Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The esophagus is designed to simply serve as a conduit to carry food into the stomach. It does not have any digestive, endocrine, or metabolic role. As a result, most non-neoplastic disorders affecting the esophagus are a result of mechanical, chemical, or immune-mediated injury to the relatively resilient nonkeratinizing squamous mucosa. These disorders can be broadly categorized into inflammatory, infectious, congenital and acquired structural abnormalities; motility, traumatic, and vascular disorders; and those associated with systemic diseases. Inflammatory disorders and infections are by far the most common disorders encountered in daily practice. The remainder of the disorders usually require a combination of clinical, radiographic, and endoscopic examinations for accurate diagnosis, and histologic examination often does not yield specific diagnostic findings.
This chapter is organized based on broad categories of non-neoplastic esophageal disorders. It is, however, essential to note that inflammatory disorders are a manifestation of several common types of stimuli, such as reflux, infections, drugs, and systemic disorders, among others. Therefore, based on the predominant inflammatory cell, these disorders can also be categorized into neutrophil-rich esophagitis, eosinophil-rich, lymphocyte-rich, and paucicellular esophagitis. Neutrophil-rich disorders are most commonly caused by reflux disease and infections (see Chapter 9 for details). Eosinophil-rich disorders include eosinophilic esophagitis (EoE), reflux, parasitic infections, Crohn’s disease, drug hypersensitivity, hypereosinophilic syndrome, celiac disease, vasculitis, and collagen vascular disorders. Lymphocytes are a predominant component of inflammation in patients with chronic reflux, drugs or medications, Crohn’s disease (pediatric), achalasia or motility disorders, autoimmune diseases, immunodeficiency (human immunodeficiency virus [HIV], common variable immunodeficiency [CVID]), celiac disease, and dermatologic conditions. Last, some conditions, such as corrosive injury, sloughing esophagitis, graft-versus-host disease (GVHD), CVID, and certain medications may not show a significant inflammatory component and thus manifest as paucicellular esophagitis.
Reflux esophagitis, also known as gastroesophageal reflux disease (GERD), is one of the most common non-neoplastic disorders of the esophagus. Its prevalence ranges between 5% and 22% and depends on the geographic location. The reported prevalence of GERD is 22% in the United States. Pregnant women have a higher incidence. The pathophysiologic hallmark of reflux is the presence of lower esophageal sphincter (LES) dysfunction. Nonerosive reflux disease (NERD) is defined as patients with classic GERD symptoms but no evidence of mucosal injury on endoscopy.
Reflux occurs at all ages and in both genders. Typical symptoms include heartburn and regurgitation. Other uncommon or atypical symptoms include dysphagia, angina-like chest pain, chronic hoarseness or cough, asthmatic episodes, and protracted hiccups. If left untreated, reflux may lead to complications such as erosive esophagitis, strictures, Barrett’s esophagus (BE), and malignancy. Importantly, a number of individuals with reflux do not manifest symptoms, although the risk for adenocarcinoma arising from BE remains. GERD may be a secondary complication of other disorders affecting the esophagus, such as systemic sclerosis.
Gastroesophageal reflux disease is a clinical diagnosis and is often classified as erosive or nonerosive based on endoscopic or pathologic findings. The current recommendations from the American Society of Gastrointestinal Endoscopy do not support using endoscopy and biopsy to diagnose typical GERD but rather to exclude other pathologies in complicated or refractory cases. Furthermore, the degree of histologic damage may not correlate with clinical symptoms, and histologic findings alone have a low sensitivity and specificity for diagnosing GERD.
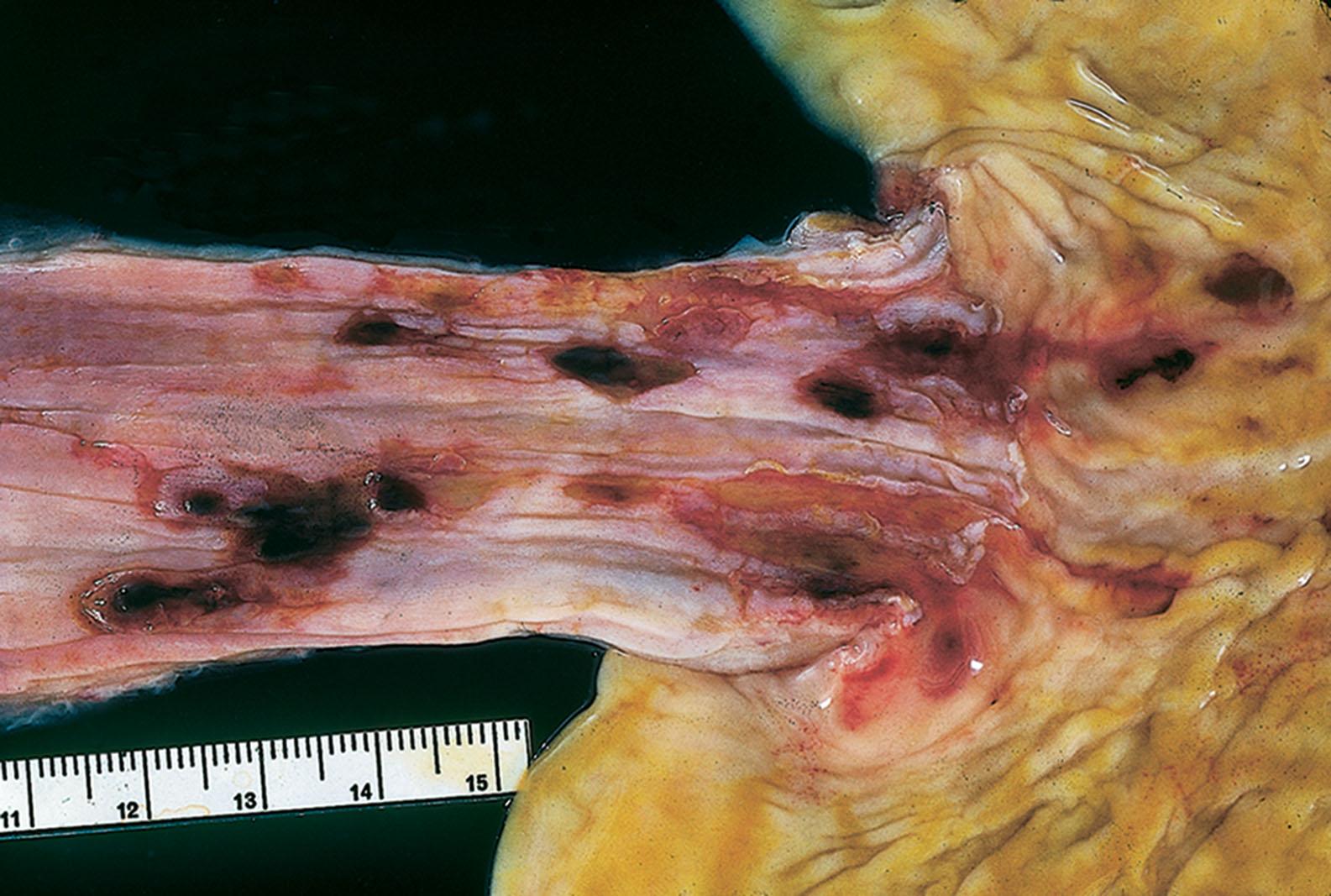
Endoscopic examination in cases of reflux is variable, depending on the severity and the chronicity of the symptoms. Some patients may have erythema, erosions, or ulceration. Deep ulcerations, bleeding, and peptic strictures are seen in severe cases ( Fig. 1.1 ). Patients with NERD by definition have normal white-light endoscopy, although high-definition endoscopy or narrow-band imaging may reveal subtle changes, including prominent vascularity and irregularity of the gastroesophageal junction (GEJ), creating a group of patients with so-called minimal change esophagitis.
