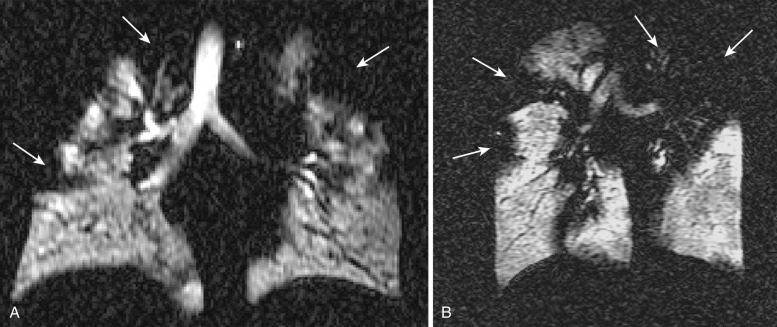
Despite the fact that magnetic resonance imaging (MRI) has a clear advantage over other imaging modalities in evaluating cardiac and vascular disorders of the chest, it continues to play a limited role in parenchymal lung evaluation. Reasons for the limited use of pulmonary MRI include respiratory motion and magnetic susceptibility effects caused by air-tissue interfaces. Assessment of chest wall disease and peridiaphragmatic processes, which do not suffer as much from respiratory or magnetic susceptibility artifacts, already benefits from the multiplanar capabilities of MRI and its improved soft tissue contrast. Hyperpolarized gas MRI of the lung provides high temporal and spatial resolution images of the airspaces of the lung and can be used to elucidate both lung ventilation and morphology. In patients with asthma and cystic fibrosis, areas of diminished gas ventilation from airflow obstruction are readily demonstrated and visualized as ventilation defects ( Fig. 36-3 ).
A large number of nonneoplastic lung diseases may present with a solitary or multiple pulmonary nodules or masses. These include congenital abnormalities, inflammatory diseases, pulmonary infections, vascular pulmonary lesions, and miscellaneous other conditions. Chest radiography serves as the primary technique for detecting most focal lung disease. A major challenge of lung imaging is to differentiate common and benign focal abnormalities from malignant tumors such as metastases or bronchogenic carcinoma. Although MDCT and fluorine-18 fluorodeoxyglucose positron emission tomography (FDG PET) have changed the evaluation of focal lung disease, optimal radiographic evaluation and management of these cases remain challenging clinical problems.
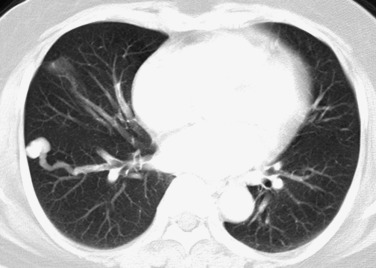
Congenital pulmonary AVMs are fistulous vascular communications between a pulmonary artery and vein (95%) or systemic artery and pulmonary vein, bypassing the capillary bed. About 50% of pulmonary AVMs are associated with Osler-Weber-Rendu syndrome (hereditary hemorrhagic telangiectasia).
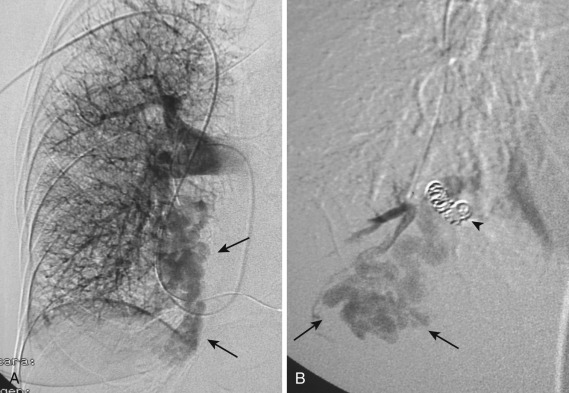
The diagnosis is usually straightforward on CT. The typical features are a smooth, lobulated, oval/round mass, or even serpiginous nodule, less than 1 cm to several centimeters in size, associated with a visible enlarged feeding artery and an enlarged draining vein; the feeding artery is usually less than half the diameter of the nodule ( Fig. 36-4 ). Complex AVMs having more than one feeding artery are rare. CT pulmonary angiography performed after a bolus injection of contrast material can be used to confirm the vascular nature of the lesion. Multiple AVMs can be confused with metastases if the enlarged feeding vessels are overlooked. MIP reconstructions can also be obtained to scrutinize the pulmonary parenchyma for malformations and depict the size and number of the supplying artery(ies) ( Fig. 36-5 ). Pulmonary AVMs can be successfully treated with embolotherapy ( Fig. 36-6 ).