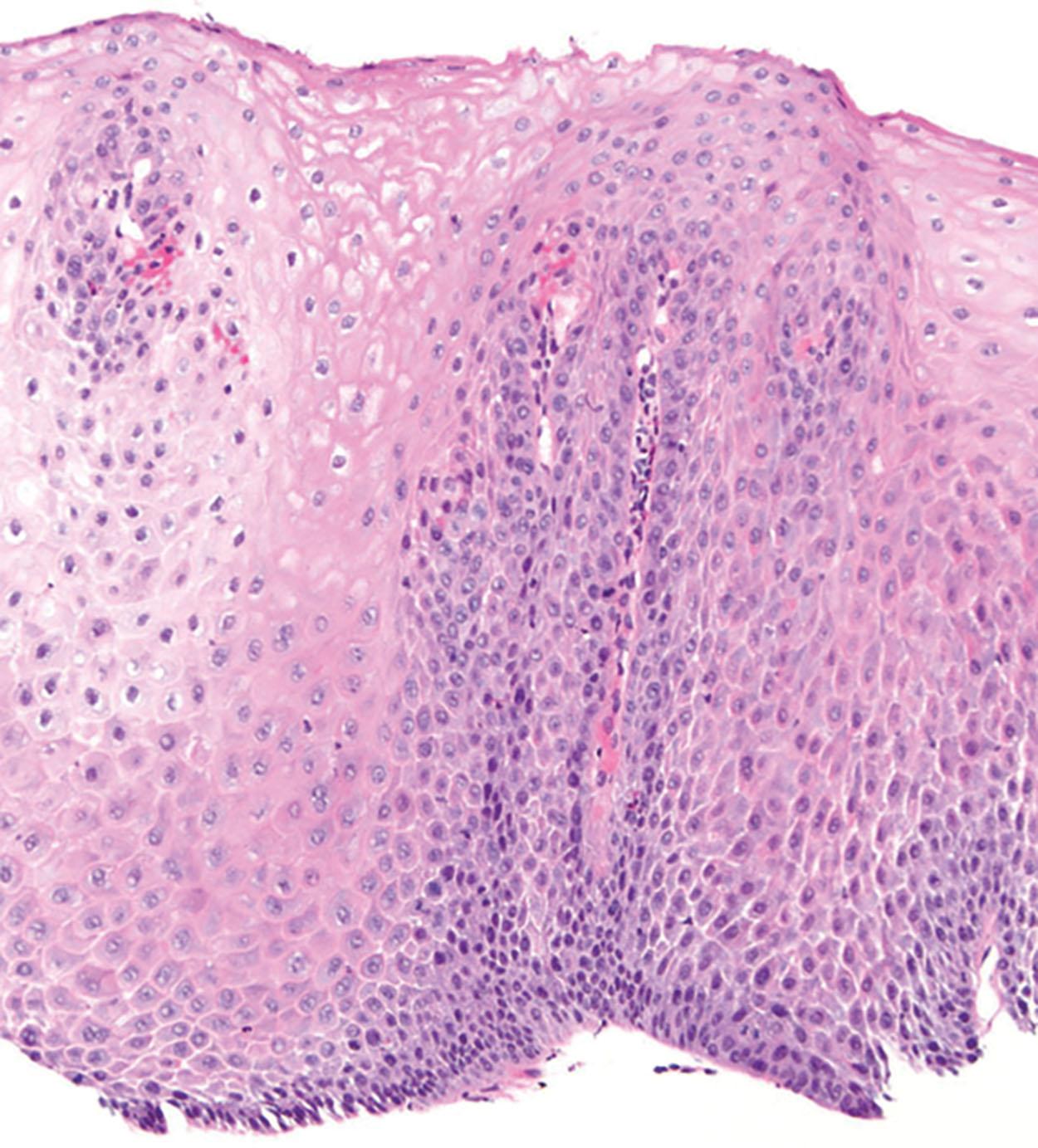
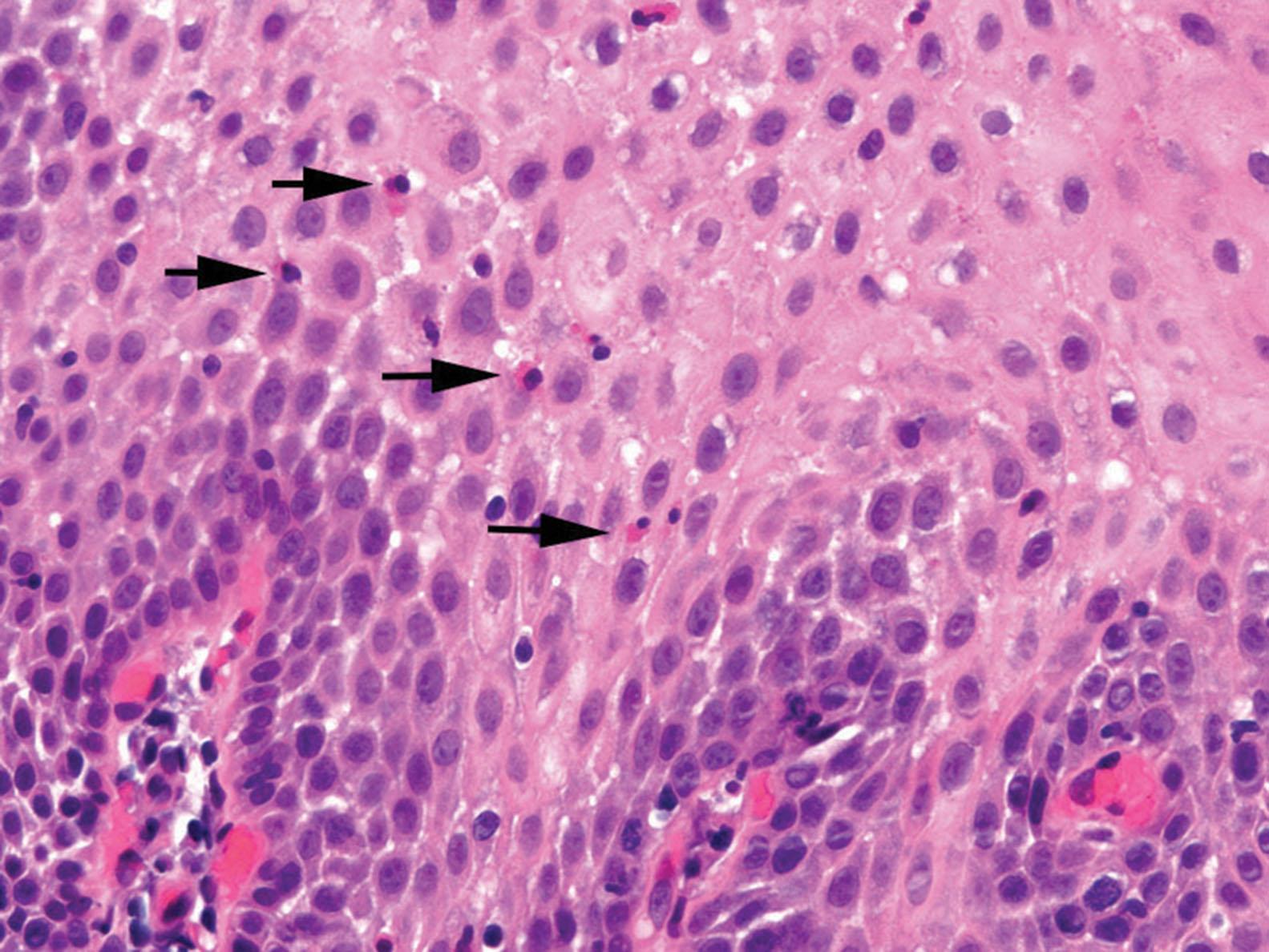
Histologic findings are usually localized to the lower esophagus and taper off or are virtually absent in the proximal segment of esophagus. The typical histologic features include basal cell hyperplasia (thickening of the basal layer to >15% of the total epithelial thickness or more than four to six basal cell layers in well-oriented sections), elongation of the papillae (>60% of the total epithelial thickness), and spongiosis ( Fig. 1.2 ). Inflammatory changes include increased numbers of lymphocytes ( Fig. 1.3 ), neutrophils, and eosinophils ( Fig. 1.4 ). Erosions or ulcers are typically associated with severe GERD. Intraepithelial lymphocytes are predominantly T cells, which tend to acquire an elongated shape (“squiggly lymphocytes”) while traversing between the intercellular spaces. Additional findings including balloon cell change ( Fig. 1.5 ) and hyperkeratosis.
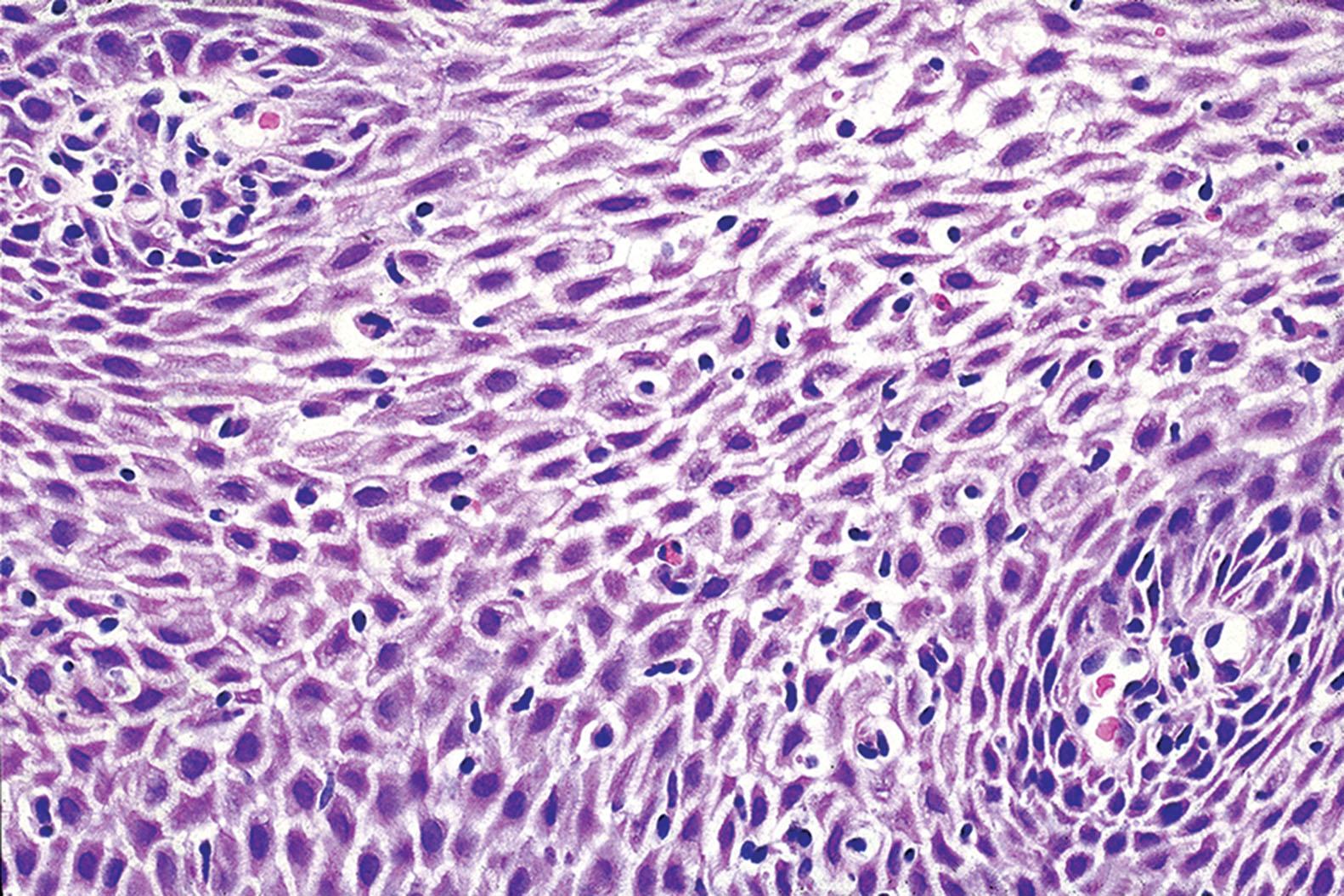
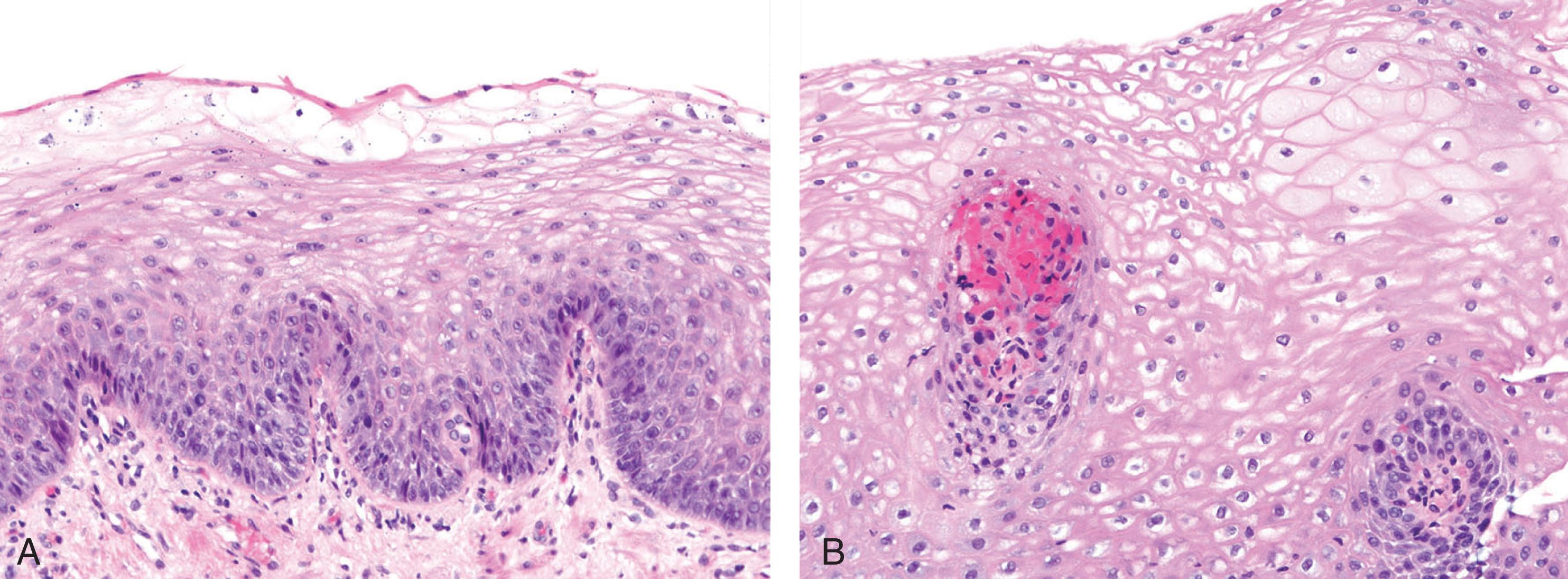
The presence of dilated intercellular spaces (spongiosis; see Fig. 1.3 ) was once considered to be a promising histologic marker of early GERD. It may be the predominant histologic finding in a patient with GERD; however, given the low interobserver agreement in assessing this feature, it remains less helpful compared with other typical features of GERD described already. It should be noted that many patients undergoing endoscopic biopsy have been on a trial of proton pump inhibitors (PPIs) and may have been asked to discontinue the medications 1 or 2 weeks or before endoscopy. In this setting, the most common histologic features are increased intraepithelial lymphocytes, basal layer hyperplasia, and elongation of the papillae. The finding of basal layer hyperplasia, elongation of the papillae, and a few eosinophils within 1 to 2 cm of the GEJ may also represent physiologic reflux. This finding is of no clinical significance. In a recent prospective evaluation of 336 patients with clinical symptoms of GERD, found that total epithelial thickness of 400 µm or greater at 0.5 cm and 430 µm or greater at 2.0 cm above the Z line was the best histologic feature to reliably identify patients with GERD.