The incidental finding of a pulmonary nodule on chest radiograph or CT is becoming an increasingly frequent event. A solitary pulmonary nodule has been defined as a spherical intraparenchymal opacity 3 cm or less in diameter, completely surrounded by lung, without associated mediastinal lymphadenopathy or atelectasis. Lesions larger than 3 cm in diameter usually are defined as masses, and most represent bronchogenic carcinoma. The goals of imaging techniques in evaluating an undiagnosed pulmonary nodule are: (1) to make a specific diagnosis and (2) if a specific diagnosis cannot be made, to distinguish benign nodules from those that are indeterminate or potentially malignant. In the early stage, bronchogenic carcinoma may also appear as a small nodule (70% in stage I). In patients younger than 30 years, the incidence of lung cancer is very low, so a solitary pulmonary nodule may not require further evaluation unless the patient has a known extrathoracic malignancy. New guidelines have been proposed by the Fleischner Society for follow-up and management of small pulmonary nodules detected on CT scans.
Despite high false-positive rates, computer-aided detection has been shown to be useful in the detection of lung nodules on chest radiographs. Bone suppression on conventional chest radiographs will be a helpful technique in detecting more pulmonary nodules by minimizing or eliminating the overprojection of the clavicles and ribs.
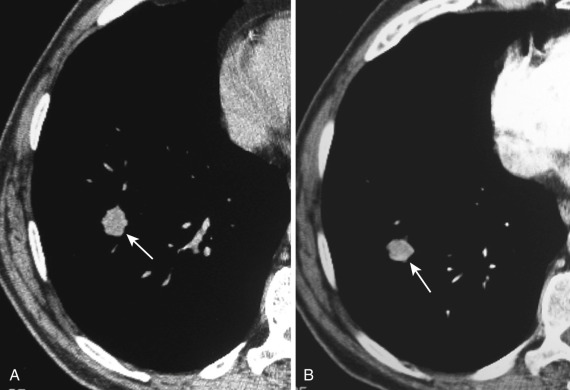
Because of differences in the vascularity of benign and malignant nodules, noncalcified nodules may show some degree of enhancement after administration of IV contrast material ( Fig. 36-7 ). Malignant nodules tend to enhance more than benign inactive granulomas, which show very little or no enhancement. However, inflammatory nodules such as active granulomas resulting from fungal infection or tuberculosis (TB) may also show significant contrast enhancement. Although false-negative results have been reported, absence of significant lung nodule enhancement (≤15 HU) at CT is strongly predictive of benignity.
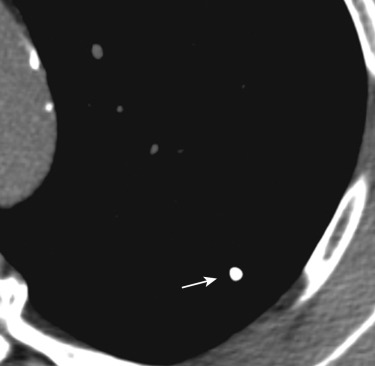
Postinflammatory granulomas due to TB or fungal infections are the most common cause of pulmonary nodules. Thin-slice section CT is much more sensitive than conventional radiography for the detection of calcification within a nodule. On unenhanced CT, a well-circumscribed calcified pulmonary nodule measuring 2 cm or less in diameter is usually a benign calcified granuloma ( Fig. 36-8 ). The CT pattern of calcification in a solitary pulmonary nodule can help differentiate benign from malignant lesions. Patterns for benignity are homogeneous calcification throughout the nodule, laminated or concentric rings of calcification, and a “popcorn-like” appearance. Exception occurs in the specific clinical setting of metastatic osteogenic sarcoma; lung metastases from osteogenic sarcoma are typically calcified in a homogeneous pattern. Although lung cancers and other malignancies may contain small areas of calcification, they rarely demonstrate calcification in one of the benign patterns described previously.
Focal areas of fat within a nodule with attenuation values ranging from −40 to −120 HU, sometimes associated with chondroid (“popcorn”) calcification, are a reliable indicator of a hamartoma ( Fig. 36-9 ). In patients with chronic rhinitis and a history of mineral oil or oily nose drop use, the presence of focal areas of fat within a pulmonary mass or focal consolidation strongly suggest exogenous lipoid pneumonia ( Fig. 36-10 ).
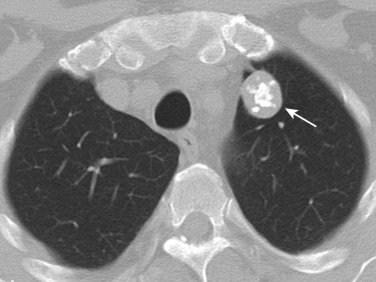
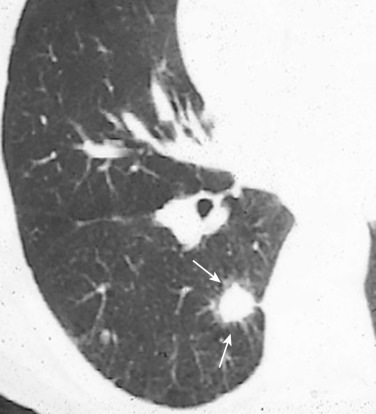
The feeding vessel sign is defined as a vessel coursing directly into a nodule. This sign indicates either that the lesion has a hematogenous origin or that the disease process occurs in close proximity to small pulmonary vessels. A number of vessel-related nonneoplastic disorders of the lung produce focal disease, including pulmonary infarcts, septic emboli, and pulmonary vasculitis.
Pulmonary infarction is associated with thromboembolic obstruction of a medium-sized pulmonary artery and implies necrosis of lung tissue. Classic radiographic findings include Westermark's sign, an area of focal hypoperfusion, and Hampton's hump, a peripheral wedge-shaped density above the diaphragm. Evidence of a dilated pulmonary artery is occasionally observed. CT findings have been reported in only 10% of cases of documented PE and include segmental and/or subsegmental atelectasis and focal ill-defined ground-glass attenuation or parenchymal consolidation, often peripheral and triangular in distribution ( Fig. 36-11 ). The reversed halo sign, characterized by a central ground-glass opacity surrounded by denser airspace consolidation, was first described on HRCT as being specific for cryptogenic organizing pneumonia. Since then, it has been reported in association with a wide range of pulmonary diseases, including pulmonary infarction. Infarcts may completely resolve or persist over a period of weeks, resulting in a residual scar that may be mistaken for a pulmonary nodule or mass.
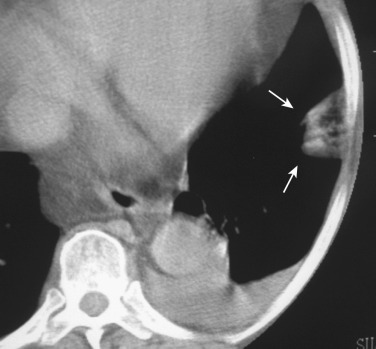
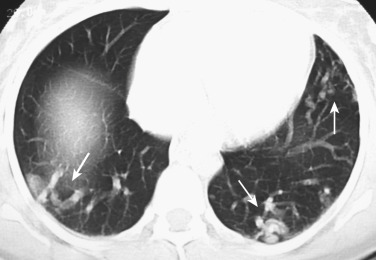
Septic PE generally presents with insidious onset of fever, cough, and pulmonary opacities. It is seen most commonly in patients with indwelling catheters, tricuspid valve endocarditis, alcoholism, skin infection, and in IV drug users; less common causes include pelvic thrombophlebitis, periodontal disease, and suppurative processes in the head and neck. Septic PE from the lesions of periodontitis should be included in the differential diagnosis of septic PE.
The radiographic manifestations usually consist of bilateral nodular opacities, variable in size, which are frequently cavitated. Septic embolism is often complicated with empyema. CT is an important modality for confirming the presence of pulmonary septic emboli even when conventional chest radiographs remain negative. The CT features of septic PE include multiple peripheral nodules varying in size from 0.5 to 3.5 cm (85%), a feeding vessel sign (60%-70%), cavitation (50%), wedge-shaped peripheral lesions abutting on the pleura (50%), and extension into the pleural space (39%) ( Fig. 36-12 ). The nodules may be circumscribed or poorly defined and may be associated with patchy areas of consolidation. MPRs show that most of these vessels course around the nodule and that the others are pulmonary veins.
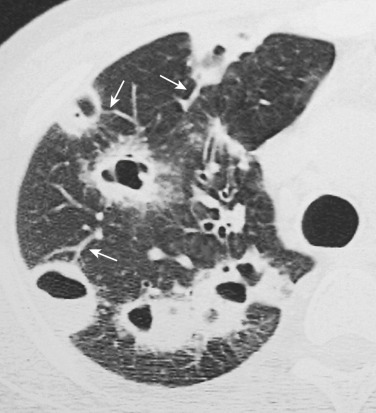
Angioinvasive pulmonary aspergillosis, generally occurring in the neutropenic phase, is the most common opportunistic infection of respiratory complications following bone marrow transplantation. Angioinvasive pulmonary aspergillosis is associated with high morbidity and mortality, reaching 50% to 60% in severely immunocompromised patients. Major risk factors for the disease include severe or prolonged neutropenia (absolute neutrophil count < 500), prolonged corticosteroid therapy, graft-versus-host disease after allogeneic bone marrow transplantation, and late-stage AIDS. The diagnosis of angioinvasive aspergillosis is based on clinical, radiologic, and mycologic data. Affected patients report nonspecific symptoms of fever, cough, and dyspnea. The clinical diagnosis is very difficult owing to its nonspecific symptoms, low frequency of positive blood cultures, and high frequency of contamination. Thrombocytopenia of these patients may preclude invasive diagnostic procedures such as percutaneous or transbronchial biopsy. Cultures of bronchoalveolar lavage (BAL) fluid are positive in 30% to 68% of infected patients. Galactomannan and nucleic acid detection in serum or BAL fluid are useful in the early identification of invasive aspergillosis in the immunocompromised host; however, a definite diagnosis of invasive aspergillosis still requires demonstration of the fungus in tissue specimens.
Radiographically, angioinvasive pulmonary aspergillosis characteristically manifests as multiple ill-defined 1- to 3-cm peripheral nodules that gradually coalesce into larger masses or areas of consolidation. An early CT finding (best seen on thin-section images) is a rim of ground-glass opacity surrounding the nodules (CT halo sign ) ( Fig. 36-13 ). The halo represents hemorrhage around a focal area of lung infarction caused by the Aspergillus organism invading pulmonary vessels. In the nonleukemic nonneutropenic patient, the CT halo sign is less specific. This finding has also been described in patients with other types of angioinvasive infections, hemorrhagic metastases, TB, mucormycosis, and Wegener's granulomatosis.