Although endoscopically normal, patients with NERD may have dilated intercellular spaces (spongiosis), as well as basal layer hyperplasia and elongation of the papillae of the squamous epithelium, often grouped together as reactive epithelial change, without significant inflammation. Reporting these findings may be helpful to distinguish patients with NERD from those with functional heartburn.
Eosinophilic esophagitis, infectious esophagitis, and pill esophagitis are in the differential diagnosis. In EoE, there is an increased density of eosinophils per high-power field (hpf) along with eosinophil microabscess formation and superficial layering of eosinophils. More importantly, EoE affects both the distal as well as proximal segments of the esophagus, is associated with characteristic rings and furrows on endoscopy, and is resistant to PPI therapy.
Infectious esophagitis, such as that caused by Candida , herpes simplex virus (HSV), and cytomegalovirus (CMV) shows specific features. Candida esophagitis reveals yeast and pseudohyphal forms that invade the mucosa and are accompanied by severe acute inflammation. Squamous epithelial cells infected with HSV show multinucleation, nuclear molding, and margination of chromatin. Viral cytopathic effect of CMV is best appreciated in stromal and endothelial cells within granulation tissue where large, infected cells show intranuclear and intracytoplasmic eosinophilic inclusions.
Pill esophagitis can be associated with prominent eosinophilia, spongiosis, and ulceration. These changes are nonspecific and need to be analyzed in light of the clinical presentation. Polarizable crystalline material may be seen in alendronate-related injury, and crystalline stainable iron can be found in ferrous sulfate-induced esophagitis. Lymphocytic esophagitis (LE), skin disorders such as lichen planus, and esophageal dysmotility states, such as achalasia and strictures, are in the differential diagnosis when increased intraepithelial lymphocytes are present. Esophagitis can also be seen in Crohn’s disease, sarcoidosis, GVHD, collagen vascular disease, or Stevens-Johnson syndrome.
Prognosis depends on the degree of LES pressures. Extremely low pressures (6 mm Hg) predict a more severe degree of reflux and worse prognosis. Early diagnosis, before the onset of extensive ulcers and strictures, is essential for best patient outcome. Conservative therapy includes significant lifestyle modifications, such as elevation of the head of the bed, avoiding recumbence after meals, weight loss in obese patients, avoiding dietary triggers, and avoiding tobacco and alcohol consumption. PPIs, histamine 2 receptor antagonists, and antacids are the mainstay medical therapy for GERD. Nissen fundoplication and laparoscopic sphincter augmentation are surgical options for those who have failed medical or endoscopic therapy,
Inflammation of the lower esophagus resulting from damage caused by acid reflux from the stomach
The most common form of esophagitis, with prevalence of about 22% in the United States
Localized to the distal esophagus
Affects both sexes and all age groups
Heartburn and regurgitation are the typical symptoms; dysphagia also occurs
Atypical presentation includes angina-like pain, hoarseness, cough, asthma, and hiccups
Some individuals are asymptomatic
Prognosis depends on the degree of lower esophageal sphincter pressure
Early detection prevents complications
If left untreated, severe ulcerations, strictures, Barrett’s esophagus, and adenocarcinoma may develop
Treatment includes lifestyle modifications, proton pump inhibitors, and surgical procedures (Nissen fundoplication) in severe cases
Half of symptomatic patients have normal endoscopic examinations
Erythema, erosions, or ulceration can be seen
Deep ulcers are followed by strictures in severe disease
Barrett’s esophagus (salmon-colored mucosal tongues) may be present in long-standing cases