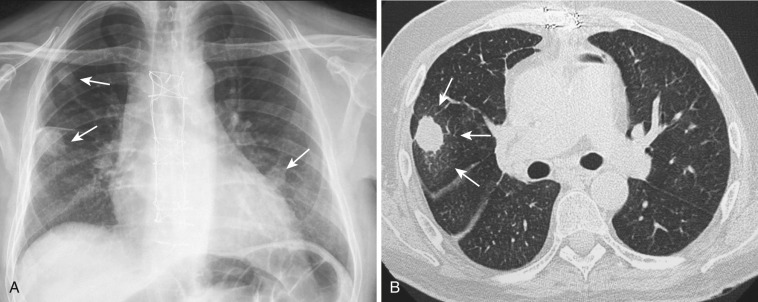
Cavitations are usually a late finding in these patients and represent the resolution phase of the infection. As the bone marrow recovers from aplasia and the white blood cell count returns to normal, the pulmonary lesions begin to cavitate and often have a distinctive radiographic appearance, the air crescent sign ( Fig. 36-14 ). This finding, resulting from an intracavitary mass composed of invasive pulmonary aspergillosis and necrotic lung, characteristically occurs 2 to 3 weeks after initiation of treatment and concomitant with resolution of the neutropenia; it usually indicates a good prognosis.
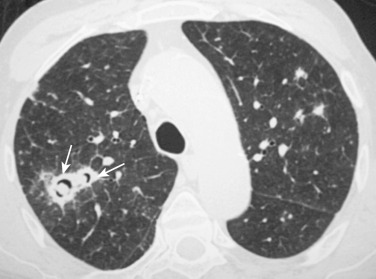
The vasculitides are disorders characterized by blood vessel inflammation leading to tissue or end-organ injury. In the Chapel Hill classification, large vessels refers to the aorta and its largest branches, medium-sized vessels to the main visceral arteries, and small vessels to the capillaries, venules, and arterioles. Thoracic involvement is most commonly seen with primary idiopathic large-vessel vasculitides (Takayasu's arteritis and giant cell arteritis) and primary antineutrophil cytoplasmic autoantibody (ANCA)-associated small-vessel vasculitides (Wegener's granulomatosis, microscopic polyangiitis, Churg-Strauss syndrome). The radiologic findings in primary pulmonary vasculitis vary widely and can include vessel wall thickening, nodular or cavitary lesions, ground-glass opacities, and consolidations, among others. Diffuse alveolar hemorrhage usually results from primary small-vessel vasculitis, specifically microscopic polyangiitis and Wegener's granulomatosis.
Wegener's granulomatosis is a multisystem disease characterized clinicopathologically by the combination of a granulomatous necrotizing and ulcerative process of the respiratory tract and the internal organs, generalized necrotizing granulomatous vasculitis, and a usually focal necrotizing glomerulonephritis. A limited (nonrenal) form of Wegener's granulomatosis affecting only the respiratory tract may also occur. Active vasculitis associated with Wegener's granulomatosis may cause abnormalities of pulmonary perfusion at the subsegmental level, resulting in ischemia or frank infarctions, leading to parenchymal scars. The characteristic CT manifestations consist of bilateral subpleural or peribronchovascular nodules or masses (1-4 cm in diameter), sometimes spiculated ( Fig. 36-15 ); in approximately 50% of cases, masses may cavitate. Other common CT features are diffuse bilateral ground-glass opacities, bilateral nonsegmental or peribronchovascular areas of airspace consolidation, peribronchial thickening, and small centrilobular nodules and branching linear structures (so-called tree-in-bud pattern). Ground-glass opacities and airspace consolidation correlate with focal or diffuse pulmonary hemorrhage (DPH). This occurs when pulmonary capillaries are primarily involved by the vasculitic process.
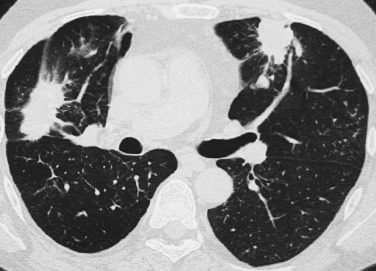
PE is a common condition, representing the third most lethal disease in the United States. It results from a thrombus formed in the venous system. PE is a frequently missed clinical diagnosis that has absent or nonspecific radiographic findings. Chest radiography is of limited value in the diagnosis; radiographic findings include elevation of the diaphragm, subsegmental atelectasis, patchy opacities (due to associated edema, hemorrhage, or infarction), pleural effusion, and segmental or larger areas of oligemia. Radiographs are normal in 10% to 15% of cases with a proven diagnosis; their major importance lies in excluding other disease processes that can mimic PE (e.g., pneumonia, pneumothorax) and for interpretation of V/Q scintigraphy.