Architectural changes of basal cell layer hyperplasia, elongation of papillae, and spongiosis
Increased numbers of intraepithelial eosinophils, lymphocytes, and/or neutrophils
Erosion or ulceration
Balloon cell change, hyperkeratosis, and increased total epithelial thickness may be seen
Eosinophilic esophagitis has proximal esophageal involvement and often has more severe eosinophilic infiltrates, superficial eosinophil layering, and eosinophilic microabscesses
Infectious esophagitis ( Candida , herpes simplex virus, and cytomegalovirus) can be assessed on hematoxylin and eosin and confirmed by special stains and immunohistochemical stains
Lymphocytic esophagitis and lymphocyte-rich skin disorders typically have only lymphocytes without neutrophils or eosinophils but require clinical correlation
Pill esophagitis and Crohn’s disease require clinical correlation
Eosinophilic esophagitis is a primary clinicopathologic disorder of the esophagus that has been associated with an increasing prevalence and has gained significant recognition over the past few years. It is defined as a chronic immune and antigen-mediated esophageal disease characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation. Three specific criteria are required to diagnose EoE: symptoms related to esophageal dysfunction, a peak eosinophil count of at least 15 eosinophils/hpf on esophageal biopsy, and eosinophilia limited to the esophagus with other causes of esophageal eosinophilia excluded. Although one of the clinical features of EoE is the lack of response to PPIs, recent studies have shown that one-third or more patients with esophageal eosinophilia can show response to PPIs. This phenomenon has been termed PPI-responsive esophageal eosinophilia . A recent transcriptome analysis study by found significant molecular overlap between PPI-responsive esophageal eosinophilia and EoE, suggesting these two entities represent a diagnostic continuum or that PPI-responsiveness is a subphenotype of EoE. However, this relationship is yet to be fully characterized.
Eosinophilic esophagitis occurs in all age groups but is seen more frequently in young children with atopic symptoms such as eczema, asthma, and food allergies. Symptoms manifest differently in different age groups: whereas infants and children often present with feeding difficulties, regurgitation, dyspepsia, abdominal pain, and vomiting, adults usually describe dysphagia and food impaction with or without chest or abdominal pain. If not recognized early, EoE can progress to odynophagia and stenosis.
A form of allergic esophagitis associated with atopic symptoms
Can present alone or as part of eosinophilic gastroenteritis
1% to 2% of patients undergoing esophageal biopsy
The entire esophagus is involved; proximal eosinophil count may be higher than distal; eosinophils may be patchy in distribution
Occurs in all age groups but is more common in the children and young adults
Men are more frequently affected than women
Symptoms in children include vomiting, abdominal pain, dyspepsia, and solid food impaction
Symptoms in adults include dysphagia, food impaction, and chest and abdominal pain
Associated with food allergies and atopic symptoms
Best outcome if diagnosed and treated early
May lead to severe esophageal strictures if untreated
Elimination of food allergens and topical corticosteroids are the treatments of choice
When strictures occur, dilation is indicated
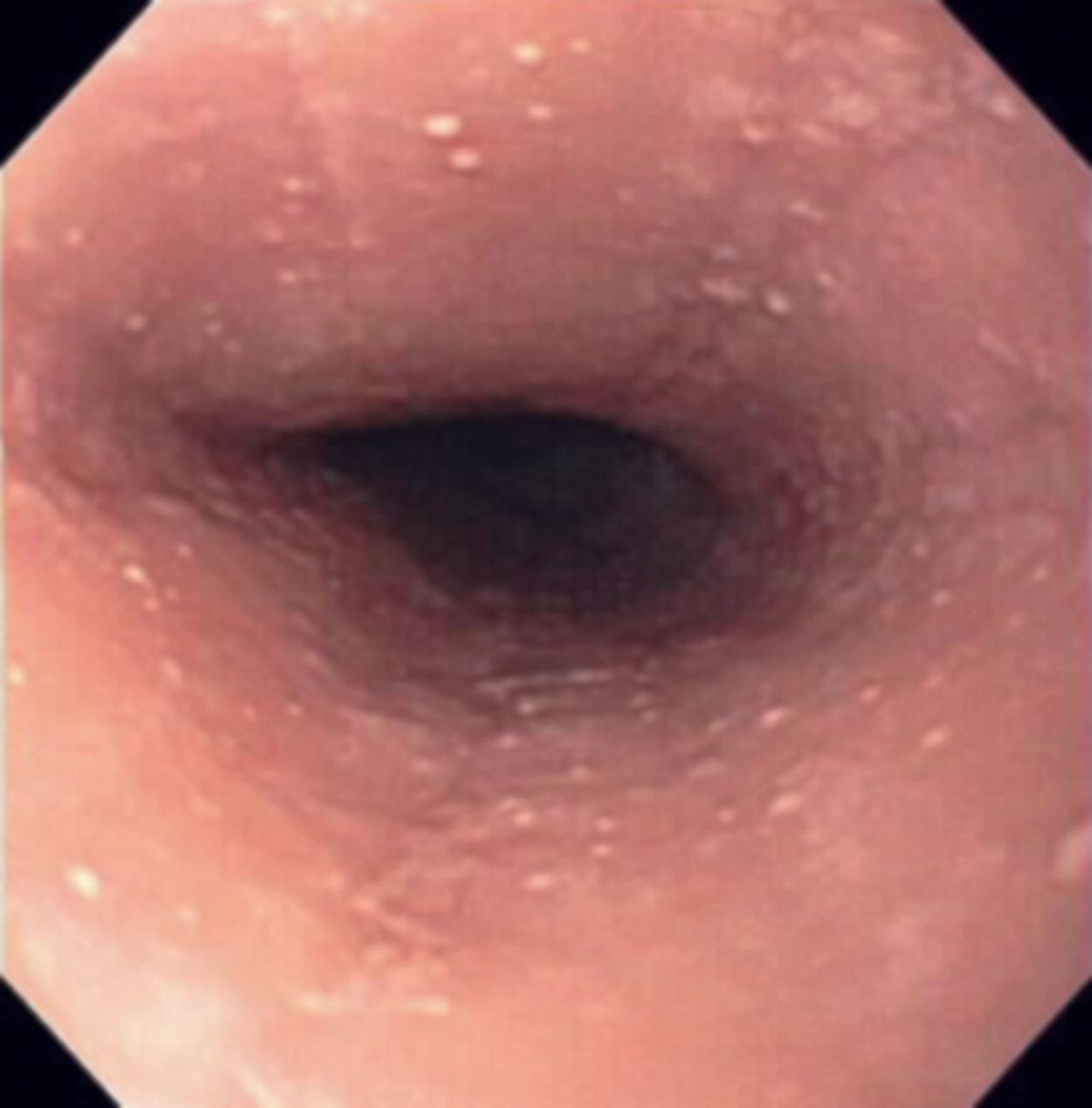
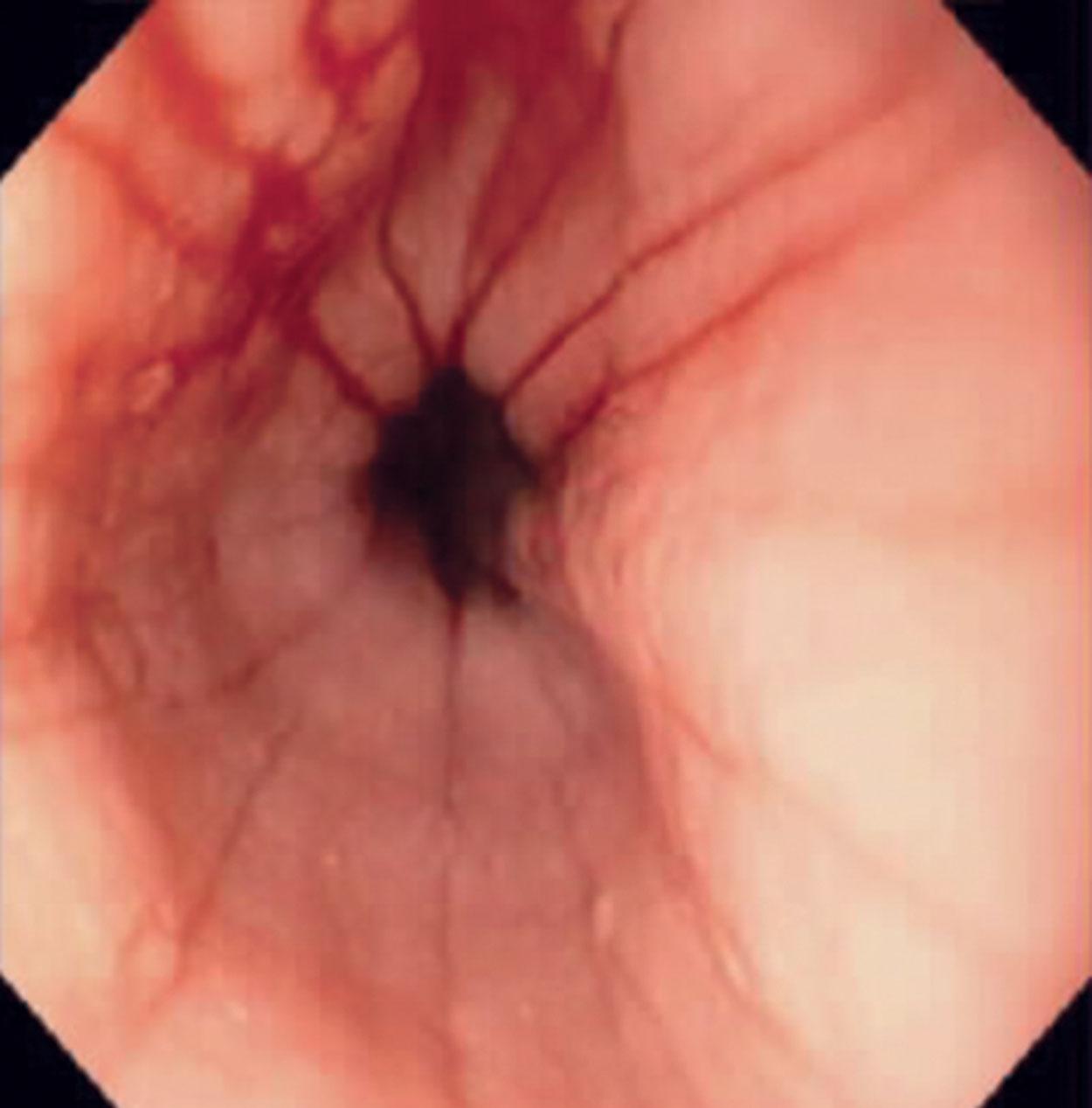
Classic endoscopic findings include mucosal rings, furrows (also known as “trachealization” of the esophagus), granularity, exudates, and mucosal fragility ( Figs. 1.6 and 1.7 ). However, in some patients, the endoscopic findings can be completely normal. In long-standing cases, stricture formation may be seen.