Compared with single-detector CT scanners used in the 1990s, a new generation of CT scanners with multiple detector rows has dramatically changed the diagnostic accuracy of PE. MDCT has a high degree of accuracy in identifying central pulmonary emboli down to the second-order to fourth-order pulmonary arteries. Initial reports pointed out the suboptimal detection of PE in subsegmental arteries. Actually the use of narrow slice reconstruction has led to progressive improvement in visualization of subsegmental arteries. Numerous studies have corroborated significantly improved detection of subsegmental emboli (75%-95%) using MDCT, with overall sensitivities and specificities ranging between 90% and 100%, respectively. Advanced image processing techniques, such as MPR or MIP reconstructed from isotropic volumetric data sets, also facilitate the demonstration of subsegmental pulmonary emboli. Suboptimal visualization of peripheral emboli in subsegmental arteries will be of potential clinical significance only when embolic disease is limited to vessels of this caliber.
The accuracy of MDCT for diagnosing PE depends on the size of the artery affected and the size of the emboli. Optimal assessment of the pulmonary vessels on MDCT requires careful attention to several parameters, including scan collimation, imaging volume, and contrast enhancement. The lung volume that is scanned should be large enough to include all segmental and subsegmental pulmonary arteries. This goal can be achieved by scanning from the top of the aortic arch to the dome of the diaphragm.
Respiratory motion exacerbates partial volume artifacts of arteries in or near the scan plane, leading to loss of continuity of arteries in the z-axis and resulting in interpretative difficulty. Although the scans can be performed in the craniocaudal direction, scanning in the caudal-to-cranial direction helps minimize motion artifacts (respiratory motion is most marked in the lung bases), particularly in patients unable to hold their breath for the duration of the scan. The current generation of MDCT devices (up to 64 detectors) can obtain thin sections up to 0.5 mm with a resolution down to 0.4 × 0.4 × 0.4 mm; thin sections and a high spatial resolution improve the depiction of small peripheral emboli down to sixth-order branches (subsegmental pulmonary emboli).
A basic understanding of contrast medium dynamics is crucial for the rational design of contrast medium injection. Incorrect timing of image acquisition after the contrast bolus injection is a common cause of suboptimal studies.
The diagnosis of acute PE on contrast-enhanced CT scan is based on direct visualization of partial or complete filling defects within the pulmonary arteries ( Figs. 36-16 to 36-18 ). Whereas a central thrombus when seen in cross section shows a round intraluminal filling defect surrounded by contrast (the “doughnut” sign), when the thrombus is imaged along its axis shows a linear intraluminal filling defect outlined by contrast (the “railroad track” sign). Other useful CT signs of acute PE include acute angles with the vessel wall, complete cutoff of vascular opacification, and increased diameter of the occluded vessel. Occasionally, hyperdense pulmonary clots can be seen in nonenhanced CT scans.
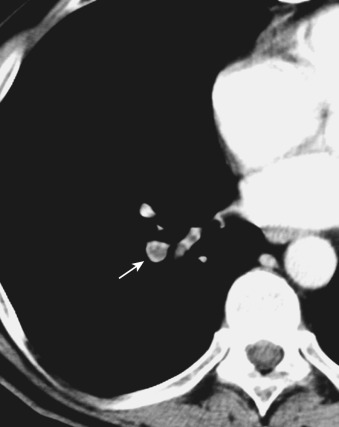
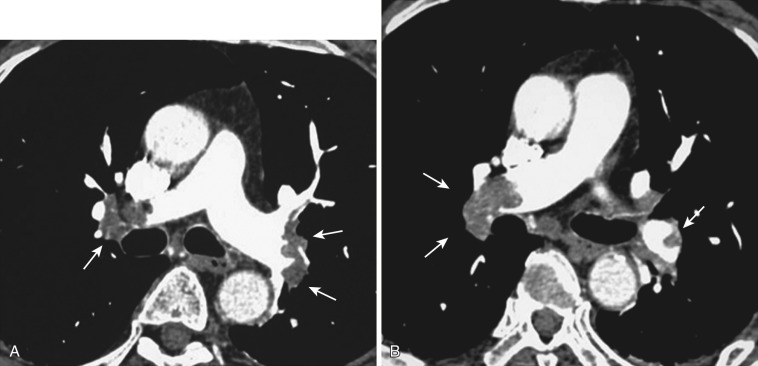
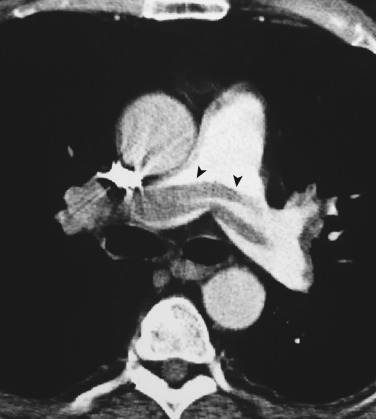
Chronic thrombi often appear as crescentic or laminar filling defects adherent to the walls of the pulmonary artery. Calcification may be identified in chronic clots in patients with long-standing pulmonary hypertension ( Fig. 36-19 ). Pitfalls in the diagnosis of PE may include poor arterial opacification caused by incorrect bolus timing and the presence of nonopacified blood within the pulmonary vasculature arriving from the inferior vena cava and right atrium (transient interruption of contrast). The lymphatic and connective tissue located adjacent to the pulmonary arteries may also mimic the appearance of PE.