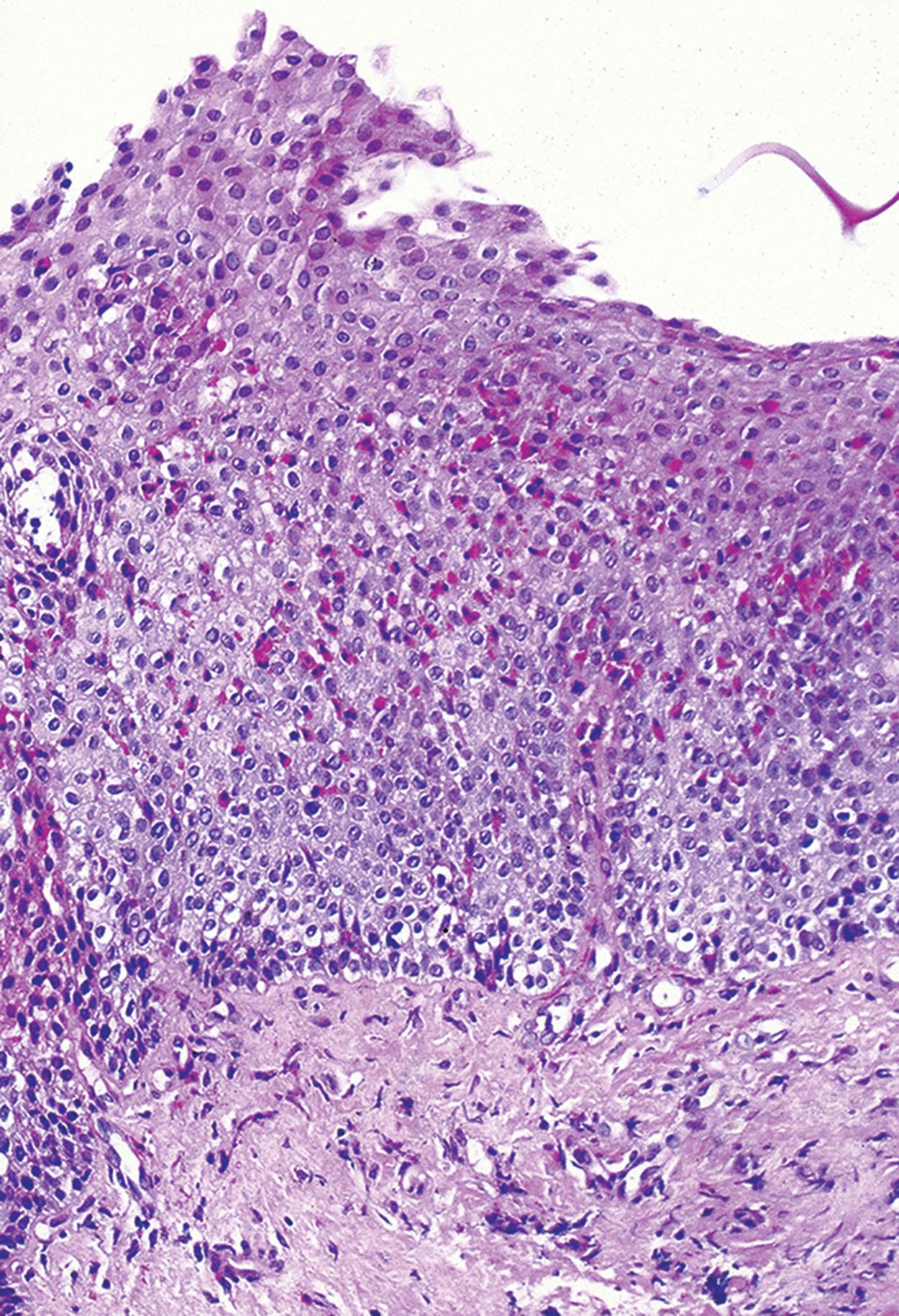
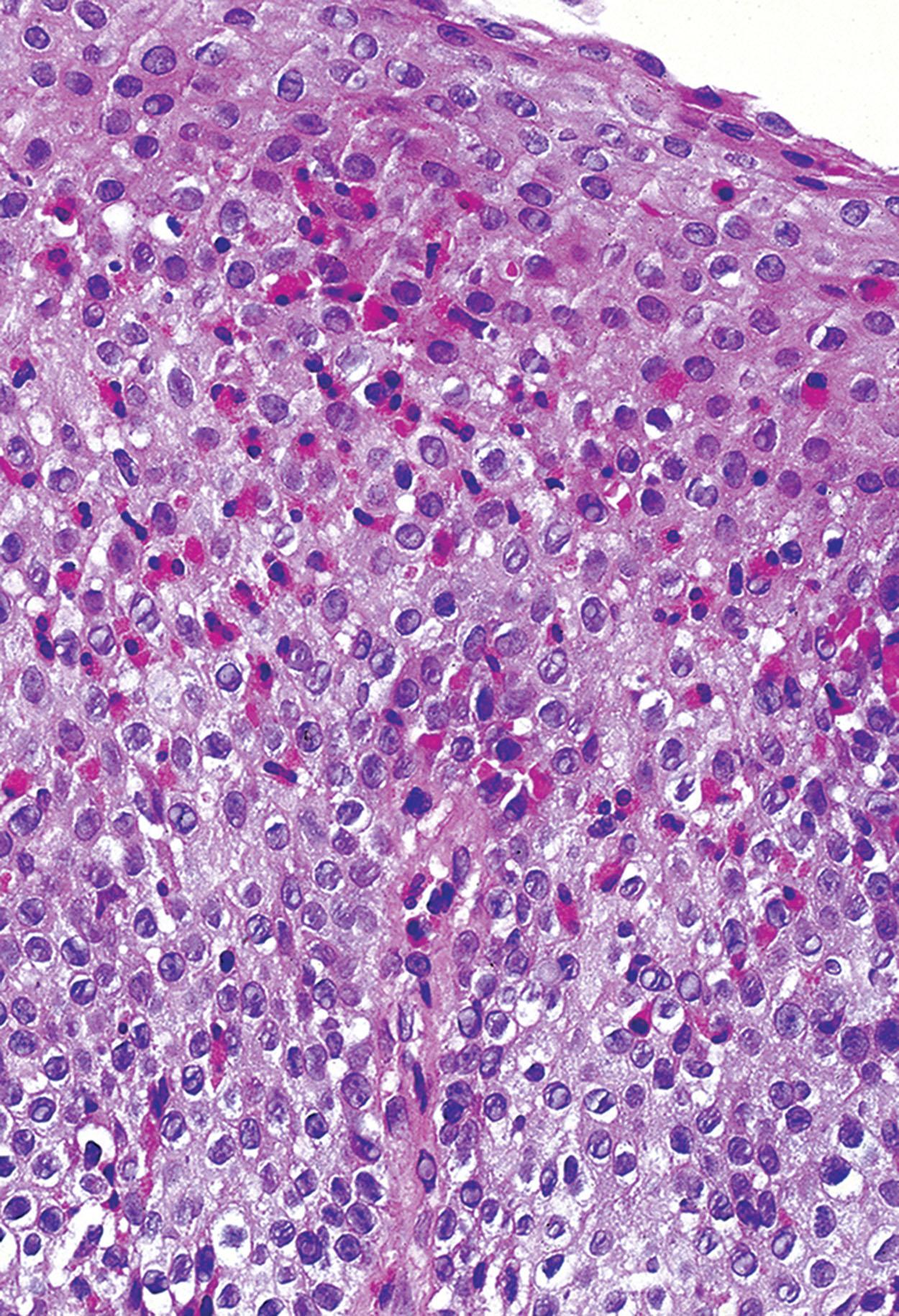
Biopsies show increased intraepithelial eosinophils (≥15 eosinophils/hpf) with concentration of eosinophils toward the luminal aspect of the epithelium (superficial layering). Eosinophilic microabscesses and degranulation of eosinophils ( Fig. 1.8 ) are frequently present. The density of eosinophils can vary with anatomic location of biopsy and within biopsy fragments. In general, biopsies from the proximal segment reveal more eosinophilia than the distal segment. It is therefore recommended that the total number of eosinophils per high-power field be generated by examining the fragments at low magnification and selecting the high-power field with maximum number of eosinophils ( Fig. 1.9 ). Care should be taken to avoid counting eosinophils within the papillae. Other findings include basal cell hyperplasia, elongation of the papillae to greater than 50% the thickness of the squamous epithelium, spongiosis, lamina propria, and submucosal fibrosis. In patients who have received diet elimination or steroid therapy, follow-up biopsies may be performed to evaluate response to therapy, in which case, giving the exact eosinophil count may be helpful.
Endoscopic examination reveals mucosal rings, furrows (“trachealization” of esophagus), erythema, and granularity
In long-standing cases, strictures are seen
Marked increase in intraepithelial eosinophils (≥15/hpf)
Eosinophil infiltrates may be more prominent in the proximal than in the distal esophagus
Superficial layering of eosinophils, eosinophilic microabscesses, and degranulation
Additional findings include basal cell hyperplasia, elongation of the papillae, spongiosis, and fibrosis of the lamina propria and submucosa
Reflux esophagitis changes are mostly seen in biopsy samples from the distal esophagus or gastroesophageal junction
In eosinophilic gastroenteritis, eosinophils are also present in other segments of the gastrointestinal tract
Drug-induced injury to the esophagus requires clinicopathologic correlation
Parasitic infections do not typically affect the entire esophagus. Biopsy specimens may show parasitic organisms
Eosinophilic esophagitis must be distinguished from reflux esophagitis, pill-induced esophagitis, eosinophilic gastroenteritis, and parasitic infections. It is important to note that any of the histologic features of EoE can also be seen in patients with reflux esophagitis. Distal esophagus-predominant mucosal changes with unremarkable proximal esophageal biopsy favors a diagnosis of reflux esophagitis. Pill-induced esophagitis is often accompanied by ulcer and granulation tissue. Some medications (alendronate, iron supplements) can be visualized on light microscopy. However, confirmation of drug-induced injury requires clinicopathologic correlation. Eosinophilic gastroenteritis is usually associated with peripheral blood eosinophilia and affects the rest of the gastrointestinal (GI) tract. Parasitic infections tend to be a localized phenomenon, and deeper levels may reveal the organism.