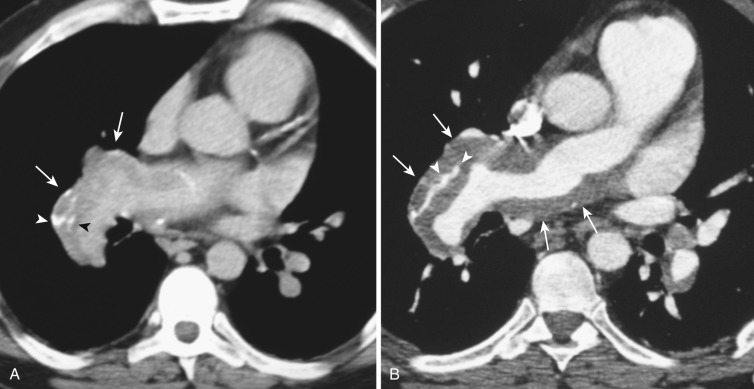
Ancillary CT findings that may suggest PE include pleural-based wedge-shaped images, linear parenchymal bands, and central pulmonary arterial enlargement. Although a triangular pleural-based parenchymal image may represent a pulmonary infarct, this finding is not specific, and other entities such as pneumonia, tumor, fibrosis, hemorrhage, or edema should also be considered. Pleural effusion is common but does not occur significantly more frequently in the presence of PE. In chronic thromboembolic disease, the lung parenchyma may demonstrate mosaic attenuation, with areas of decreased attenuation associated with “pruned” or attenuated vessels.
Nonthrombotic PE is an uncommon condition but is sometimes associated with specific imaging findings, including discrete nodules with cavitation (septic embolism), widespread homogeneous and heterogeneous areas of increased opacity or attenuation that typically appear 12 to 24 hours after trauma (fat embolism), fine miliary nodules that subsequently coalesce into large areas of increased opacity or attenuation (talcosis), or opaque tubular and branching structures (methyl-methacrylate PE after percutaneous vertebroplasty). Fat embolism secondary to long bone fractures occurs in 1% to 3% of patients with simple tibial or femoral fractures but in up to 20% of individuals with more severe trauma. Imaging findings are similar to those in acute respiratory distress syndrome (ARDS). The radiographic findings resemble those in ARDS. Hydatid PE is a rare complication of cardiac or hepatic echinococcosis.
Pulmonary tumor embolism and pulmonary tumor thrombotic microangiopathy are uncommon manifestations of neoplasms. Although pulmonary intravascular tumor emboli are seen in up to 26% of autopsies, its diagnosis is difficult to establish both clinically and on conventional radiographic studies. The major CT findings consist of multiple multifocal dilatation and beading of peripheral pulmonary arteries, primarily in a subsegmental distribution.
A vascular cause of the tree-in-bud pattern on thin-section CT has been described in a patient with pulmonary tumor thrombotic microangiopathy (PTTM) ( Fig. 36-20 ). Histopathologically, PTTM is characterized by widespread fibrocellular intimal hyperplasia of small pulmonary arteries (so-called carcinomatous endarteritis) induced by tumor microemboli.
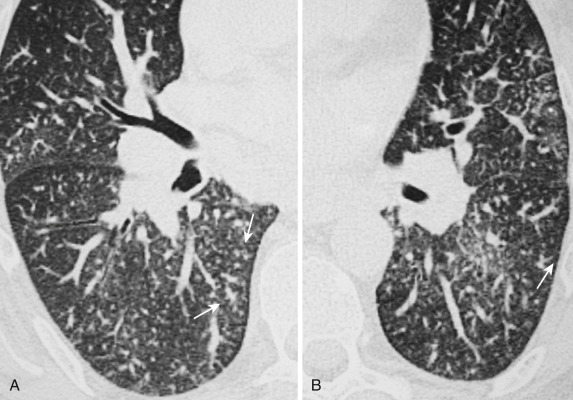
Rounded atelectasis refers to a form of nonsegmental peripheral pulmonary collapse that may mimic a primary lung cancer. It is always found adjacent to an area of pleural thickening, and the process is thought to begin with an adhesive pleural effusion or pleural reaction that entraps the adjacent atelectatic lung. Although many cases of rounded atelectasis are the result of asbestos-related pleural disease, other causes of pleural reaction may produce similar findings. Usually this pulmonary pseudotumor can be differentiated from malignancy using CT criteria such as: (1) a rounded or wedge-shaped mass that forms an acute angle with the adjacent thickened pleura, (2) vessels and bronchi emanating from the hilar region, swirling around and converging in a curvilinear fashion into the lower border of the mass, forming a comet's tail or vacuum cleaner sign, (3) air bronchogram visible in the central portion of the mass, (4) homogeneous contrast enhancement of the atelectatic lung, and (5) volume loss in the ipsilateral hemithorax ( Fig. 36-21 ). However, percutaneous needle biopsy, radiologic follow-up, or surgical resection are required for indeterminate cases.