The prognosis is excellent when treatment is given promptly. Dietary elimination of the six common offending foods (milk, egg, wheat, soy, peanuts and tree nuts, and seafood) and topical steroids leads to dramatic improvement in symptoms and histology. Rarely, patients refractory to steroid therapy may show disease progression in the form of esophageal strictures that require repeated dilation procedures.
Lymphocytic esophagitis is a poorly defined clinicopathologic entity. A variety of clinical diagnoses may be associated with increased intraepithelial lymphocytes on biopsy; therefore, lymphocytic esophagitis pattern of injury is the preferred diagnostic terminology used by many pathologists. Some studies have demonstrated that the increased intraepithelial lymphocytes of LE in patients with dysmotility are predominantly CD4+ T cells, in contrast to the normally present scattered intraepithelial lymphocytes, which are CD8+ T cells. In patients with LE and normal motility, both CD4+ and CD8+ T cells are increased.
Symptoms include dysphagia, chest pain, heartburn, nausea, and odynophagia. Symptoms can lead to a clinical impression of EoE. Adults and children both can be affected, and most patients are diagnosed in the fifth or sixth decade of life. Men and women are equally affected. Many patients diagnosed with LE also have potentially confounding diagnoses, including GERD, inflammatory bowel disease (IBD), hypothyroidism, allergies or asthma, history of radiation or chemotherapy, and connective tissue disease.
In about a quarter of patients, the endoscopic impression of the mucosa is normal. Endoscopic findings can mimic those seen in EoE and include esophageal rings, esophagitis, and strictures. Findings suggestive of motility disorder may be identified. Erythema, nodularity, plaques, furrows, and webs have also been reported.
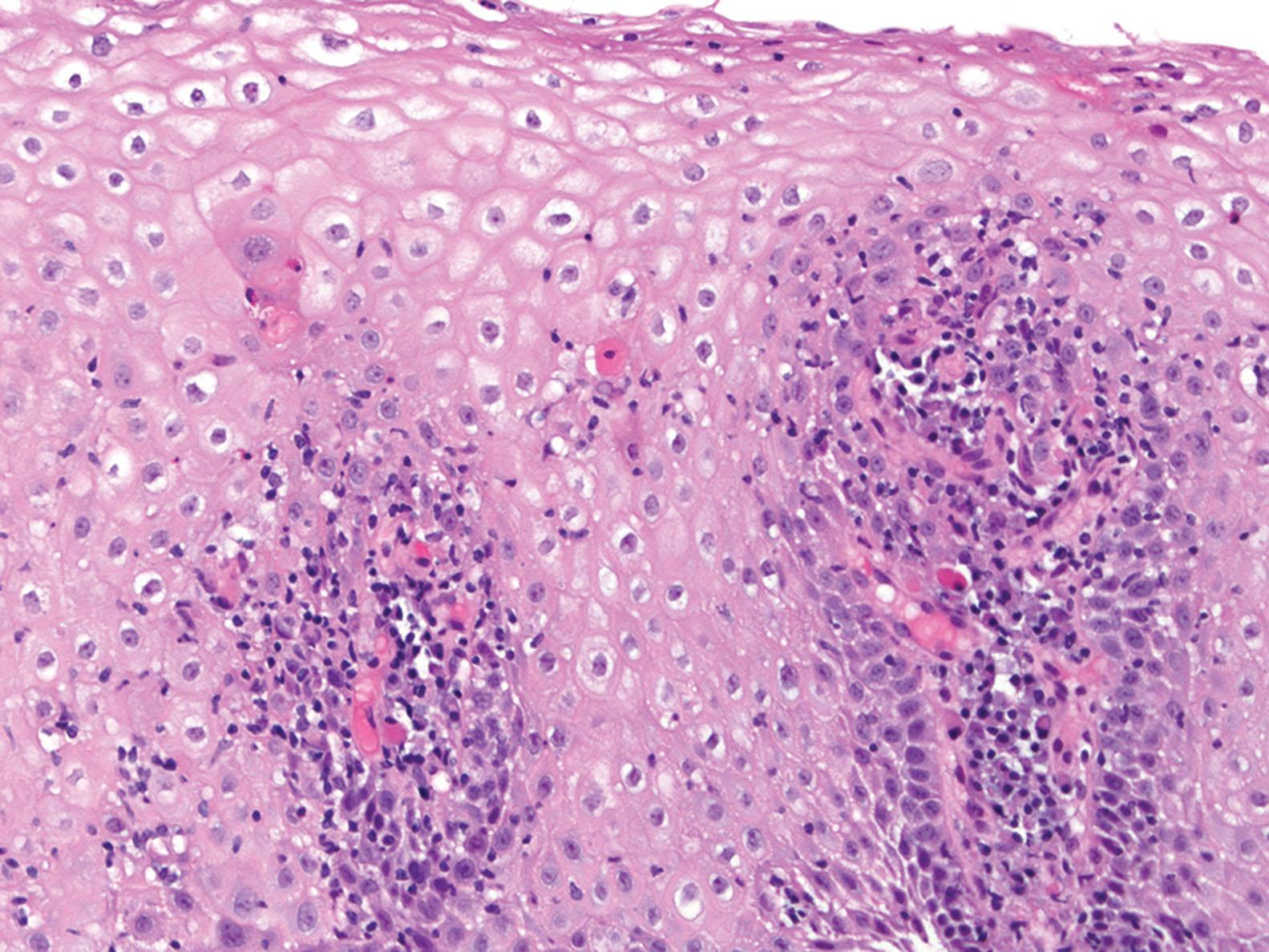
The esophageal biopsy shows increased intraepithelial lymphocytes, predominantly in a peripapillary distribution, with spongiosis of the associated peripapillary squamous epithelium ( Fig. 1.10 ). Similar to EoE, the distribution of intraepithelial lymphocytes is usually patchy and can vary between different biopsy fragments, with some papillae being unaffected. Neutrophils or eosinophils are rare or even absent. There may be accompanying basal cell hyperplasia and spongiosis. Unfortunately, there is no standard number of intraepithelial lymphocytes to diagnosis LE, and studies have included various minimum cut-offs of 20 lymphocytes, 30 lymphocytes, or 50 lymphocytes per high-power field. It is therefore appropriate to render a descriptive diagnosis of “LE pattern of injury” with a comment consisting of the various conditions that may result in this pattern of injury.
Increased intraepithelial lymphocytes can be seen in reflux esophagitis, which typically shows more neutrophils and eosinophils. Esophageal Crohn’s disease is characterized by increased intraepithelial lymphocytes, especially in the pediatric population. Granulomas may be seen in some cases. However, involvement of the rest of the GI tract is helpful in confirming a diagnosis of Crohn’s disease. Achalasia and other motility disorders can have increased intraepithelial lymphocytes on biopsy but usually show radiographic and endoscopic evidence of dysmotility. Inflammatory disorders of the skin, including lichen planus, can affect the esophagus resulting in increased intraepithelial lymphocytes. The presence of interface activity, hyperkeratosis, parakeratosis, dyskeratotic keratinocytes, or a history of inflammatory skin disease can be helpful.
More than half of patients have symptomatic improvement with treatment, which most often includes a PPI. Patients with IBD may benefit from immunomodulatory therapy. Dilation is often helpful in patients with strictures. Follow-up endoscopic biopsies with the LE pattern of injury have shown persistence of LE, progression to reflux or Crohn’s disease, or complete resolution of histologic findings.