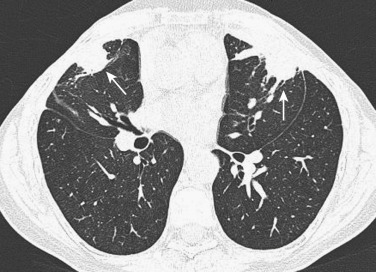
DLD may result from a variety of acute and chronic causes, including ILD, airspace processes, airway diseases, and emphysema. There are over 200 clinical forms of DLD of known causes (e.g., collagen vascular disease, environmental or drug related), as well as disorders of unknown cause. The latter include idiopathic interstitial pneumonias (IIPs), granulomatous lung disorders (e.g., sarcoidosis), and other forms of ILD, including lymphangioleiomyomatosis (LAM), Langerhans cell histiocytosis (LCH), and eosinophilic pneumonia.
HRCT (1- to 2-mm collimation) is more sensitive than both conventional thick-section CT and chest radiography for the detection and characterization of ILD. With the advent of MDCT scanners it is also feasible to perform volumetric HRCT through the entire chest; this approach allows assessment of the entire lung parenchyma and therefore minimizes the risk of a false-negative examination. In addition, volumetric HRCT allows use of advanced imaging techniques such as MPRs and MIPs or MiniPs. There is no significant difference in overall image quality between volumetric HRCT and standard axial HRCT techniques. Follow-up CT studies can be performed by using low-dose technique whenever feasible.
A basic knowledge of the anatomy of the interstitium and secondary lobule is essential for understanding pathologic alterations in DLDs and their radiographic appearances. In general, DLD can be divided into disease involving primarily the airspace, interstitium, or small airways.
Attenuation (density) on a CT scan is expressed in terms of Hounsfield units (HU); under normal circumstances, water is 0 HU and air is −1000 HU. CT scans of the lung are usually obtained during maintained full inspiration. Normally the attenuation of lung parenchyma is determined by its relative proportions of blood, gas, extravascular fluid, and pulmonary tissue. The normal lung parenchyma has a fairly homogeneous attenuation that is slightly greater than that of air. As lung gas volume is reduced, lung attenuation increases. A significant increase in attenuation of the lung parenchyma is seen on expiratory scans. Attenuation values are variable and influenced by the pulmonary volume and different parameters such as kilovoltage, patient size, and the particular region of the lung being assessed. Appearance of CT images on the monitor or film (hard copy) depends on the display parameters used (window level ands window width) and is limited to 256 shades of gray. For clinical practice a window level of −600 to −700 HU and a window width of 1000 to 1500 HU are recommended. Window levels of 30 to 50 HU and window widths of 350 to 500 HU are the recommended parameters to evaluate mediastinum, hila, and pleura.
The secondary pulmonary lobule is a fundamental unit of lung structure defined as the smallest portion of the lung that is surrounded by connective tissue septa, made up of 3 to 12 acini, and supplied by 3 to 5 terminal bronchioles. A pulmonary acinus is defined as the portion of the lung parenchyma distal to a terminal bronchiole and supplied by a first-order respiratory bronchiole or bronchioles and comprising respiratory bronchioles, alveolar ducts, alveolar sacs, and alveoli. Secondary pulmonary lobules are irregularly polyhedral in shape and somewhat variable in size (1-2.5 cm in length). An understanding of lobular anatomy is essential to interpretation of thin-section CT of the lung. The most typical secondary pulmonary lobules are recognized in the outermost part of the lung, where each lobule is bounded by the visceral pleura, interlobular septa, and pulmonary veins. The secondary lobule of the lung is separated incompletely by the interlobular septa from adjacent lobules.
Structures in the centrilobular region include terminal and proximal respiratory bronchioles, accompanying pulmonary arteries, interstitial tissue enveloping these bronchovascular structures, and immediately adjacent alveolar ducts, sacs, and alveoli. The smallest pulmonary arteries and accompanying bronchioles are situated in the centrilobular area, but bronchioles cannot be seen in normal conditions because they are no more than 1 mm in diameter and their walls are less than 0.1 mm thick. Lambert's canal connects airspace to bronchioles. The number of terminal bronchioles included in one lobule varies from 3 to approximately 30, depending upon the size of the lobule. The interlobular septa define the margins of the secondary pulmonary lobule, whose sides measure approximately 1 to 2.5 cm in length and contain pulmonary venules and lymphatics. Normal intralobular bronchioles and interlobular septa usually are not detected on CT. Their presence usually indicates abnormal thickening of the bronchiolar walls or septal interstitium.
The imaging findings associated with DLDs have been extensively described. A number of studies have shown that HRCT can demonstrate the presence, distribution, and extent of parenchymal abnormalities, reflecting the pathologic findings closely. The best approach to diagnosing DLD is recognition and analysis of the different patterns. In an attempt to arrive at a differential diagnosis, DLDs can be further characterized into two broad categories based on their CT appearances: (1) increased lung attenuation, including linear and reticular opacities, nodular opacities, ground-glass opacity, and airspace consolidation, and (2) decreased lung opacity, including cysts, cavities, emphysema, and mosaic perfusion.