Increased number of intraepithelial lymphocytes, predominantly peripapillary, within the squamous esophageal mucosa, with associated spongiosis, and rare to no neutrophils or eosinophils
Incidence has been increasing over time
Can be seen anywhere along the esophagus
Men and women are equally affected
Any age can be affected; most patients are diagnosed in the fifth to sixth decade of life
Symptoms include dysphagia, odynophagia, chest pain, and heartburn
Patients may also carry a diagnosis of gastroesophageal reflux disease, inflammatory bowel disease, or allergy
Most patients have symptomatic improvement with proton pump inhibitors
Dysphagia is likely to resolve
Gastrointestinal symptoms and histologic findings persist in some patients
Normal mucosa, esophageal rings, esophagitis, strictures, features of motility disorder, erythema, nodularity, plaques, furrows, and webs
Increased peripapillary lymphocytes associated with spongiosis and rare to no neutrophils or eosinophils on biopsy of esophageal squamous mucosa
The lymphocytosis is patchy, with some unaffected papillae
Gastroesophageal reflux disease has more than rare granulocytes
Crohn’s disease may have granulomas and almost always has involvement of other gastrointestinal sites
Motility disorders have radiographic and endoscopic evidence of dysmotility
Inflammatory disorders of skin may have interface activity, hyperkeratosis, and dyskeratotic keratinocytes
Lichen planus of the esophagus, or lichen planus esophagitis, can occur with or without concurrent cutaneous lichen planus. For both lichen planus and lichenoid esophagitis pattern of injury, girls and women are affected about three times more often than boys and men. Adults and children can be affected, with a median age of about 64 years. Clinical symptoms include dysphagia and stricture and less commonly, esophagitis, heartburn, chest pain, and hiatal hernia. Whereas comorbidities including viral infections (HIV, hepatitis B, and hepatitis C) have been reported in patients with lichenoid esophagitis, hypothyroidism and rheumatologic diseases have been reported in patients with lichen planus esophagitis. Polypharmacy is associated with both conditions.
The squamous epithelium and lamina propria are involved by a dense band of predominantly T-cell lymphocytic infiltrates. Lymphocytic inflammation can be patchy or diffuse and affect the upper and lower esophagus. Apoptotic or otherwise degenerating squamous cells (Civatte bodies) in a lichenoid background are diagnostic of lichen planus esophagitis. The background squamous epithelium can be atrophic. Other inflammatory cell types are not prominent. Direct immunofluorescence demonstrates globular immunoglobulin M (IgM) deposits at the squamous–subsquamous interface in lichen planus and cases with negative direct immunofluorescence but with the other histologic features of lichen planus have been termed lichenoid esophagitis pattern of injury .
Graft-versus-host disease, achalasia and other motility disorders, Crohn’s disease, and reflux esophagitis with increased intraepithelial lymphocytes are prominent. LE has a peripapillary lymphocytosis, rather than bandlike, and lacks Civatte bodies.
Lichen planus is a chronic progressive disease that can result in esophageal stricture or even squamous cell carcinoma (SCC). Immunomodulatory medications are the mainstay of treatment.
Esophageal involvement in Crohn’s disease is uncommon, affecting about 6% of patients with Crohn’s disease.
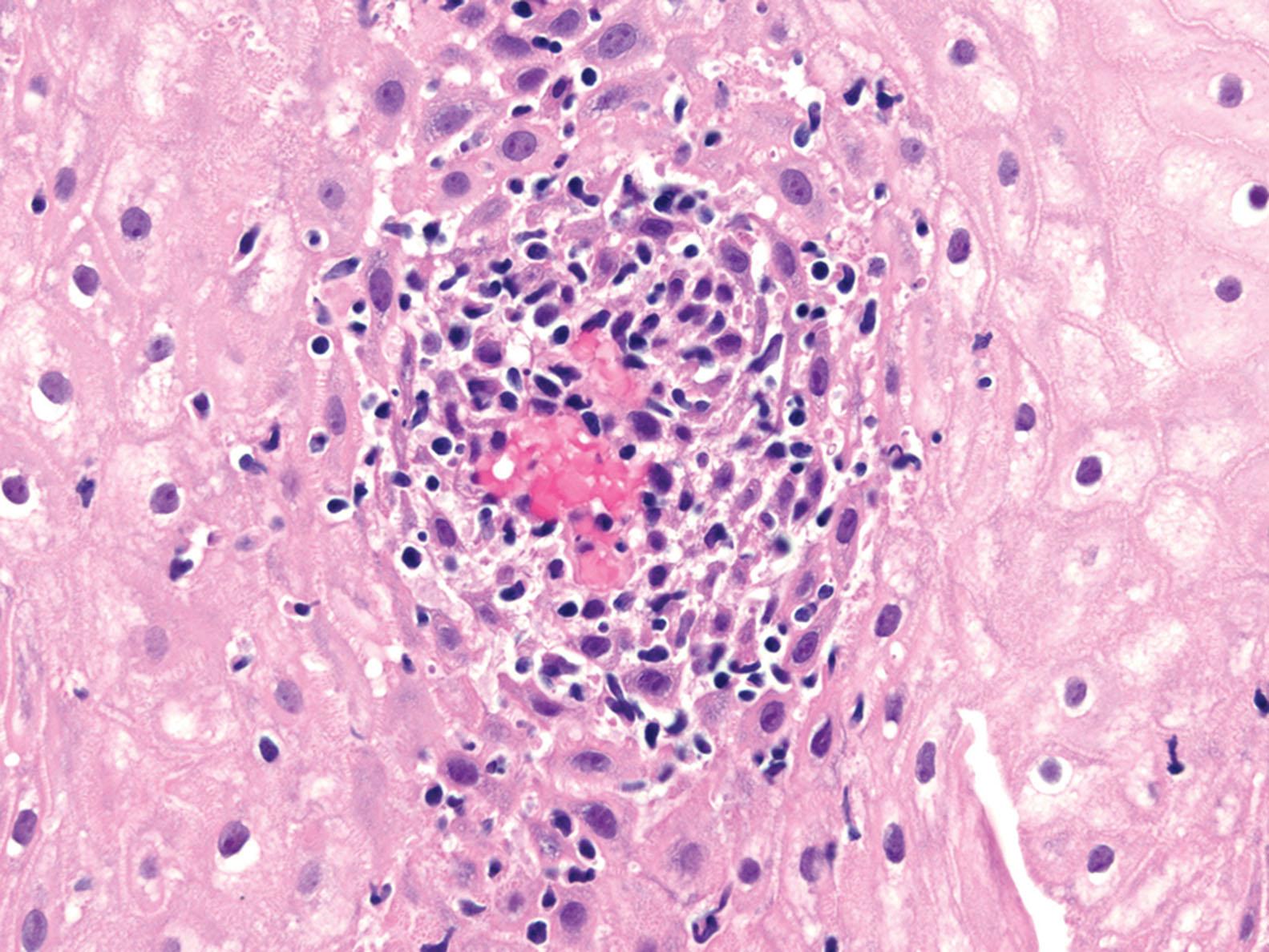
Esophageal biopsies may show increased intraepithelial lymphocytes, especially in pediatric patients ( Fig. 1.11 ). Well-formed, non-necrotizing epithelioid granulomas may also be present. Active inflammation consisting of intraepithelial neutrophils, erosion, or ulceration can be seen.
Knowledge of a patient’s diagnosis of Crohn’s disease, by demonstrated involvement in other organs, is useful when considering the differential diagnosis. Granulomatous esophagitis and LE are the main considerations if there is no other evidence of Crohn’s disease in other organs. Granulomatous esophagitis can be caused by infections, such as mycobacteria and fungus, sarcoidosis, Wegener’s granulomatous, chronic granulomatous disease, or some medications.
Patients with Crohn’s disease who have esophageal involvement are treated similar to those with disease elsewhere in the GI tract, including antiinflammatory agents and immunomodulators. There is no reported difference in prognosis for patients with Crohn’s disease who have esophageal involvement.
Esophageal involvement is uncommon in patients with GVHD and usually accompanies involvement of other parts of the GI tract. Symptoms of esophageal involvement include dysphagia and chest pain. On endoscopy, the mucosa appears friable and may be ulcerated. Rarely, severe cases may show prominent sloughing of the esophageal mucosa.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here