Interlobular septum marginates part of a secondary pulmonary lobule. Normally only a few septa are seen. Thickening of interlobular septa (septal lines) is commonly seen in patients with a variety of ILDs and may be smooth, irregular, or nodular. Thickened septa can result from fibrosis, interstitial fluid, and infiltration by cells or other material. Septal lines may be smooth, nodular, or irregular. Smooth septal thickening is commonly seen in patients with pulmonary edema, hemorrhage, lymphatic spread of carcinoma, and amyloidosis; less common causes include lymphoma, leukemia, Churg-Strauss syndrome, acute lung rejection, lymphangiectasia, and metabolic storage diseases such as Niemann-Pick and Gaucher's diseases. Irregular septal thickening, often accompanied by distortion of lung architecture, is characteristic of diseases causing interstitial pulmonary fibrosis. Nodular or beaded thickening of the interlobular septa (beaded septum sign) occurs in sarcoidosis, lymphangitic carcinomatosis, amyloidosis, and occasionally silicosis. As noted earlier, the combination of ground-glass opacity with a superimposed linear pattern is termed crazy paving . Although initially described as typical of alveolar proteinosis, once again, this appearance is nonspecific and may be seen in a variety of acute (e.g., edema, hemorrhage, P. jiroveci pneumonia, ARDS) or chronic (alveolar proteinosis, exogenous lipoid pneumonia) lung diseases.
A reticular pattern (intralobular linear opacities) represents thickening of the intralobular interstitium within the secondary pulmonary lobule. Although intralobular interstitial thickening is a common HRCT finding in patients with usual interstitial pneumonia (UIP) it may also be seen in patients with nonspecific interstitial pneumonia (NSIP). Intralobular interstitial thickening usually indicate fibrosis; however, it can also be due to infiltration of such structures by infection, malignant cells (lymphangitic carcinomatosis), pulmonary edema, an abnormal protein (alveolar proteinosis), or simply edema fluid from cardiac failure.
A nodular pattern refers to multiple round opacities ranging in diameter from 1 to 10 mm. Nodules can be characterized according to their margins (e.g., well defined or poorly defined), presence of associated findings, and distribution. Three main types of distribution can be identified on HRCT: centrilobular, perilymphatic, and random. Multiple small smooth or irregularly marginated nodules in a perilymphatic distribution are characteristic of sarcoidosis, lymphangitic carcinomatosis, silicosis, and coal worker's pneumoconiosis (CWP). Centrilobular nodules are characterized on HRCT by their location within the secondary pulmonary lobule several millimeters away from the pleura, interlobar fissures, and interlobular septa. Morphologically, centrilobular nodules may have either a poorly defined ground-glass density or a tree-in-bud configuration; the tree-in-bud pattern is characteristic of acute cellular bronchiolitis, representing dilated centrilobular bronchioles impacted with mucus, fluid, and/or pus and usually associated with a peribronchiolar inflammation. Predominantly numerous small nodules of ground-glass opacity in a centrilobular distribution are characteristic of the subacute stage of extrinsic allergic alveolitis or respiratory bronchiolitis, and rarely cryptogenic organizing pneumonia (COP). Tree-in-bud opacities are characteristic of infectious bronchiolitis, bronchopneumonia, and endobronchial spread of TB or Mycobacterium avium-intracellulare complex (MAC) infection. Miliary nodules as small as 1 mm in diameter can be seen with hematogenous spread of TB, fungal infection, or metastases.
Nodules larger than 1 cm in diameter may result from conglomeration of small nodules, as occurs in sarcoidosis, silicosis, or talcosis. Larger nodules usually result from metastases, septic emboli, or Wegener's granulomatosis. In immunocompromised patients, nodules surrounded with a halo of ground-glass attenuation are caused by invasive pulmonary aspergillosis; small nodules with halo have been described in diverse viral infections.
Ground-glass opacity is defined as a hazy increased attenuation of lung, with preservation of bronchial and vascular margins. The significance of ground-glass opacity depends on the clinical scenario. This finding can be made with HRCT while plain films are still negative. Ground-glass opacity may be caused by partial filling of airspaces, interstitial thickening, partial collapse of alveoli, normal expiration, or increased capillary blood volume. It should not be confused with consolidation, in which bronchovascular margins are obscured and may be associated with an air bronchogram. Because it is a nonspecific CT finding, a large number of diseases can be associated with this pattern. In acute DLD, ground-glass attenuation as a main pattern is suggestive of acute interstitial pneumonia, acute or subacute hypersensitivity pneumonitis (HP), pulmonary edema, pulmonary hemorrhage, drug-induced disease, and Pneumocystis jiroveci pneumonia in patients with AIDS. The most common causes of ground-glass opacity in chronic DLD include NSIP, desquamative interstitial pneumonia (DIP), and respiratory bronchiolitis–associated interstitial lung disease (RBILD). A “crazy-paving” pattern represents superimposition of a linear pattern in a background of ground-glass opacity. This pattern, initially described in alveolar proteinosis, can also be seen in a variety of pulmonary disorders including bronchioloalveolar cell carcinoma and exogenous lipoid pneumonia.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here